Hydrides and Dihydrogen as Ligands: Hydrogenation Catalysis

Hydrides and Dihydrogen as Ligands: Hydrogenation
Catalysis
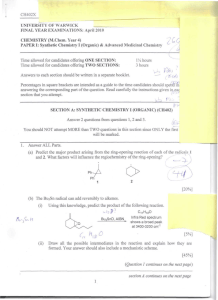
Synthesis of Organometallic Complex Hydrides
• Reaction of MCO with OH , H , or CH
2
CHR
2
– M(CO) n
+ OH = M(CO) n-1
(COOH) = HM(CO) n-1
+ CO
2
–
M(CO) n
+ H = M(CO) n-1
(C(=O)H) - = HM(CO) n-1
+ CO
– M(CO) n
CH
2
CHR
2
= HM(CO) n-1
- + CH
• Protonation of MCO anion*
2
=CR
2
+ CO
– M(CO) n
+ H + = M(CO) n
H
• Hydrogenation of MCO dimer**:
– M
2
(CO)
2n
+ H
2
= M(CO)
• Oxidative addition of H n
H
2
to (typically) d 8 metal
– M(PR
3
)
3
X + H
2
= M(CO) n
X(H)
2
*
Oxidative addition of a proton. If a dianion, the resultant MCO hydride will be anionic and may react as a hydride transfer reagent.
** The resultant neutral hydride may have acidic characteristics (i.e., the hydrogen may be removed by a base (reductive deprotonation)
Properties of the M-H functionality
• Stereochemically active
• M-H distance range (3d transition metals): 1.5 -1.7 Ǻ
• M-H stretch: 2100 – 1600 cm -1
• M-H hydride resonance: typically upfield, -1 to -20 ppm, but little correlation with electron density
• M-H Bond Dissociation Energy: 60 -100 kcal/mol (Contrast M-C BDE of ca. 26-30 kcal/mol
• Homolytic cleavage can initiate radical chain reactions
• Acid/Base character: Varies. HCo(CO)
HFe(CO)
.
4
– is weak; Cp
2
W(H)
2
4
is strong acid, pK a
<1;
forms Lewis Base/Acid adduct with
AlMe
3
• Proton loss is slow as in carbon-based acids.
Acidity of MCO Hydrides
HM(CO) n
+ OH H
2
O + M(CO) n
-
HCo(CO)
4
HCo(CO)
3
PPh
3
HMn(CO)
5
HRe(CO)
5
H
2
Fe(CO)
4
CpCr(CO)
3
H
CpMo(CO)
3
H
CpW(CO)
3
H
K a
~2
1 × 10 -7
8 × 10 -8 very weak
3 × 10 -5 ; 1 × 10 -14
10 -13.3
10 -13.9
10 -16.1
The M-H Bond Functionality: Reactivity
H
M
No change in M oxidation state; reverse is β-elimination
M• + H•
Oxidation state of M reduced by 1; can yield H
2 or initiate radical rxns
MH
M + H +
Formal reduction of M oxidation state by 2; electron
Withdrawing ligands stabilize
M + + H -
M oxidation state is unchanged; electron donating ligands stabilize
Hydride Transfer Reactivity
HM(CO) n
+ RX XM(CO) n
+ RH
Rate = k
2
[HM ][RX]
Organometallics , 1984 , 3 , 646
Suppose one protonates the anionic metal hydride of HW(CO)
5
-
. Is it possible that the resultant H
2
would remain bound to the metal?
Heinekey, JACS, 2005, 850-851;
Heinekey, JACS, 2006, 2615-2620
The
2 -Dihydrogen as Ligand Story
Kubas, JACS , 1984 , 10 , 451.
The η 2 -H
2
Complexes
- Typically d 6 , Oh structures of Cr 0 , Mo 0 , W 0 , Fe II , Ru II , Ir III .
Kubas, LANL
- Bonding: Delicate Balance Required for Stability
H
M
H
H
M
H
M
H
H
Morris, U. Toronto
- donor * acceptor M
2+
(H
-
)
2
- Examples of
2
-H
2
complexes
++
R
3
P
O
C
H H
C
O
W 0
PR
3
C
O
Kubas
P
P
H H
Fe II
C
N
H
Morris
P
P
Ph
3
P
H H
Ir III
N
C
+
Crabtree, Yale
H
PPh
3
Crabtree
Every Molecule Has a Story: The 2 -H
2
Complexes
R
3
P
O
C
H H
C
O
W
PR
3
C
O
R
3
P
O
C
H
W
H
C
O
PR
3
C
O
- stability towards H
2
dissociation
- oxidative addition to dihydride
Kubas, LANL
P
P
H H
Fe
C
N
H
P
P
+ H + - H +
H
P P
Fe
P P
C
N
H
- strong acid
- CNH ligand
+
Morris, U. Toronto
+ +
R
3
P
N
H H
Ir
H
PR
3
C
+
R
3
P
N
H H
Ir
H
PR
3
C
+
- resonance forms
- H/D exchange
Crabtree, Yale
H
2
Ir III
Vaska’s Complex and Its Oxidative Addition Reactions
Catalysis
© 2009 W.H. Freeman
Catalyst Development
Catalyst Development
Inorganic Chemistry Chapter 1: Figure 26.16
Schematic representation of physisorption and chemisorption of Hydrogen
on a nickel metal surface
Schematic representation of
Diverse sites exposed on a
Metal surface — a) different
Exposed planes, edges; b) steps,
And kinks from irregularities
© 2009 W.H. Freeman
Hydrogenation of alkenes on supported metal
Involves H
2
dissociation and migration of H-atoms
to an adsorbed ethene molecule. (Paul Sabatier, 1890)
Mechanism: All isotopomers are seen, therefore highly
Reversible prior to loss of the ethane.
Volcano diagrams relate stability of products on
Surface: Temp. for a set rate of release vs. the
Enthalpy. Intermediate values of ΔH f
, with the rate being a combination of the rate of adsorption and the rate of desorption gives best catalyst.
Just right
Surface adducts
Very strong
Surface adducts
Very weak
Surface adducts
The Goldilocks Effect
© 2009 W.H. Freeman
A Prominent Example of Heterogeneous http://www.greener-
Hydrogenation Catalysis: Ammonia Synthesis
CH
4
+ H
2
O 3 H
2
+ CO
CO + H
2
O H
2
+ CO
2
CO/H
2
500 x 106 tons/year; Known as “population detonator” http://www.greenerindustry.org.uk/pages/ammonia/6AmmoniaPMHab er.htm
http://www.greenerindustry.org.uk/pages/ammonia/6AmmoniaPMHab er.htm
Conditions
According to the equation, the equilibrium mixture will contain more ammonia:
• When the temperature is lower (the reaction is exothermic in the ammonia direction)
• When pressure is higher (4 moles of reactant gas make 2 moles of product gas)
In practice, the equilibrium is run under conditions of moderate temperatures and pressure.
Low temperatures affect the equilibrium favorably, but the reaction would be too slow. Very high pressures, though favoring product creation, increase the costs of plant construction, and present a greater risk to plant workers.
With the conditions used, a yield of approximately 20 - 30 % is achieved from each pass over the catalyst. http://www.greenerindustry.org.uk/pages/ammonia/6AmmoniaPMHab er.htm
Some homogeneous catalytic processes
(Adapted from J. Halpern, Inorg. Chim. Acta 1981, 50 , 11)
Hydrogenation of Alkenes: Wilkinson’s catalyst and
(one of several versions of) the mechanism
Hydrosilation of Terminal Alkenes
Mechanism :
H
2 activation prior to olefin addition
Mechanism :
Olefins add first to cationic catalyst
Wilkinson ’ s Catalyst: Mechanism for Olefin Hydrogenation
Pre-catalyst
With the Rh(I) cationic precursor:
Olefin adds prior to H
2
oxidative addition.*
* This mechanistic route followed by asymmetric Hydrogenation process
Halpern, Science , 1982
Halpern
( Science , 1982 , p. 401)
Halpern
( Science , 1982 , p. 401)
from Collman, Stanford
Wacker process: Oxidation of olefins
Inorganic Chemistry Chapter 1: Figure 26.2
Olefin Isomerization
© 2009 W.H. Freeman
from Collman, Stanford