P V
advertisement

PHGN341: Thermal Physics
Exam II - April 14, 2010
NAME: KEY
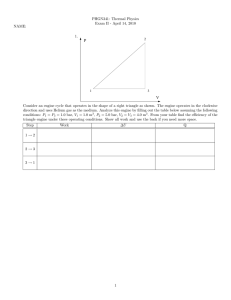
1. (30 points)
2
P
1
3
V
Consider an engine cycle that operates in the shape of a right triangle as shown. The engine operates in the clockwise
direction and uses Helium gas as the medium. Analyze this engine by filling out the table below assuming the following
conditions: P1 = P3 = 1.0 bar, V1 = 1.0 m3 , P2 = 5.0 bar, V2 = V3 = 4.0 m3 (1 bar = 105 Pa). From your table find
the efficiency of the triangle engine under these operating conditions. Show all work.
Step
Work (kJ)
∆U (kJ)
Q (kJ)
1→2
-900
+2850
+3750
2→3
0
-2400
-2400
3→1
+300
-450
-750
R
Solution: The work done on the system is given by: W = − P dV which is nothing morethan the area under the
P-V curve for that step. For the 1 → 2 step: W1→2 = − 21 (P2 − P1 )(V2 − V1 ) + (P3 − P1 )V1 = −900 kJ. There is no
work done in step 2 → 3 since the volume is constant. For the 3 → 1 step: W3→1 = −(P1 − P3 )V1 = 300 kJ.
From the equipartition theorem for an ideal gas, we have U = f2 N kT where here f = 3 because Helium is a monoatomic gas. Using the ideal gas law, P V = N kT , we get: U = 23 P V . Thus the change in internal energy is given by:
∆Ui→f = 32 ∆(P V ) = 32 (Pf Vf − Pi Vi ). From this we find: ∆U1→2 = 23 (P2 V2 − P1 V1 ) = 2850 kJ. Likewise, we find:
∆U2→3 = 23 (P3 V3 − P2 V2 ) = −2400 kJ and ∆U3→1 = 23 (P1 V1 − P3 V3 ) = −450 kJ. Note that ∆Ucycle = 0.
Finally, for each step we can use the first law of thermodynamics to find Q = ∆U − W which gives the values shown
in the table above.
The efficiency is defined as the net work done by the system (+600 kJ) divided by the heat put into the system. Only
step 1 → 2 puts heat into the system; so e = 600/3750 = 0.16.
1
Substance
Calcite
Aragonite
2. (20 points)
∆f H (kJ)
-1206.9
-1207.1
∆f G (kJ)
-1128.8
-1127.8
V (cm3 )
36.93
34.15
S (J/K)
92.9
88.7
Calcium Carbonate (CaCO3 ) can exist in two crystalline forms: calcite and aragonite. The thermodynamic properties
of one mole of these forms at 298 K and 1 bar of pressure are given in the table. Assume that the temperature of
the earth increases by 16 C per kilometer of depth while the pressure increases by 32 MPa per kilometer of depth. At
ground level the temperature is -10 C (263 K) and the pressure is 1 bar (105 Pa). At what depth is Aragonite the
stable form? Show all work.
Solution: The stable phase is the one with the lowest Gibbs free energy. According to the table above, at standard
temperature and pressure, calcite, is the stable phase. To evaluate the Gibbs free energy at different values of the
pressure and temperature, use a Taylor exapansion:
G(P, T ) ' G(P0 , T0 ) +
∂G
∂P
(P − P0 ) +
T,N
Using the thermodynamic identify for dG, we have
∂G
= V,
∂P T,N
∂G
∂T
∂G
∂T
(T − T0 ).
P,N
= −S.
P,N
From the information given in the problem, we have the depth dependence of the pressure and temperature: P (z) =
P0 + αz and T (z) = Ts + βz where z is the depth, α = 32 MPa/km, Ts is the surface temperature (-10C=263 K), and
β = 16 K/km. The condition for the phase to transition from calcite to aragonite is when the respective Gibbs free
energies are equal: GA (z) = GC (z). Using the Taylor expansion and the derivative expressions, we have
GC (P0 , T0 ) + VC (P (z) − P0 ) − SC (T (z) − T0 ) = GA (P0 , T0 ) + VA (P (z) − P0 ) − SA (T (z) − T0 ),
or, using the expressions for the pressure and temperature as functions of depth,
GC (P0 , T0 ) + VC αz − SC (βz + Ts − T0 ) = GA (P0 , T0 ) + VA αz − SA (βz + Ts − T0 ).
Solving for z gives:
z=
GA (P0 , T0 ) − GC (P0 , T0 ) + (SC − SA )(Ts − T0 )
= 39.2 km.
α(VC − VA ) − β(SC − SA )
3. (20 points) From the Classisus-Clapeyron relation and the ideal gas law, the liquid-vapor saturation pressure as a
function of temperature is well approximated by:
L
Ps (T ) = P0 e− RT ,
where L is the molar latent heat of vaporization, R = 8.315 J/K is the molar gas constant, and T is the temperature.
a. For the water-steam phase transition one measures Ps = 0.1234 bar at T = 50 C (323 K), with L = 42.92 kJ/mol.
From these values find P0 .
4.292×104
Solution: From the above equation and the given data, one can solve for the constant: P0 = (0.1234)e (8.315)(323) =
1.076 × 106 bar.
b. Suppose the temperature is 30 C and the relative humidity is 50%. What is the dew point (temperature)? (Recall
that the relative humidity is the ratio of the actual water vapor pressure to the saturation pressure.)
Solution: Using the value of P0 found in part a, we have the saturation pressure at 30 C: Ps (303K) = 1.076 ×
4.292×104
106 e (8.315)(303) bar = 0.04299 bar. Since the relative humidity is 50%, the actual water vapor pressure is Pw = 0.0215
bar. To find the dew point temperature (TDP ) at which the actual vapor pressure is the saturation pressure, one inverts
the starting equation to find:
L
4.292 × 104
TDP =
=
= 291 K.
6
R ln PPw0
8.315 ln( 1.076×10
0.0215 )
2
4. (30 points) Consider a system of N spin-3/2 paramagnetic atoms at temperature, T, in an external magnetic field,
B0 . The energy of such an atom in an external magnetic field is U = −µ0 B0 mj , where mj is the quantum number
for the z-component of the angular momentum and µ0 is the Bohr magneton. (Recall that a spin-3/2 atom can have
four possible m-substates: mj = {−3/2, −1/2, +1/2, +3/2}. (Possibly useful information: N ! ' N N e−N (Sterling’s
approximation).)
a. Find the single atom partition function.
Solution: The partition function for a single atom is:
mj =+3/2
Z1 =
X
e−β(−mj µ0 B0 )
mj =−3/2
Explicitly performing the sum gives:
1
3
1
3
Z1 = e+ 2 βµ0 B0 + e+ 2 βµ0 B0 + e− 2 βµ0 B0 + e− 2 βµ0 B0 = 2(cosh
3βµ0 B0
βµ0 B0
+ cosh
).
2
2
b. Find the average energy of one atom in thermal equilibrium with a reservoir at temperature, T .
1
Solution: The average energy is given by: E = − Z11 ∂Z
∂β , which gives:
µ0 B0
E=−
2
sinh (βµ0 B0 /2) + 3 sinh (3βµ0 B0 /2)
cosh (βµ0 B0 /2) + cosh (3βµ0 B0 /2)
.
c. What is the partition function of the N-atom system assuming the atoms are indistinguishable?
Solution: The N-atom partition function assuming all the atoms are indistinguishable is:
ZN =
Z1N
Z N eN
' 1N
N!
N
where Z1 is defined in part a and where Sterling’s approximation has been used.
d. What is the Helmholtz free energy, F , for this N-atom system?
Solution: The Helmholtz free energy can be determined from the partition function by F = −kT ln ZN = −N kT (ln (Z1 /N ) + 1),
where Z1 is given in part a.
e. What is the chemical potential?
Solution: The chemical potential can be obtained from the Helmholtz free energy: µ =
Helmholtz free energy from part d gives:
µ = −kT (ln Z1 − ln N ) ,
where Z1 is given in part a.
3
∂F
∂N V,T .
Applying this to the