Chapter 1
advertisement

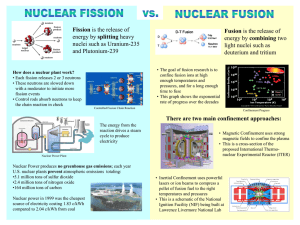
Chapter 1 Learning Objectives • Historical Recap • Understand the different length scales of nuclear physics • Know the nomenclature for isotopes and nuclear reactions • Know the different types of neutron nuclear collisions and their relationship to each other • Basic principles of nuclear reactor Learning Objectives • • • • • • • • • Neutron Sources Basic Principles of Nuclear Reactor Binding g energy gy curve Liquid drop model Fission Reaction ODE Review Radioactive Decay Decay Chains Chart of Nuclides Historical Recap • Driscoll handout • New programs – GNEP/AFCI – Gen-IV – Nuclear Power 2010 Why nuclear? • Power density – 1000 MW electric • 10 000 ttons off coall per DAY!! • 20 tons of uranium per YEAR (of which only 1 ton is U U-235) 235) A typical pellet of uranium weighs about 7 grams (0.24ounces). It can generate as much energy as…… 3.5 barrels of oil or…… 17,000 cubic feet of natural gas, or….. 1,780 pounds of coal. Why nuclear? • Why still use coal – Capital cost – Politics – Public perception of nuclear nuclear, nuclear waster issue 50% What do you think is the biggest barrier to constructing the next U.S. nuclear power plant? 40% Nuclear Waste Political Disposal Resistance to Issues Nuclear Energy Cost of Nuclear Power Plant Construction Fear of Nuclear Accident 14% 10% 0% 15% 10% 16% 20% 44% 30% Other Image by MIT OpenCourseWare. - 2008 surveyy of energy professionals “Eighty-two Eighty two percent of Americans living in close proximity to nuclear power plants favor nuclear energy energy, and 71 percent are willing to see a new reactor built near them, according to a new public opinion them survey of more than 1,100 adults nationwide. nationwide ” – NEI, NEI September 2007 Basic Principles of Nuclear Reactor • Simple device – Fissioning fuel releases energy in the “core” – Heat is transported away by a coolant which couples the heat source to a Rankine steam cycle – Very similar to a coal plant, with the exception of the combustion process – Main complication arises from the spent fuel, a mix of over 300 fission products Public domain image from wikipedia. Main Turbine Electric Generator Discharge Main Condenser Large Body of Water (Ocean, Lake, etc.) Intake Circulating Water Pump • Power plants will often often discharge their circulating water directly back to the ocean – Strict environmental prottecti tion regullatitions – Temperature increases by 5-10 10 Farenheits Image by MIT OpenCourseWare. Main Turbine Electric Generator Cooling Tower Main Condenser Circulating Water Pump Image by MIT OpenCourseWare. • If far from a water source, cooliling towers are used d to transfer the heat to air. – Water vapor is visible at the contact of the warm wet air inside the tower with the cool dry air outside Reactors Concepts • Fuel – Uranium – Plutonium – Thorium • Moderator (optional) – – – – Light water Heavy water Graphite Be • Coolant – – – – – – – – Light water Heavyy water Sodium Molten salt Helium CO2 Lead-Bi d Bismuth th … M Macroscopic i tto microscopic i i world ld Cutaway of PWR pressure vessel and Internals. Fission chain reaction Public domain image from wikipedia. Public domain image from wikipedia. Shield Wall Reactor Vessel Wall Control Rod Moderator (Water) Neutrons in a reactor Fuel Rods Image by MIT OpenCourseWare. Nomenclature Isotopes Nomenclature--Isotopes A Z 12 6 X such as C or 235 92 Z is the atomic number A is the atomic mass N = A-Z is the number of neutrons Nuclei with the same Z and different A are called isotopes. 238 U and E.g. 235 92 U 92 1 1 H and 12 H and 13 H U Nuclear Stability Image removed due to copyright restrictions. • As Z increases, the long range Coulomb repulsion between protons is balanced by the presence of additi dditionall neuttrons to provide additional short--range attractive short nuclear forces. Distribution of Stable Nuclides A Z N # nuclides Even Even Even 159 Odd Even Odd 53 Odd Odd Even 50 Even Odd Odd 4 266 Nuclear Collision Reactions a+b 1 0 c+d n+ 235 92 U→ 235 92 a(b, c)d 236 92 U +g 236 92 U (n, g ) U Fundamental Laws • Conservation of nucleons – Total “A” remains the same • Conservation of charge – Total “Z” remains the same • Conservation of momentum gy • Conservation of Energy – Energy, including rest mass, is conserved Rest Mass _ 2 2 2 E (pc) = (mc ) 2 Mass is a characteristic of the total energy and momentum of an object or a system of objects that is the same in all frames of reference. 2 m0 = E/c The invariant mass of the system is equal to the total system energy divided by c2. This total energy in the center of momentum frame, is the minimum energy which the system may be observed to have. Special Relativity Mass m= m0 2 _ ν 1 /c 2 A body’s mass increases when it is in motion with speed v relative to an observer at rest. Q - value Exothermic reaction produces energy Endothermic reaction requires energy An exothermic reaction is defined with Q < 0 therefore it is important to understand the concept: E = mc2 Q = [(Ma + Mb) _ (Mc + Md)]c2 Q > 0 exothermic Q < 0 endothermic Ea + Eb + Mac2 + Mbc2 = Ec + Ed + Mcc2 + Md c2 Examples of Q-value Exothermic _ _ ( Be) + M ( He ) M ( C) mn]c2 Q = [M _ Q = [9.012182u + 4.002603u 12.000000u _ 1.008664u]931.5MeV/u 9 4 4 2 12 6 Q = 5.702MeV 9 Be 4 + 4 He 2 12 C 6 + 1 n 0 Examples of Q-value Endothermic 13 _ _ Q = [M ( O) + mn M ( 6C) M(42He)]c2 16 8 _ Q = [15.994915u + 1.008664u 13.003354u _ 4.002603u]931.5MeV/u Q = _2.215MeV 16 8O + 1 n 0 13 C 6 + 4 He 2 Examples of Q-value 16 16 8O(n,p) 7N Assumption Q = ⎡⎣ mn + M ( 168O ) − M ( 167 N ) − mp⎤⎦ c 2 Why is this incorrect? 1 0 n + 168 O → 167 N + −10 e + 11 p This is approximately equivalent to 1 0 n+ O → N + H 16 8 16 7 1 1 Q = ⎡⎣ mn + M ( 168 O ) − M ( 167 N ) − M ( 11H )⎤⎦ c 2 Most important Reactions An Example 235U 92 + 10n 129 53I 1n + 104 Y + 3 39 0 Nuclear fission (n, fission) 1 0 n + ZA X → A1 Z1 X + ZA22 X + neutrons + 200MeV Radiative Capture An Example 238 92U + 1 0n 239 ( 92U)* Radiative capture (n, γ) 1 0 n+ X →( A Z A+1 Z 239 92U X) → * A+1 Z + 0 0γ X +g Scattering Examples elastic 126C + 10n 1 inelastic ( 240 Pu + 94 0n 12 1 C + 6 0n 240 1 Pu)* + 94 0n 240 0 1 Pu + β + 94 0 0n Scattering (n, n) or (n, n') 1 0 1 0 A Z 1 0 A Z 1 0 A Z n+ X ν n+ X elastic scattering (n,n) n + X ν n + ( X ) ν 01n + ZA X + γ A Z * inelastic scattering (n,n') Beta decay When the weak interaction converts a neutron into a proton and emits an electron and an anti-neutrino, Beta (minus) decay occurs. This happens when an atom has an excess of neutrons. A Z 0 −1 X → e+ Cs 137 55 A Z +1 _ Y + u + Energy _ 137 0 56Ba + -1e + υ Positron Emission Positron emission cannot occur in isolation unlike Beta decay. This happens because it requires energy (the mass of the neutron is greater than the mass of the proton). Positron emission happens inside the nuclei when the absolute value of the binding energy of the mother nucleus is lower than that of the daughter nucleus. A ZX + Energy 22 11 Na 22 10 A Z-1Y 0 1 + 0 1e Ne + e + υ +υ Capture of Electron A ZX + 0 -1e + Energy A Z-1Y +u In cases where ß+ decay is allowed energetically, it is accompanied by the electron capture process. 22 Na 11 + 0 -1e 22 10Ne +u If the energy difference between initial and final states is low (less than 2mec2), then b+ decay is not energetically possible and electron capture is the sole mode decay. Alpha Decay Coulomb repulsion increases ~ Z2 Alpha decay occures only in heavy atoms (A > 100 amu ) Alpha particle has small mass relative to parent nucleus and has very high binding energy Nuclear binding force increases ~ A A ZX a+ 238 92U A-4 Z-2Y + Energy 234 90Th +a Gamma decay 60 Co 27 eV β 1.17 MeV γ 0.31 M 1.33 MeV γ 5.26 a Ni 60 28 Image by MIT OpenCourseWare. • Gamma decay is the emission of a gamma ray (photon) from a nucleus • Occurs when nucleus transitions from a higher to lower energy state • Energy of photon(s) equal to the change in energy of nuclear states • Nuclear structure does not change so parent and daughter are the same Predicting type of decay 90 α 80 Proton Number (Z) 70 60 ure 50 + β 40 or n ro ect El pt Ca Line of Stability β − 30 20 10 0 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 Neutron (N) Image by MIT OpenCourseWare. Binding Energy D = ZMp + NMn - MX The weights of these constituent masses exceeds the weight of the nucleus if we add the masses of Z protons and N neutrons that make up a nucleus. The difference is the mass defect which is positive for all nuclides. Multiplying by c2 yields the binding energy of the nucleus. When the nucleus is formed, the loss in mass is due to the conversion of mass to binding energy. It is defined as the energy that is supplied to a nucleus to completely separate its nucleons. A measure of nuclear stability is obtained when the binding energy is normalized to the number of nucleons. Calculate mass defect and binding f uranium-235 i 23 energy for Mass of neutron 1 1.008665 008665 amu Mass of proton 1.007826 amu Mass off one atom off U U-235 235.043924 M t 235 235 043924 Binding energy = mass defect x c2 • Mass defect = 1 1.91517 91517 amu • BE = 1 1.91517 931.5 MeV 91517 amu x 931 5M V / 1 amu • BE = 1784 MeV 1 amu = 1.66054 1 66054 x 10-27kg = 931.5 931 5 MeV / c2 Binding Energy Curve • Exothermic reactions result in reaction products with higher binding energy ti • T Two options – Fission of heavy nuclides – Fusion of light nuclides First-Order First Order ODE - Review • Appendix A of Lewis + Handout Radioactive Decay • Lewis Lewis, Section 1 1.7 7 Decay chain (a) λB = 5 x λA; t1 = 5 x t1 2 Normalized activity 1 A 2 0.75 0.5 0.25 0 0 1 2 t 3 4 (b) λB = λA /5; t1 = t1 /5 2 A 2 (c) λB = λA; t1 = t1 B 0.75 0.5 0.25 0 0 1 2 t 3 2 1 Normalized activity Normalized activity 1 B 4 A 2 B 0.75 0.5 0.25 0 0 1 2 t 3 4 AA (t)/AA (0) AB (t)/AA (0) Image by MIT OpenCourseWare. Decay Chains Definition of Decay Chain: The radioactive decay of different discrete radioactive decay products as a chained series of transformations. Decay Chains _ Thorium series or 4n _ Neptunium series or 4n + 1 _ Uranium or Radium series 4n + 2 _ Actinium series or 4n + 3 Chart of Nuclides 3 He in − t out β out p in d in n out Original Nucleus n in d out p out β out + α in t in α, 3n p, n γ, n n, 2n He out He, n α, n p, γ d, n 3 He, np a, np t, n 3 He, p d, p Target Nucleus n, γ t, np γ, np γ, p n, α n, He 3 α out α, 2n 3 t, p n, p 3 Image by MIT OpenCourseWare. Chart of Nuclides • Gray shaded square (stable nuclide) Symbol, mass number Pd 108 Percent abundance 26.71 Activation cross-section in "bars" to two isomers Mass (C-12 scale) σ (0.2 + 12) 107.9030 Fission product, slow neutron fission of U-235 Image by MIT OpenCourseWare. • White or "color" square: ( Artificially Produced Radioactive Nuclide)) Symbol, mass number Half life Modes of decay, radiation, and energy in MeV. ( ) means radiation from short-lived daughter. Disintegration energy in MeV. Fe 52 Fe852 h B 0.80, (263), ε γ 0.17, 380, (1.43) E 2.38 Image by MIT OpenCourseWare. Chart of Nuclides • Black rectangles across the top of square – On gray-shaded square: Radioactive nuclide with long half life (Considered Stable) Symbol Ce 142 11.07 Half life Thermal neutron absorption cross-section in barns Percent abundance 5x1015yrs α, 1.5 Modes of decay σ1 141.9090 Mass Image by MIT OpenCourseWare. – On white square: Radioactive nuclide found in nature with relatively short half life Chart of Nuclides • Smaller black rectangle g near top p of square q ((Nuclide is a member of a natural radioactive decay chain) Po 218 Symbol Ra A Modes of decay and energies α 6.00 β 3.05m 218.0089 Symbol, mass number Half life Mass Image by MIT OpenCourseWare. • Black triangle at bottom corner of square (nuclide is formed by y fission of U-235 or Pu-239)) Pd 108 Percent abundance 26.71 σ (0.2 + 12) Mass (C-12 scale) Symbol, mass number 107.9030 Activation cross-section in "bars" to two isomers Fission product, slow neutron fission of U-235 Image by MIT OpenCourseWare. Chart of Nuclides • Verticallyy divided square q – Two isomeric states, one stable Sn 117 Symbol Percent abundance 7.61 Half life Modes of decay radiation, and energies in Mev 14 d IT 0.159 γ 0.161 e- Mass 116.9031 Radioactive upper isomer Stable lower isomer Image by MIT OpenCourseWare. – Two isomeric states, both radioactive Pm 145 Symbol ? 16d β+ 0.45 Radioactive upper isomer Half life, ? means uncertainty 18y ε ,eγ 0.068, 0.073 E 0.14 Modes of decay radiation, and energies in Mev Disintegration energy in Mev Stable lower isomer Image by MIT OpenCourseWare. Neutron Sources Definition of Spontaneous Fission Spontaneous fission (SF) is a form of radioactive decay characteristic for very heavy isotopes. In practice, only energetically feasible for atomic masses above 230 amu. It is theoretically possible for any atomic nucleus with mass >= 100 amu. Radioisotopes for which spontaneous fission is a nonnegligible decay mode may be used as neutron sources notably Cf-252 (half-life 2.645 years, SF branch ratio 3.09%) Intensity (ARB. UNITS) 102 101 100 1 2 3 4 5 Neutron energy (MeV) Measured neutron energy spectrum from the spontaneous fission of 252Cf. Image by MIT OpenCourseWare. • Alpha Neutron Source – Neutrons are produced when alpha particles impinge upon any of several low atomic weight isotopes • beryllium, carbon and oxygen • Must have loosely y bound neutron – Alpha emitters must be long-lived • Radium, polonium, plutonium, americium – The low A material and alpha emitter are usually mixed in powdered form – Typical emission rates for alpha reaction neutron sources range from 1×106 to 1×108 neutrons per second. Th size i and d costt off these th t l comparable bl – The neutron sources are also to spontaneous fission sources. – Usual combinations of materials are plutonium-beryllium (PuBe), americium-beryllium (AmBe) (AmBe), or americium-lithium (AmLi) – Radium is not used as much now because of its high gamma emission rate Relative neutron intensity (MeV-1) 8 6 Stilbene 4 Emulsions 2 0 2 4 6 8 10 Neutron energy (MeV) Measured energy spectra for neutrons from a 239 Pu/Be source containing 80g of the isotope. Image by MIT OpenCourseWare. • Photoneutrons – A photon that is absorbed by the nucleus creates an excited state from which a neutron is emitted. There are two such sources: – 9Be + >1.7 Mev photon → 1 neutron + 2 4He – 2H (deuterium) + >2 >2.26 26 MeV photon → 1 neutron + 1H – The resulting neutron energies are discrete if the photons are ti R hl one gamma ray iin 106 interacts. i t t S monoenergetic. Roughly, So, the gamma ray source needs to be very large (as in a fission reactor) for these sources to be appreciable. The most common use is the deuterium reaction as a source of neutrons for the startup of light-water reactors. The source of the photons would be fission products. (Note: Sufficient D2O exists in light water for this source to be effective in LWRs.) • • • • Accelerated charged particles Fission Fusion F i … Fission • Consider the following g example p of U-235 fission • From binding energy curve, energy released is about ~200 MeV (235*(8.9 – 8)) – Most of the energy leaves in the form of kinetic energy of the fission products – Rest goes to particles emitted during fission • A distinction must be made between energy produced and energy recuperated – Fission products are large ionized particles that travel a short distance, thus energy is deposited locally – The electrons released by beta decay of the fission products are also absorbed locally. – The gamma rays (photons) travel much greater distances and are sometimes b b d by b th t shield. hi ld absorbed the reactor – The neutrinos escape entirely Energy Released Energy Recuperated 168 168 Beta (FP) 8 8 Gamma (FP) 7 7 Neutrinos ~12 - Prompt Gammas 7.5 7 Prompt Neutrons 5 5 (n, gamma) - 3-12 ~207 198-207 Fission Products Image by MIT OpenCourseWare. • For fission to occur, we must provide some energy to the nucleus. A potential p barrier exists that p prevents spontaneous fission from happening very frequently. • Liquid drop model: A water drop doesn’t separate in two spontaneously even if its energetically favorable favorable. The superficial tension of the drop acts as a barrier that tries to keep the fragments from splitting. Liquid Drop Model of Fission A + +B B + + C Image by MIT OpenCourseWare. • In nuclear fission, the short nuclear bonds of the nucleons keeps the together. Initially Initially, the potential energy of the nucleus is nucleus together equal to the binding energy of the nucleons (no kinetic energy). To deform the nucleus, energy must be provided in an effort to increase the average distance between the nucleons, thus increasing the nucleus. However However, the strong nuclear forces potential energy of the nucleus are very short. Thus when the separation starts, the repulsive forces diminish and the potential energy diminishes as well. There is thus a threshold energy required (about 6 MeV) for fission t h i also l explains l i h t fifission i can • Q Quantum mechanics how spontaneous happen, but with very-low probability, thru a tunnelling effect without any energy input. • When a neutron interacts with a nuclide, it forms a compound d nucleus. l Energy E is i given i to t the th nucleus l b by the binding energy of the incident neutron and its kinetic energy – If the binding energy is sufficient to get above the fission threshold of the nuclide, than the nuclide is fissile to thermal neutrons – If it requires additional kinetic energy, than it is said to be fissile to ffast neutrons or ffissionable • Fissile nuclides – – – – U-235: only naturally occurring fissile isotope P 239 radiative di i capture off U 238 Pu-239: U-238 U-233: radiative capture of Th-232 Pu-241: radiative capture of Pu-240 Critical Energy Critical Energies Compared to Binding Energy of Last Neutron Target Nucleus Critical Energy Ecrit Binding Energy of Last Neutron BEn BEn _ Ecrit 232 90 Th 7.5 MeV 5.4 MeV -2.1 MeV 238 92U 7.0 MeV 5.5 MeV -1.5 MeV 235 92U 6.5 MeV 6.8 MeV +0.3 MeV 233 92U 6.0 MeV 7.0 MeV +1.0 MeV 239 94Pu 5.0 MeV 6.6 MeV +1.6 MeV Image by MIT OpenCourseWare. Fission cross sections for fi i bl nuclei l i fissionable 3 236U 240Pu 238U 234U 232Th σf , barn 2 242Pu 1 0 0 2 4 6 8 10 Neutron energy, Mev Image by MIT OpenCourseWare. Fertile Materials • Materials that can undergo transmutation to become fissile materials. 233 239 92 94 U β- N Pu β- 233 239 91 93 Pa N β232 90 Th N n, Np β- 233 238 90 92 Th U n, 239 92 U N Image by MIT OpenCourseWare. Fission Products • Generally observe only two fission fragments – Note the logarithmic scale 10 Fission Yield, % 1 0.1 0.01 14 MeV Thermal 0.001 0.0001 70 90 110 130 150 170 Mass Number Image by MIT OpenCourseWare. Stability of fission products Image removed due to copyright restrictions. • Neutron rich fission products beta decay towards stability Criticality – Neutron Multiplication k ≡ multiplication factor ≡ • • • number of neutrons in one generation number of neutrons in preceding generation Critical, k=1 Sub-critical k<1 Super-critical k>1 1 235U N(t) Supercritical X 238U 2 235U X k>1 N(0) k=0 Critical 3 235U 235U k<1 Subcritical t Image by MIT OpenCourseWare. Neutron generation • Lewis Lewis, p p.12 12 Neutrons released from fission • Prompt – Spectra – Average energy • Delayed – Delay discussion to kinetics Neutrons released from fission 5 v 233U 4 Pu-239 produces more neutrons per fission than U-235. 239Pu v 3 235U 2 0 v49 = 2.874 + 0.138 E v25 = 2.432 + 0.066 E (0 < E < 1) = 2.349 + 0.15E (E > 1) 23 v = 2.482 + 0.075 E (0 < E < 1) = 2.412 + 0.136 E (E > 1) 5 10 v(E)=average number of neutrons produced per fission 15 E (MeV) Image by MIT OpenCourseWare. Fission prompt neutron energy spectrum χ (E ) = 0.453e −1.036E χ ( E )dE ≡ 0.5 Average number of fission neutrons emitted with energy in E to 0.4 E + dE per fission neutron. sinh 2.29E χ (E) 0.3 0.2 0.1 0 1 2 3 4 5 E (MeV) Image by MIT OpenCourseWare. Delayed fission neutrons 87 Br 55s β β (87Kr)* Neutron Less than 1% of neutrons from fission 87 are considered delayed. Delayed neutrons appear long after the fission event through the emission 86 Kr + Neutron Kr β decay of certain fission products, also called neutron precursors. These delayed neutrons are essential to the control of nuclear reactors since they appear many orders of magnitude later than the prompt neutrons. 87 Rb β 87 Sr Image by MIT OpenCourseWare. MIT OpenCourseWare http://ocw.mit.edu 22.05 Neutron Science and Reactor Physics Fall 2009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.