CBEN 408 Quiz #2 Name (1 point): ___________________________________
advertisement

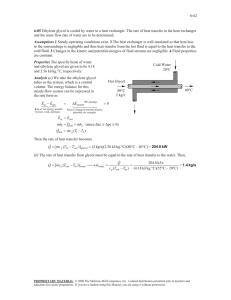
CBEN 408 Quiz #2 Name (1 point): ___________________________________ February 22, 2016 1. (4 points) What are two (2) functions of the inlet slug catcher at the front end of a gas processing plant? 2. (2 points) Convert 20 MMscfd to normal conditions (i.e., Nm³/day). Remember there is also a change in the standard temperature. 3. (3 points) You’ve added ethylene glycol (C2H6O2) to suppress the formation of hydrates by 20°F. What must be the mass fraction of glycol in the produced water phase? Solution 1. Protect plant from large, sudden liquid influxes Do initial separation of gas, oil, & water 2. Remember that the “standard” temperature is 60°F & the “normal” temperature is 32°F (0°C): 3 3 m 32 459.67 °R 6 ft 6 3 20 10 0.3048 0.538 10 Nm /day day ft 60 459.67 °R 3. The molecular weight of ethylene glycol is approximately 62 (212 + 212 + 6). Using the Hammerschmidt equation (with value as mass fraction): X EG t F Mi 2062 0.347 t F Mi 2335 20 62 2335 If you use the Nielsen and Bucklin equation (as mole fraction water): T F° 20 xw exp exp 0.8570 129.6 129.6 Now converting to mass fraction glycol: X EG 1 0.8570 62 xEG MEG 0.365 xw Mw xEG MEG 0.8570 18.015 1 0.8570 62