UNIVERSITY OF MALTA
advertisement
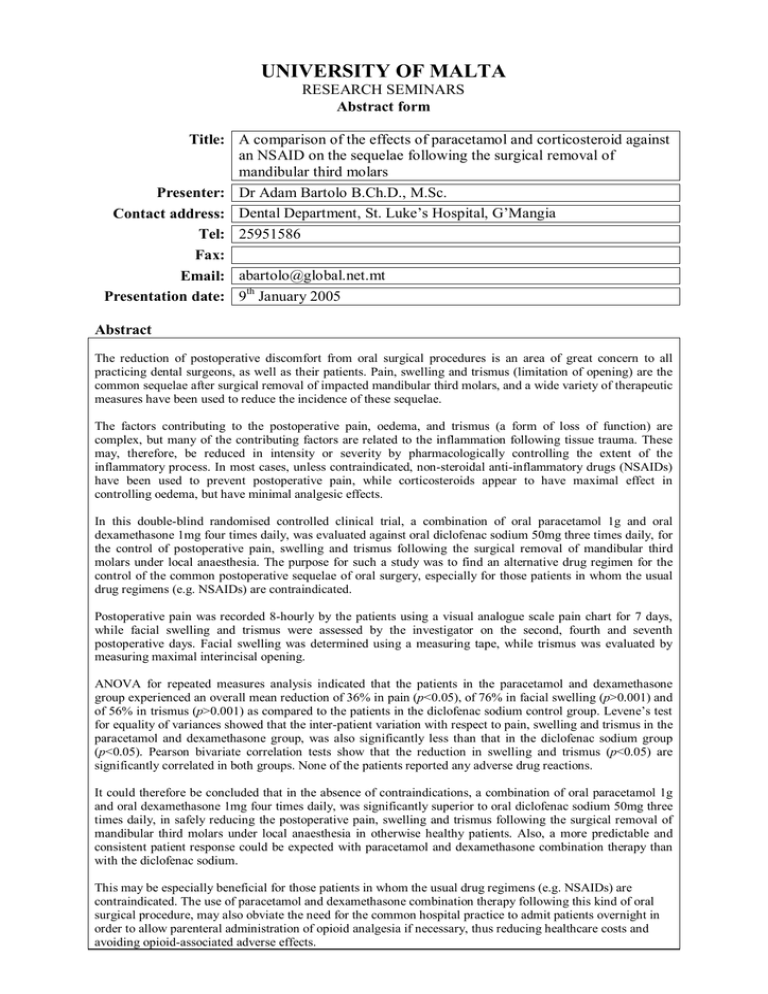
UNIVERSITY OF MALTA RESEARCH SEMINARS Abstract form Title: A comparison of the effects of paracetamol and corticosteroid against an NSAID on the sequelae following the surgical removal of mandibular third molars Presenter: Dr Adam Bartolo B.Ch.D., M.Sc. Contact address: Dental Department, St. Luke’s Hospital, G’Mangia Tel: 25951586 Fax: Email: abartolo@global.net.mt Presentation date: 9th January 2005 Abstract The reduction of postoperative discomfort from oral surgical procedures is an area of great concern to all practicing dental surgeons, as well as their patients. Pain, swelling and trismus (limitation of opening) are the common sequelae after surgical removal of impacted mandibular third molars, and a wide variety of therapeutic measures have been used to reduce the incidence of these sequelae. The factors contributing to the postoperative pain, oedema, and trismus (a form of loss of function) are complex, but many of the contributing factors are related to the inflammation following tissue trauma. These may, therefore, be reduced in intensity or severity by pharmacologically controlling the extent of the inflammatory process. In most cases, unless contraindicated, non-steroidal anti-inflammatory drugs (NSAIDs) have been used to prevent postoperative pain, while corticosteroids appear to have maximal effect in controlling oedema, but have minimal analgesic effects. In this double-blind randomised controlled clinical trial, a combination of oral paracetamol 1g and oral dexamethasone 1mg four times daily, was evaluated against oral diclofenac sodium 50mg three times daily, for the control of postoperative pain, swelling and trismus following the surgical removal of mandibular third molars under local anaesthesia. The purpose for such a study was to find an alternative drug regimen for the control of the common postoperative sequelae of oral surgery, especially for those patients in whom the usual drug regimens (e.g. NSAIDs) are contraindicated. Postoperative pain was recorded 8-hourly by the patients using a visual analogue scale pain chart for 7 days, while facial swelling and trismus were assessed by the investigator on the second, fourth and seventh postoperative days. Facial swelling was determined using a measuring tape, while trismus was evaluated by measuring maximal interincisal opening. ANOVA for repeated measures analysis indicated that the patients in the paracetamol and dexamethasone group experienced an overall mean reduction of 36% in pain (p<0.05), of 76% in facial swelling (p>0.001) and of 56% in trismus (p>0.001) as compared to the patients in the diclofenac sodium control group. Levene’s test for equality of variances showed that the inter-patient variation with respect to pain, swelling and trismus in the paracetamol and dexamethasone group, was also significantly less than that in the diclofenac sodium group (p<0.05). Pearson bivariate correlation tests show that the reduction in swelling and trismus (p<0.05) are significantly correlated in both groups. None of the patients reported any adverse drug reactions. It could therefore be concluded that in the absence of contraindications, a combination of oral paracetamol 1g and oral dexamethasone 1mg four times daily, was significantly superior to oral diclofenac sodium 50mg three times daily, in safely reducing the postoperative pain, swelling and trismus following the surgical removal of mandibular third molars under local anaesthesia in otherwise healthy patients. Also, a more predictable and consistent patient response could be expected with paracetamol and dexamethasone combination therapy than with the diclofenac sodium. This may be especially beneficial for those patients in whom the usual drug regimens (e.g. NSAIDs) are contraindicated. The use of paracetamol and dexamethasone combination therapy following this kind of oral surgical procedure, may also obviate the need for the common hospital practice to admit patients overnight in order to allow parenteral administration of opioid analgesia if necessary, thus reducing healthcare costs and avoiding opioid-associated adverse effects.










