Caoimhe Nic a’ Bháird, Isla Wallace , Dr Penny Xanthopoulou...
advertisement

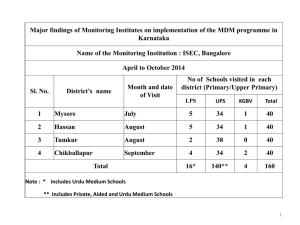
Caoimhe Nic a’ Bháird, Isla Wallace , Dr Penny Xanthopoulou and Professor Rosalind Raine Department of Applied Health Research, University College London, 1-19 Torrington Place, London WC1E 7HB Aim Using Consensus Methods To develop a set of feasible recommendations for improving multidisciplinary team meetings (MDMs) for patients with chronic diseases Phase 1 findings • Formal consensus methods are structured facilitation techniques that explore levels of consensus among a group of experts by synthesising opinions (Campbell et al., 2003). They are designed to minimise some of the limitations associated with group decision making. • Their main purpose is to define levels of agreement, particularly where there is an insufficient or contradictory evidence base (Jones and Hunter, 1995). Background Relevant research literature Relevant policy 68 potential recommendations for improving MDMs • This is particularly important in the field of health care, where clinical practice varies widely, and clinicians are often faced with uncertainty about the value of different options (Murphy et al., 1998). • MDMs are widely established in the NHS and have been endorsed by the Department of Health as the core model for managing chronic diseases (Department of Health, 2004; 2007). • Three main approaches have been used in health research since the 1950s: o the Delphi Method o the Nominal Group Technique o the RAND appropriateness method • It is believed that MDMs ensure higher quality decision making and improved outcomes. However, the evidence underpinning the development of MDMs is not strong and the degree to which they have been absorbed into clinical practice varies across conditions and settings. Expert panel rates recommendations in two rounds • In practice, formal consensus studies often adopt elements from each of these methods to optimally address specific research objectives (Murphy et al., 1998). We adopted such an approach to develop a set of recommendations that are both desirable and feasible. • We conducted a large mixed-methods observational study of multidisciplinary teams (in Cancer, Heart Failure, Memory, and Mental Health services) to investigate current MDM practice and examine the determinants of effective MDM decision making (Phase 1). 1st round: No interaction 2nd round: Panel meeting Final recommendations are those that are rated highly and with a high level of agreement among the expert panellists • Drawing on this data, we applied consensus methods to develop recommendations to improve MDM decision making and effectiveness (Phase 2). Phase 2: Developing recommendations for improvement Figure 3. Phase 2: Developing recommendations • The steps of Phase 2 are illustrated in Figure 3. Phase 1: Examining current MDM practice Phase 1 involved the collection and analysis of qualitative and quantitative data sources to identify areas for improvement in MDMs. This process is illustrated in Figure 1. Review of medical records of 3184 Team Climate Inventory patients to assess MDM decision implementation, clinical data and sociodemographic details Questionnaire completed by 161 team members Interviews with 53 team members • We established a panel of 16 expert stakeholders including patient representatives, clinicians and policymakers. • MDMs are widespread across the NHS. This study, the largest of its kind, compared MDTs in a range of clinical specialties, allowing us to develop recommendations that are generalisable to a broad range of disease types. • A Questionnaire Pack was sent to the panelists, containing a summary of research and policy and the recommendations relevant to each of the 16 themes. • The study developed recommendations both from empirical evidence and the experience of a diverse group of expert stakeholders. • Panellists privately rated the feasibility and desirability of each recommendation on a scale of 1 to 9, where 1 indicated strong disagreement with the recommendation and 9 indicated strong agreement (Round 1 ratings, see Figure 2a). • This process allowed us to identify recommendations that are both desirable and feasible, making it more likely that they can be practically implemented. • Observation of 370 MDMs of 12 teams (qualitative field notes and quantitative data on MDM and decision characteristics ) 16 themes identifying potential areas for improvement Conclusions • Drawing on the Phase 1 findings and a review of the relevant research and policy literature, we developed a list of 68 potential recommendations. Interviews with 20 patients and carers A meeting was then convened to discuss the ratings, focusing on areas of low consensus among panellists. The discussion was chaired by the principal investigator, and was designed to ascertain whether discrepant ratings were due to real clinical disagreement or to misunderstandings. Further information • At the meeting, each panellist received a second, personalised version of the questionnaire pack showing the distribution of all panellists' first round ratings, together with his/her own ratings (see Figure 2b). 1. Lack of clarity regarding purpose of MDMs 2. Attendance and participation in MDMs 3. Chairing the MDM 4. Administrative support and the coordinator role 5. Agreeing which patients should be discussed 6. Preparing and presenting cases 7. Discussing comorbidities in the MDM 8. Discussing patients holistically 2 (a) 9. Incorporating patient preferences into discussions MDT discussions should result in a documented treatment plan for each patient discussed 13. The role of research and evidence in MDMs 14. Teaching as a function of MDMs 15. Recruitment to trials in MDMs 16. Monitoring the quality of MDMs Figure 1. Phase 1: Examining current MDM practice • Follow the Dept. of Applied Health Research on Twitter @ucl_dahr • visit tinyurl.com/improvingmdts or scan the QR code on the right References • The extent to which each respondent agrees with each statement (i.e. 7-9 on the Likert scale), and the extent to which respondents agree with each other will be used to generate a final list of recommendations. 11. Patient attendance at MDMs 12. Providing feedback to patients email c.bhaird@ucl.ac.uk • Having discussed each theme as a group, panellists individually rated each recommendation a second time (Round 2 ratings). Potential areas for improvement identified in Phase 1 10. Patient awareness of MDMs • Strongly …………...……… Disagree 1 2 2 (b) 3 4 5 6 Strongly ………………..…… Disagree Strongly Agree 7 Don’t know 8 9 Strongly Agree Don’t know MDT discussions should result in a documented treatment plan for each patient discussed 1 1 2 1 2 3 4 5 Department of Health. (2007). Cancer Reform Strategy. London: Central Office of Information. Department of Health (2004). Improving chronic disease management. London: Author. Campbell, S., Braspenning, J., Hutchinson, A. & Marshall, M. (2003). Research methods used in developing and applying quality indicators in primary care. British Medical J ournal, 326, 816-819. Jones, J. & Hunter, D. (1995). Consensus methods for medical and health services research. British Medical Journal, 311, 376. Murphy, M., Black, N., Lamping, D., McKee, C., Sanderson, C., Askham, J. & Marteau, T. (1998). Consensus development methods and their use in clinical guideline development. Health Technology Assessment , 2, i. 3 2 7 6 7 8 9 Figure 2. Rating response scales for Round 1 (a) and Round 2 (b) Acknowledgments The researchers would like to thank Natalie Austin-Parsons, Sophie Bostock, Mike Galsworthy, and Khadija Rantell. Guidance was provided throughout by the study co-applicants and Expert Advisory Group members: Julie Barber, Anne Lanceley, Alex Clarke, Gill Livingston, Archie Prentice, Jane Blazeby, Dave Ardron, Miriam Harris, Michael King, Susan Michie, Simon Gibbs, and Ewan Ferlie. We would also like to acknowledge the support of the Comprehensive Clinical Research Network.