PHGN341: Thermal Physics NAME:
advertisement

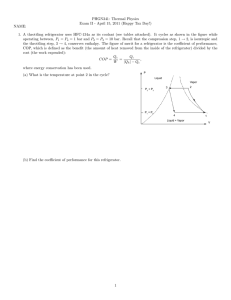
NAME: PHGN341: Thermal Physics Exam II - April 15, 2011 (Happy Tax Day!) 1. A throttling refrigerator uses HFC-134a as its coolant (see tables attached). It cycles as shown in the figure while operating between, P1 = P4 = 1 bar and P2 = P3 = 10 bar. Recall that the compression step, 1 → 2, is isentropic and the throttling step, 3 → 4, conserves enthalpy. The figure of merit for a refrigerator is the coefficient of performance, COP, which is defined as the benefit (the amount of heat removed from the inside of the refrigerator) divided by the cost (the work expended): Qc Qc = , COP = W |Qh | − Qc where energy conservation has been used. P (a) What is the temperature at point 2 in the cycle? Liquid Solution: The 1 → 2 step is adiabatic (isentropic); thus S1 = S2 . From Table 2 we have S1 = 0.94 kJ/K. Extrapolate from the data in Table 3 to find the temperature at point 2 that has the same entropy as point 1: S1 = αS40 + (1 − α)S50 . Solving gives: α = (S1 − S50 )/(S40 − S50 ) = 0.0833. This gives: T2 = α40o C + (1 − α)50o C = 49.17o C. Vapor P2 = P3 P1 = P4 2 3 4 Liquid + Vapor 1 V (b) Find the coefficient of performance for this refrigerator. Solution: As state above, COP = 1 Qc , = |Qh | − Qc |Qh |/Qc − 1 where |Qh | = H2 − H3 and Qc = H1 − H4 . Points 1 and 3 are at the phase boundaries; so we can read their values directly from Table 2: H1 = 231 kJ (pure gas) and H3 = 105 kJ (pure liquid). Because the throttling step conserves enthalpy, we also have H4 = H3 = 105 kJ. From part (a) we have the interpolation constant needed to find H2 = αH40 + (1 − α)H50 = 279.08 kJ. Plugging these into the expression for the coefficient of performance gives: COP = 2.62. 2. When the volume of the liquid can be neglected compared to that of the gas phase, the Clausius-Clapeyron relation for a liquid-gas phase transition becomes dP nL nL = ' , dT T (Vg − Vl ) T Vg where n is the number of moles of the substance and L is the molar latent heat of vaporization. (a) Treating the gas as ideal, P Vg = nRT , where R = 8.315 J/K-mol, is the molar gas constant, find a differential equation for the saturation pressure as a function of temperature. (Do not solve.) Solution: Use the ideal gas law to write: Vg = nRT /P , then substitute this into the Clausius-Clapeyron relation to find: dP L = 2 P. dT T R (b) The solution to your equation gives the liquid-vapor saturation pressure as a function of temperature which can be written in the form L Ps (T ) = P0 e− RT , where T is the absolute temperature. At a pressure of Ps = 0.0317 bar one finds that water boils at T = 25 C. By analyzing the energy needed for the phase transition at this temperature, one determines L ' 44.0 kJ/mol. From this data find P0 . 1 L Solution: From the above equation we have: P0 = Ps (T )e+ RT . Substituting the values above gives: 44×103 P0 = (0.0317bar)e+ 8.315·298 = 1.633 × 106 bar. (c) The dew point is the temperature where the saturation vapor pressure is equal to the actual vapor pressure. Suppose the temperature is 28 C and the relative humidity is 80%. What is the dew point (temperature)? (Recall that the relative humidity is the ratio of the actual water vapor pressure to the saturation pressure. ) Solution: If the humidity is 80% at 28 C, then the absolute vapor pressure is: P = 0.80Ps (T = 28) = 0.03027 bar. The temperature where this pressure saturates is: T =− L = 297.2 K or 24.2 C. R ln(P/P0 ) A 2 3. (a) Recall the particle-in-a-box result for the density of states in two-dimensions: d2 n = (2πh̄) 2 d p, where p is the momentum and A is the area of the system. Use this to calculate the partition function for a single particle in two p2 . dimensions by integrating the boltzmann factor times the density of states over all p-space. For a free particle E = 2m Solution: Perform the indicated integration: Z Z ∞ p2 p2 A A 2 −β 2m −β 2m Z= d p e = 2π dp p e . (2πh̄)2 (2πh̄)2 0 2 p to obtain p dp = Let x = β 2m m β dx. Thus, A m Z = 2π (2πh̄)2 β Z ∞ dx e−x = 0 mA . 2πβh̄2 (b) Using your result from part (a), find the the N-particle partition function assuming that all the particles are indistinguishable. Solution: For indistinguishable particles the N-particle partition function is: ZN = Z1N /N !. (c) From N-particle partition function, find the Helmholtz free energy, F = −kT ln Z, as a function of the area, A, temperature, T , number of particles, N , mass, m, and physical constants. Use the approximation, N ! ' N N e−N , to simplify. Solution: Using the stated approximation in the expression for the N-particle partition function gives: mA N −N N mA F = −kT ln ( ) N e = −N kT ln − ln N + 1 . 2πβh̄2 2πβh̄2 (d) From the Helmholtz free energy calculate the two-dimensional “pressure” (force per unit length), λ = − Simplify to obtain the ideal gas law in two dimensions. Solution: To simplify the derivative, break out the logarithm: m − ln N + 1 . F = −N kT ln A + ln 2πβh̄2 Thus, λ=− ∂F ∂A or, in standard ideal gas law form: λA = N kT . 2 = +N kT N,T 1 , A ∂F ∂A N,T . Thermodynamic Tables Substance C (graphite) C (diamond) CaCO3 (calcite) CaCO3 (aragonite) Al2 SiO5 (kyanite) Al2 SiO5 (andalusite) Al2 SiO5 (sillimanite) H2 O (l) H2 O (g) H2 (g) O2 (g) CH4 (g) CO2 (g) ∆f H (kJ) 0.0 1.895 -1206.9 -1207.1 -2594.29 -2590.27 -2587.76 -285.83 -241.82 0.0 0.0 -74.81 -393.51 ∆f G (kJ) 0.0 2.900 -1128.8 -1127.8 -2443.88 -2442.66 -2440.99 -237.13 -228.57 0.0 0.0 -50.72 -394.36 S (J/K) 5.74 2.38 92.9 88.7 83.81 93.22 96.11 69.91 188.83 130.68 205.14 186.26 213.74 CP (J/K) 8.53 6.11 81.88 81.25 121.71 122.72 124.52 75.29 33.58 28.82 29.38 35.31 37.11 V (cm3 ) 5.30 3.42 36.93 34.15 44.09 51.53 49.90 Table 1. Thermodynamic properties of selected substances for one mole of the substance at standard temperature and pressure. T (◦ C) -26.4 -18.8 -10.1 8.9 21.6 31.3 39.4 46.3 P (bar) 1.0 1.4 2.0 4.0 6.0 8.0 10.0 12.0 Hliquid (kJ) 16 26 37 62 79 93 105 116 Hgas (kJ) 231 236 241 252 259 264 268 271 Sliquid (kJ/K) 0.068 0.106 0.148 0.240 0.300 0.346 0.384 0.416 Sgas (kJ/K) 0.940 0.932 0.925 0.915 0.910 0.907 0.904 0.902 Table 2. Thermodynamic properties of saturated HFC-134a refrigerant for 1 kg of material at the boiling point temperatures for each pressure. P (bar) 8.0 10.0 12.0 Property H (kJ) S(kJ/K) H (kJ) S(kJ/K) H (kJ) S(kJ/K) Temperature 40 50 274 284 0.937 0.971 269 280 0.907 0.943 276 0.916 (◦ C) 60 295 1.003 291 0.977 287 0.953 Table 3. Thermodynamic properties of superheated HFC-134a refrigerant for 1 kg of material. 3