Document 13185394
advertisement

Vol. 53: 293-303, 1989
MARINE ECOLOGY PROGRESS SERIES
Mar. Ecol. Prog. Ser.
1
Published May 18
Assessment of macroalgal nitrogen limitation in
a seasonal upwelling region
Rodney M. Fujita*,Patricia A. Wheeler**,Robert L. Edwards
College of Oceanography, Oregon State University, Cowallis, Oregon 97331, USA
ABSTRACT: The relationship between nitrogen (N) availability and the growth of macroalgae in a
seasonal upwelling region (Yaquina Head, Oregon, USA) was investigated. Water column nutrient
concentrations were relatively high and stable during the winter, decreased in early spring, and were
highly variable in the summer and early fall. Periods of high nutrient availability d u e to upwelling
commenced in May and alternated with periods of low nutrient availability until September. Dissolved
inorganic nitrogen (DIN) concentration was a poor predictor of N limitation of macroalgal growth d u e to
the a b h t y of macroalgae to store N for continued growth when ambient DIN concentrations were low.
The relationship between growth and internal N content for Pelvetiopsis limitata (Phaeophyta : Fucaceae) and Ulva rigida (Chlorophyta : Ulvales) depended on the N source and culture conditions.
Results of a n in situ N enrichment experiment indicated that P. limitata growth may have been Nlimited in April and May. A nitrogen budget for P. limitata indicated that NH; was the major N source
for growth at Yaquina Head, due to its rapid uptake, despite the greater abundance of NO: The cntical
N value (tissue N level during the transition from N limiting to non-N limiting conditions) for growth of
U. rigida on NO: ( 5 2 . 4 % dry wt) was much lower than that for growth on NH: (3.0 % dry wt).
Ammonium was the major N source for growth of U. rigida at Y a q u ~ n aHead, and growth was probably
N-limited in April and May, a s indicated by the critical N level for growth on NH: Growth of U. ~ g i d a
may also have been intermittently N-limited between upwelling events. Problems with the use of
cirtical N levels and nutrient monitoring to assess N limitation are discussed. Use of critical tissue N
levels is con~plicatedby variations in this parameter with the N source used for growth
INTRODUCTION
The seasonality of macroalgal productivity is often
controlled by light and nutrient availability. In temperate regions, irradiance and growth are usually low
during the winter and early spring, while nutrient
levels are high (e.g. Gagne et al. 1982). As light conditions improve, nutrients are utilized by both macroalgae and phytoplankton and often become depleted in
the euphotic zone. Low nutrient levels may limit primary production during the summer and fall when
adequate light is still available.
Most studies of the relationship between the availability of dissolved inorganic nitrogen (DIN) and macroalgal productivity have been carried out in Atlantic
coastal waters (e.g. Chapman & Craigie 1977, Hanisak
1979, Harlin & Thorne-Miller 1981, Davison et al. 1984)
''
Present address: Environmental Defense Fund, 257 Park
Avenue South, New York, New York 10010, USA
Corresponding author
O Inter-Research/Printed in F. R. Germany
where winter mixing provides the dominant seasonal
input of DIN to the euphotic zone. A contrasting situation occurs along the eastern boundaries of the oceans
where coastal upwelling occurs. Upwelling is particularly strong off the coast of Oregon and the upwelling
season usually spans 6 mo (May to October). During
this period upwelling is episodic, with 4 to 6 major
upwelling events and the same number of weaker
events (Small & Menzies 1981).
Other natural environments (such as salt ponds and
estuaries) and cultivation systems also have periodic
episodes of high DIN availability ('pulses') superimposed on a background of low DIN availability. In these
environments, macroalgae take u p DIN rapidly when
available and store it to support growth between pulses
(Rosenberg & Ramus 1982, Rosenberg et al. 1984,
Fujita 1985a, Lapointe 1985). Gerard (1982) has suggested that the giant kelp Macrocystis pyrifera is able
to use transient pulses of NOT supplied by upwelling,
thermocline excursions (Zimmerman & Kremer 1984),
and runoff to alleviate N starvation in southern Califor-
294
Mar. Ecol Prog. Ser 53. 293-303, 1989
nia waters. However, some species have a limited
capacity to store N perhaps d u e to their high growth
rates (Fujita 1985a). Thus, the frequency with which
DIN pulses occur in the environment may determine
whether or not a particular species is N-limited. For
example, growth of the red alga Gracilaria tikvahiae
continued unabated in a salt pond when enriched with
DIN only once every 5 d , while abundance of the fastgrowing green algae Ulva lactuca and Enteromorpha
spp. greatly decreased (Fujita 198513).Nutrient storage
uncouples growth from nutrient availability, complicating the assessment of nutrient limitation. To determine
and predict the effects of nutrient availabihty on macroalgal growth in the field, it is necessary to measure
nutrient concentrations on a n ecologically relevant
scale, assess the kinetics of nutrient uptake, determine
which nutrient species are being utilized, and measure
the length of time that growth can be supported in the
absence of nutrients (i.e. the nutrient storage capacity).
The relationship between internal nutrient levels and
growth rates has been analysed extensively for both
higher plants and for phytoplankton. For microalgae, a
well-defined hyperbolic relationship between cell nutrient content and growth rate is found in continuous
cultures (Droop 1974). The relationship for mineral
content of plant tissue, on the other hand, is more
complex and is divided into three or more 'zones' of
nutrient status (Smith 1962), with the 'critical level'
usually being defined as the level separating the zone
of deficiency and the zone of adequacy (Ulrich 1952).
Here, we follow Hanisak's (1979) definition of the 'critical nutrient' content as the internal concentration that
just limits maximal growth for macroalgae.
In this paper, w e describe the temporal variability of
nutrients in a n intertidal macroalgal community at
Yaquina Head (Oregon, USA) over a seasonal cycle.
We determined critical tissue N levels by growing
macroalgae in continuous culture systems with varylng
levels of N supply, then used these values to predict the
onset of N limitation in situ. We also report 'subsistence' levels, tissue nutrient levels at zero growth rate,
as used in the phytoplankton literature (Eppley &
Strickland 1968), and conducted a n in situ N enrichment experiment to evaluate N limitation.
MATERIALS AND METHODS
Site description. Water samples and algal specimens
were collected from Yaquina Head, Oregon, USA
(44"41' N, 124O05' W). Yaquina Head is a rocky headland extending about 2 km into the Pacific Ocean. The
field experiment was conducted on a relativly flat rock
platform in the midst of a dense bed of Pelvetiopsis
limitata (Gardn.) (Phaeophyta Fucaceae) at the south
end of Yaquina Head. P. limitata, with Fucus d ~ s t i c h u s
(L.) (Powell) (Phaeophyta : Fucaceae), dominates the
high intertidal zone and is present year-round. All P.
limitata thalli were collected from the vicinity of thls
site. Ulva rigida C. Ag. (Chlorophyta : Ulvales) was
present lower in the intertidal at the same site, and
abundance was highly vanable, but was generally
greater during the summer than during spring and fall.
U. rigida was scarce during the winter. U. rigida thalli
were collected from the vicinity of the field experiment
and nutrient monitoring site for tissue N analysis; thalli
for growth experiments were collected in nearby
Yaquina Bay, where they were present in much greater
abundance. Other Ulva species also occurred at
Yaquina Head, but were not as abundant.
Nutrient concentrations and tissue N content at the
field site. We collected water samples from Yaquina
Head in polypropylene bottles over various temporal
scales. In August 1985, we sampled surface water
hourly for 24 h to characterize tidal variations in nutrient concentrations during a n upwelling event. For
high-tide samples, w e used a garden hose fitted with a
perforated bottle to minimize intake of debris. For low
tide samples debris was not evident. Water samples
were taken 6 m seaward from the edge of the rock
ledge at a depth of ca l m below the surface through
hosing connected to a hand-held bilge pump. All samples were packed in ice and filtered through Whatman
GF/C glass-fiber filters within 4 h of collection. They
were analysed for NH:, NO:, NO;, and PO:- within
6 h of collection on a Technicon Autoanalyzer I1 using
the methods of Grasshoff et al. (1983) with minor modifications. Nitrite concentrations were always less than
5 % of NOT concentrations and are not reported here.
Coefficients of variation for replicates in each nutrient
analysis were typically < 1 %.
We sampled water in the same manner at both high
and low tides every 1 to 3 d during June, July and
August. Samples were taken throughout the rest of the
year at intervals of 1 to 30 d, usually at or near low tide.
These samples were filtered and frozen within l h of
collection and stored for later analysis. The differences
in nutrient concentrations observed between fresh and
frozen samples were small for NO; (1.7 OO/ greater in
fresh samples) but rather high for NH: and PO:- (10.3
and 10.5 OO/ greater in fresh samples, respectively).
We collected whole specimens of Pelvetiopsis
Limitata and Ulva rigida periodically from the general
vicinity of our experimental site, taking care not to
damage the thalli. Debris was removed by hand and
the thalli rinsed in nutrient-free seawater (see 'Critical
N content'). Excess water was removed by shalung or
spinning in a salad spinner for 15 S. The thalli were
frozen at 7 0 "C for at least 2 h, freeze-dried for at least
24 h , and ground to a fine powder in a Spex Industries
Fujita et al: Assessment of nutrient limitation
mixermill. Carbon and N contents were measured with
a Perkin-Elmer 240B elemental analyzer. For some
samples, N content was measured as NO; after persulfate digestion (Grasshoff et al. 1983), which gave
results comparable to those obtained using the elemental analyzer.
Outdoor culture experiments. Pelvetiopsis limitata
and Ulva r-igida were grown in outdoor flow-through
tanks at a density of 10 g wet wt per tank (0.9 g 1-l).
The outdoor culture system was developed to provide
high turnover rates to simulate natural conditions more
closely than was possible using an indoor culture system. The tanks were injection-molded ABS plastic halfcylinders 30 cm in diameter and 43 cm in length,
arranged in sets of 4. Outflow pipes were fitted with
perforated plastic bottles to prevent clogging, resulting
in a worlung volume of 11.5 1. Agitation was provided
with compressed air; nylon needle valves were used to
equalize flow rates. Plexiglas covers kept out rain
and debris. Biomass was cropped weekly to 10 g
and growth rate (% d-l) was calculated as In
(W,W,-')t-' X 100, where W, is the wet weight at the
end of the growth period, W, is the weight at the start of
the growth period, and t is the duration of the period.
P. lin~itatawas cultured from 20 April to 20 May 1986 to
coincide with the in situ enrichment experiment. U.
rigida was cultured from 5 August to 27 August 1986.
Salinity-adjusted (30 to 32 %o) seawater from Yaquina
Bay was sand-filtered and pumped through 10- and 1pm cartridge filters into a 4-tiered, air-agitated, flowthrough nutrient stripping tank stocked with Ulva and
Enteromorpha spp. Flow rates were adjusted to maximize the residence time of the water and thus increase
the efficiency of nutrient removal. The water was then
pumped to a 600 1 head tank provided with an overflow
to maintain a constant head, and directed through a
carefully leveled manifold to the tanks. Each tank was
provided with a nylon needle valve for control of flow
rates.
Two 2000 1 reservoir tanks were filled with nutrientstripped water every 3 to 4 d and enriched to 150 pM
NH: or NOT. Phosphate (15 PM)was provided in each
case to prevent P limitation (N:P=10:1).The enriched
seawater was then pumped to separate 600 1 head
tanks with overflows and allowed to flow into the tanks
through nylon needle valves. The 2000 1reservoir tanks
were operated in tandem with two 600 1 tanks to provide nutrient-enriched water continuously.
Nominal influent N concentrations of 0, 10,20,30,40,
and 50 pMwere achieved by adjusting the relative flow
rates of enriched and unenriched seawater. The total
flow rate to each tank was held constant at 216 1 d-'
(18.8turnovers d-l). The flow rates were checked daily
and adjusted as necessary. The treatments were
assigned to tanks randomly to avoid systematic effects
of shading or other gradients in the outdoor courtyard
where the system was located. Inorganic N and P
concentrations in the seawater prior to nutrient enrichment were always less than 3.4 and 0.41 FM, respectively. Dissolved organic N (DON) and P were not
measured. The possible presence of DON may have
contributed to the fast growth of Ulva rigida in outdoor
cultures, but the use of DON by Pelvetiopsis limitata is
not likely (see 'Discussion'). Salinity ranged between
30 and 32 %o, while temperature (11 to 20 "C) and
irradiance were highly variable.
Indoor culture experiments. The critical N content
was determined by measuring tissue N content for
algae grown over a range of growth rates controlled by
nitrogen supply. We grew Pelvetiopsis limitata from 23
October to 23 December 1986 in 1.2 l plastic containers
with water input and output fittings and filtered air
agitation. Small, non-reproductive thalli (2 to 3 cm in
length) with intact holdfasts were collected from the
field site and cultured in enriched seawater in a constant temperature room. The seawater was obtained
from Yaquina Bay and mixed with high salinity seawater as needed to maintain salinity above 30 %o.
Nutrients were stripped from the water in 2000 1 outdoor tanks by dense, air-agitated batch cultures of Ulva
spp. for at least 4 d. The water was then pumped
through 10- and l-km cartridge filters, and a Millipore
PelLicon@high-volume tangential flow filter system was
used to remove > 0.2 pm particulate material. Residual
concentrations of dissolved inorganic N and P were
always less than 1.9 and 0.15 pM, respectively.
Separate polypropylene carboys were filled with seawater enriched with f/2 vitamins and minerals (Guillard
1975) and varying amounts of NH: or NOS plus sufficient PO:- to ensure N limitation (N:P = 2: 1). Medium
was pumped to the cultures at 1 2 0.1 m1 min-L (0.5 1
d-l), light was maintained at 370 FE m-' S-' (measured
in culture medium) with cool white GE fluorescent
tubes, and temperature was held constant at 13 "C.
Photosynthesis should have been saturated at the light
intensity used (Oakes & Murray 1983). However, we
found in preliminary studies that Pelvetiopsis lirnitata
became necrotic and died after about 1 wk of constant
submergence under these conditions. We subsequently
simulated natural conditions in the high intertidal by
providing a 14:10 L:D cycle (lights on between 07:OO
and 21:OO h), with 8 h of submergence in the light, 6 h of
emersion in the light, and 10 h of emersion in the dark.
Thalli were simply removed from the medium and left in
plastic weighing dishes for the emersion period. Natural
populations of P. lirnitata experienced a similar cycle of
photoperiod and submergence/emergence at Yaquina
Head during the spring.
Five to 7 thalli of ca 1.5 g wet wt each were cultured
in each container, resulting in 7.5 to 8.5 g wet wt total
Mar. Ecol. Prog. Ser. 53: 293-303, 1989
biomass and a biomass:volume ratio of about 8 g 1-'.
Growth was measured weekly immediately after
removal from the medium at 17:OO h by spinning in a
salad spinner for 15 s and weighing. We found that this
resulted in consistent weights after repeated periods of
submergence. No systematic effects of desiccation
during weighing (about l h) were observed. Tissue
was cropped at each weighing to maintain the biomass
at about 8 g. Growth rate (% d-l) was calculated as
ln(W, WO-') t p ' X 100, where t is the number of days
between weighings, W, is the initial weight, and W, is
the final weight. The cropped tissue was frozen at
-70 "C and freeze-dried in preparation for CHN
analysis.
Ulva rigida was grown from 16 September to 20
October 1986 under the same conditions of irradiance,
temperature, and nutrient loading. Photoperiod was set
at 1 3 : l l (L:D) and the algae were continuously submerged. Biomass was cropped to 6.5-8.5 g wet wt
weekly a t every weighing. Growth rate was calculated
as described above.
In situ nutrient enrichment. Naturally occurring
clumps of Pelvetiopsis limitata attached to barnacles
were removed from the field site and attached to 10 X
20 X l cm black, UV-resistant plastic plates with
Marine-Tex marine epoxy cement. Cement was
applied between the barnacles and plates and allowed
to cure for 24 h. Plates were inverted over a tank with
flowing seawater to keep the thalli submerged while
the cement dried. Initial weights were determined after
the algae were allowed to dry for 4.5 h at 20 "C. We
attached the plates directly to black basalt at the field
site with stainless steel bolts and expansion nuts. The
treatments were distributed randomly over a level platform in the midst of a dense bed of P. limitata at
Yaquina Head.
Daily at lowest low tides (Oregon's semidiurnal hdes
are asymmetrical) between 25 April and 13 May 1986
we enriched the algae by unbolting the plates and
placing them in translucent buckets filled with 7 1 of
freshly collected seawater for at least 2 h. We enriched
one bucket to 50 pM NH: and another was left unenriched to serve as a control. The buckets were swirled
every 10 to 15 min to reduce diffusion resistance.
Enrichment was continued for 18 d ; at the end of the
experiment the plates were collected and allowed to
dry in the laboratory for 4.5 h at 20 ' C before the final
weighing. Growth was calculated as the difference
between the initial and final weights. Attempts to conduct this experiment with Ulva rigida failed due to
excessive tissue loss.
In situ growth rates. We measured the growth rates
of Pelvetiopsis limitata thalli which were cemented
onto nylon bolts that were threaded into plastic plates
attached to the rock during March and early April of
1986. We found that the product of the longest frond
length and the number of fronds was linearly related to
wet weight (? = 0.9). Therefore, weight gain could be
extrapolated from frond length. We measured frond
lengths and numbers weekly during the experimental
period. We also established a permanent transect line
with markers adjacent to individual thalli. Frond length
and number of thalli along the transect were measured
weekly as an independant estimate of growth rate
under undisturbed conditions.
RESULTS
Nutrient concentrations and tissue N content
at the field site
We characterized the temporal distribution of macronutrients at Yaquina Head on time scales ranging
from hours to months. Hourly sampling, over a tidal
cycle in August (during upwelling) revealed that NO,
concentrations ranged between 15 and 22 yM with
highest concentrations generally occurring at high
tides (Fig. 1). Ammonium and POi- concentrations
were low (<1 and 3 yM, respectively) and did not vary
significantly over the tidal cycle (Fig. 1). For sampling
dates between mid-June and mid-August, low-tide
NO3 concentrations were an average of 72 % of hightide concentrations.
Upwelling events were detected and characterized by
sampling every 2 to 4 d without regard to the tidal cycle
(Fig. 2). Upwelling commenced in mid-May 1985 and
resulted in pulses of elevated nutrient concentrations
(henceforth to be called pulses) (7 to 28 yMN0: and 1 to
J
z
LLT
HHT
HLT
LHT
Fig. 1. Tidal cycle of nutrient abundance in surface water (0to
3 m depth) at Yaquina Head, 29 to 30 Aug 1985. Nitrate ( L ) ,
PO:- ( U ) , and NH; (*)concentrations are shown. LLT.lowest
low tide; H H T highest high tide; HLT: highest low tide; LHT.
lowest high tide
Fujita e t al: Assessment of nutrient limitation
297
October, high nutrient concentrations occurred sporadically, ranging from 1.3 to 10.5 P M NO,, 0.9 to 2.2 yM
PO:-, and 0 to 6.3 p M NH:. Satellite and ship-based
thermal analysis indicated that the 1985 upwelling
season ceased abruptly during the last few days of
September (NOAA 1985); the moderately high NO;
concentrations observed during October were therefore
probably residual (Fig. 2A). Nutrient concentrations
were more stable during the winter (6 to 12 p M N 0 7 and
1 to 2 yM PO2-) and decreased in March to 0 to 4 pM
NO: and 0.2 to 1 yM PO:-. The decrease in nutrients
may have resulted from a spring phytoplankton and
macroalgal bloom (Stefansson & Richards 1964). In
1987, nutrient levels were also relatively low during
March and early April (0.8 to 5.7 1MNO; and 0.6 to 1.9
pMNH:), increasing in late April ( l 5 to 23 pMNOJ and
1.9 to 4.4 pM NH: (data not shown).
Tissue N content of naturally occurring algae
100
0
I
J
I
J
I
A
I
S O
1985
200
I
I
N
I
D
300
I
J
I
F
I
M
I
A
400
I
M J
1986
I
I
J
J
A
S
30
Tissue N level of Pelvetiopsis limitata thalli collected
from Yaquina Head ranged from 1.5 to 2.2 % dry wt but
showed no significant seasonal variation (Fig. 3). The
tissue N content of Ulva rigida thalli collected from
Yaquina Head was more variable (2.1 to 4.2 OO/ dry wt),
but also showed no distinct seasonal trend (Fig. 3).
Z
W
2
.V)
-
2
F
I
TIME (days)
Fig. 2. (A) Seasonal cycle of NOT concentration and (B) NH:
(4) and PO:( 0 ) concentration at Yaquina Head. Note the
scale change. (C) Nitrate concentration during the upwelling
season. 1985
3 yM PO:-) separated by periods of low nutrient availability during July and August (0.7 to 3 p M N 0 7 and 1to
1.3 PM PO:-) (Fig. 2B, C). In June 1986, NO; concentrations were usually above 7 P M Ammonium concentrations ranged between 0 and 8 yM (Fig. 2B); these
unusually high levels may have been due to the large
deposits of guano in the area. In September and
O
Jun Mar ApMayIMar Apr
I
May
Jun
Jun Ap
l
I
Apr
May Jun
Fig. 3. Pelvetiopsis limitata and Ulva rigida. Tissue N content
of specimens collected at Yaquina Head. Two collections were
made for each of April and May 1983. Error bars indicate 1
standard deviation. CN (and horizontal lines) = critical N level
(see text)
Growth kinetics
Growth of Pelvetiopsis limitata was hyperbolically
related to influent NH: loading (concentration X
culture turnover rate) and was a linear function of
influent NO: loading in outdoor culture after 10 to 17 d
Mar. Ecol. Prog. Ser. 53: 293-303, 1989
of acclimation (Fig. 4A). Growth was faster when NH:
was the N source. Growth at nominally zero N loadings
probably resulted from residual inorganic N ( 5 3 . 3 1iM)
in the incoming medium which is equivalent to a loading of 1 0 . 7 mm01 d-'. In indoor culture growth was a
hyperbolic function of NH: and NOT loading after 64
and 54 d of acclimation, respectively, but growth rates
were much lower than for outdoor culture (Fig. 4B).
Growth of Ulva n g i d a in outdoor culture after 10 d of
acclimation was independent of NH: loading at low
levels and of NOT loadings at high levels. At higher
NH: loading and lower NO; loading, growth rates
were positively correlated with N loading (Fig. 4C).
Growth at nominally zero N loading probably resulted
from residual inorganic N in the incoming medium as
noted above for Pelvetiopsis limitata. U. rigida growth
in indoor culture was a linear function of NO: loading,
but showed a weaker positive correlation with NH:
loading after a 19 d acclimation period (Fig. 4D).
Critical N content
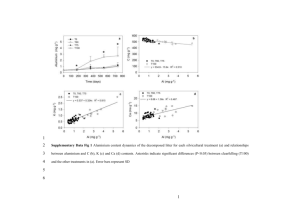
Growth rates of Pelvetiopsis limitata were relatively
high and constant at tissue N levels between 0.9 and
2.2 % dry wt for algae grown outdoors on NHT (Fig.
5A). For algae grown outdoors on NOT, growth rate
was nearly a linear function of tissue N. For algae
grown indoors, growth rate was slow and independent
of tissue N for NH: and linearly related to tissue N for
NOT (Fig. 5A). No single critical N value can be determined for P. limitata; the relationship between growth
and tissue N content depends on N source and culture
conditions such as irradiance, temperature, and culture
turnover rate. All of these factors differed between the
indoor and outdoor culture experiments. The critical N
value for growth indoors on NOT was 2 1.2 % dry wt,
but no value could be determined for growth on NH:.
Critical N values for growth on NHZ and NO.? were
<0.9 and 2 1.5 % dry wt, respectively. Subsistence
tissue N level (the level at which no growth occurs) was
ca 0 . 5 % dry wt.
The relationship between Ulva rigida growth and
tissue N also appears to vary with N source and culture
conditions (Fig. 5B). Thalli grown outdoors on NHT
exhibited N limitation at tissue N levels less than 3.0 %
dry wt. while outdoor growth on NO; resulted in a
critical N level 1 2 . 4 % dry wt. Indoor culture results
did not yield critical N levels because growth did not
saturate at the tissue N contents achieved (Fig. 5B).
Growth rates of U. rigida at the lowest N loadngs
attainable in the indoor cultures indicate that the subsistence N level is ca 1.2 % dry wt (Fig. 5B).
In situ growth rates and effects of nutrient enrichment
Growth rates of Pelvetiopsis limitata determined by
outplanting (3.7 1.5 % d-l) and transect methods
(3.9 3.6 % d-l) had high variance but agreed well
+
+
D
5-
/
4
-
3
-
2
-
0'
//.
/
'-
*
/
/O
/
0
4
8
N Loading (rnrnol/d)
12
0
/
B
P'
l
l
I
I
I
25
50
75
'00
N Loading (pmol/d)
Fig. 4 . Pelvetiopsis limitata and Ulva riglda.
Mean growth rates on NH: (m, o) and NO: (o, o)
plotted as functions of influent N loading for
flow-through cultures. Error bars for outdoor
cultures indicate 1 standard deviation. No replicates were run for indoor cultures. (A) P.
limitata, outdoor culture. ( B ) P. Limitata, indoor
culture. Note scale change. (C) U. rigida, outdoor culture. (D) U rigida, indoor culture. Note
scale changes
Fulita et al: Assessment of nutrient limitation
299
ments initially had 17 and 13 thalli, respectively). Since
biomass loss was relatively uniform between the treatments, a comparison of growth rates (with less loss
indicating more rapid growth) can be made. Algae
enriched with N lost significantly (p<0.05, MannWhitney U-test) less weight (i.e. grew more rapidly)
and had higher tissue N levels than control (unennched) algae (Table 1).
DISCUSSION
Tissue N ( % d w )
Fig. 5. Pelvetiopsis limitata and Ulva ngida. Relationship
between growth rate and tissue N content for the indoor
culture on NOS (o) and NH; (e) and outdoor culture on NOS
(D) and NH$ ( M ) . Error bars indicate 1 standard deviation.
No rephcates were run for indoor cultures. (A) P. limitata.
(B) U. ngida
Table 1. Pelvetiopsis limitata. In situ nitrogen enrichment.
Experiment conducted at Yaquina H e a d from 25 Apnl to 11
May 1986. Means f lstandard deviation are presented
Treatment
Control
N-ennched
(50 F M N
H:)
Number of
replicates
3
Weight loss
( g wet wt)
1.8 k 0 . 2 6
0.11~0.15
3
N content
(% dry wt)
1.5k0.02
1.9k 0.10
with each other and with maximum growth rates
observed in culture (3.9 0.44 % d-l).
Pelvetiopsis limitata in both the control and the Nenriched treatments showed a decrease in weight over
the experimental period (Table 1). Since few grazers
were observed on the P. limitata beds adjacent to the
experimental site, and since no obvious grazer-induced
damage was observed, we suggest that the weight loss
was due to the senescence of individual fronds with
subsequent breakage and loss. We observed senescent
fronds and frequent signs of breakage within both
naturally occurring and outplanted clumps of thalli.
Similar numbers of thalli were lost from control and Nenriched treatments (5 and 6, respectively; the treat-
+
Our studies of N availability, critical N content, and
the effects of in situ N enrichment allow us to integrate
the results from 3 methods of assessing N limitation of
macroalgal growth. The first method predicts N limitation during periods of low N availability as measured
by water column concentrations. High temporal resolution is required, because some algae may have limited
capacities to store N for growth (Fujita 1985a) and
could become N-limited between intermittent upwelling events. The second method predicts N limitation
when tissue N levels of algae from natural communities
are less than a critical value established in culture
experiments. The third method predicts N limitation
when enrichment with N results in increased growth.
We have assumed in this study that dissolved organic
N is not an important N source for rocky intertidal
macroalgae. Ulva spp. can use urea and amino acids as
nitrogen sources (Nasr et al. 1968, Mohsen et al. 1974).
However, in general the capacity of macroalgae to
utilize organic forms of N is very limited (Stephens
1981). Furthermore, concentrations of both urea (Kokkinakis & Wheeler 1988) and primary amines (measured by fluorescamine, Wheeler unpubl.) are not particularly high in the Oregon coastal waters, and we feel
justified in excluding these possible N sources in the
present discussion.
Based on water column N concentrations, we would
predict that Pelvetiopsis limitata growth would b e Nlimited only in March and April, when inorganic N
levels were consistently low. To apply the critical N
concept to field populations of P. limitata, it is necessary to determine the major N source for growth
because the N source affects the critical N relationship
(Fig. 5A). We can make a rough estimation of the
relative contributions of NH; and NO: to total N
demand by constructing a N budget (Table 2). In our
calculations, we estimated mean exposure time at low
tides by direct observation throughout the year (A.
Olsen pers. comm.) and assumed that the low tides
were symmetrical (resulting in an overestimation of
exposure time, since all observations were made at the
lowest-low tides). We further assumed that NO:
uptake is light dependent while NH4f uptake is not
Mar Ecol. Prog. Ser. 53: 293-303. 1989
300
Table 2. Pelvetiopsis lim~tata.Nitrogen budget for in situ
growth at Yaquina Head, Oregon. Uptake rates were measured a s a function of N concentration in short-term laboratory
experiments and used in conjunction with ambient nutrient
concentrations to calculate in situ uptake rates
Month
Jan
Feb
Mar
AP~
May
Jun
Jul
Aug
S ~ P
Oct
Nov
Dec
Time
Estimated in situ % N required for
submersed
uptake rate
maximum growth
per day (pmol[gdry wt]-'h-')
potentially
supplied
(h)
NO;
NHZ
NO3NH;
9
5
7
8
8
9
11
9
8
12
9"
ga
1.7
1.6
0.8
1.O
1.7
4.6
2.2
1.6
2.0
1.6
1.6
1.6
3.7
3.7
3.7
5.3
5.3
5.3
3.7
37
3.7
5.3
3.7
3.7
26
30
14
16
28
7l
28
24
33
19
25
25
113
143
132
174
174
163
96
113
122
130
116
116
NO data available; means for entire year used
(Hanisak 1983), that growth rate was constant at 4 %
d-' (growth rates in outdoor cultures varied between
2 and 4 % d-' and in situ growth rates averaged 3.9 %
d-l), and that tissue N levels averaged 1.7 % dry wt
throughout the year (mean of all tissue N values in
Fig. 3; standard deviation = 0.23). The budget indicates that N0.7 could account for 14 to 33 O/O of the N
demand, while NH: could potentially supply 96 to
174 % of the total N demand (Table 2). The uptake
rates used in the calculation of the N budget were
measured in short-term experiments and may overestimate long-term or steady-state uptake (Probyn &
Chapman 1982),resulting in percentages of the total N
demand supplied by NH: in excess of 100 % (Table 2).
Nonetheless, it is unlikely that in situ growth is Nlimited. The in situ enrichment experiment, however,
suggests that, despite high N concentrations present
during early May, P. limitata growth may have been Nlimited (Table 2). The algae in the control treatment
had a tissue N level of 1.5 % dry wt while N-enriched
thalli had a tissue N content of 1.9 % dry wt. Since the
critical N level for growth in outdoor cultures was
2 1.5 O/O dry wt, these results suggest that P, limitata
was growing on NOT in situ.
The 3 methods for the assessment of N limitation
used in this study (water column DIN concentrations,
critical tissue N level, and in situ enrichment) do not
yield the same conclusion for Pelvetiopsis limitata.
Water column DIN concentrations indlcate that P.
limitata may be N-limited in March and April (Fig. 2A).
The critical N levels obtained in outdoor culture
coupled with the results of the N budget (i.e. that NH:
is the major N source in the field) indicate that this
species is never likely to be N-limited. The results of
the in situ enrichment experiment indicate that P.
limitata may be N-limited in April and May only if NO;
is the major N source for growth in the field, contrary to
the conclusion drawn from N budget analysis (Table 2).
Based on water column N concentrations, Ulva rigida
would also only exhibit N limitation in April and May.
The relationships between growth and critical N content
depended on N source in outdoor culture (3.0 % dry wt
on NH:, 4 2 . 4 % dry wt on NO;). A N budget was
constructed to determine which N source was more
important in situ. We assumed that thalli were always
submerged, experienced 10 h of light during the spring
and 12 h during the summer, and that N content and
growth rate varied from 3 % dry wt and 20 % d-' to 4 %
dry wt and 30 O/O dC1 in the spring and summer, respectively. The budget (Table 3) indicates that NH: may
supply most of the N requirements for growth despite
much higher concentrations of NOT. Using the critical N
level derived from the outdoor cultures of U. rigida on
NH: (3.0 % dry wt), growth is just on the verge of N
Limitation most of the spring (Fig. 3).
We were unable to conduct a n in situ N enrichment
study with Ulva rigida due to excessive tissue loss.
Similarly, we were unable to measure the growth rate
of Ulva spp. in situ. However, our outdoor cultivation
studies indicate that the growth rate of these algae can
b e relatively high (up to 12 % d-l). Many other workers
have also reported high growth rates for Ulva spp. (e.g.,
Lapointe & Tenore 1981, Fujita 1985a, Duke et al.
1986). Ulva and Enteromorpha spp. are capable of
extremely rapid N uptake when N availability is high,
and continued growth for up to 5 d after N is depleted
(Fujita 1985a, O'Brien 1987). However, because N
pulses sometimes occur as much as 6 d apart at
Yaquina Head, ulvoid algae may be intermittently Nlimited if growth rates are high enough to deplete
stored tissue N and ambient N is too low to maintain
growth rates.
When N was provided only once every 5 d in a field
experiment, ulvoids decreased in abundance while the
red alga Gracilaria tikvahiae (which can grow for 14 d i n
the absence of N; Ryther et al. 1981, Fujita 1985a),
dominated mixed beds (Fujita 1985b). Nitrogen uptake
by Ulva rjgida is rapid (ca 12 ,pm01N g dry wt-l h-') even
at low concentrations (2