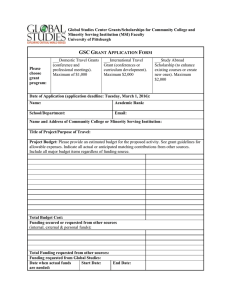
This information is required for all "Request for Services”:
advertisement
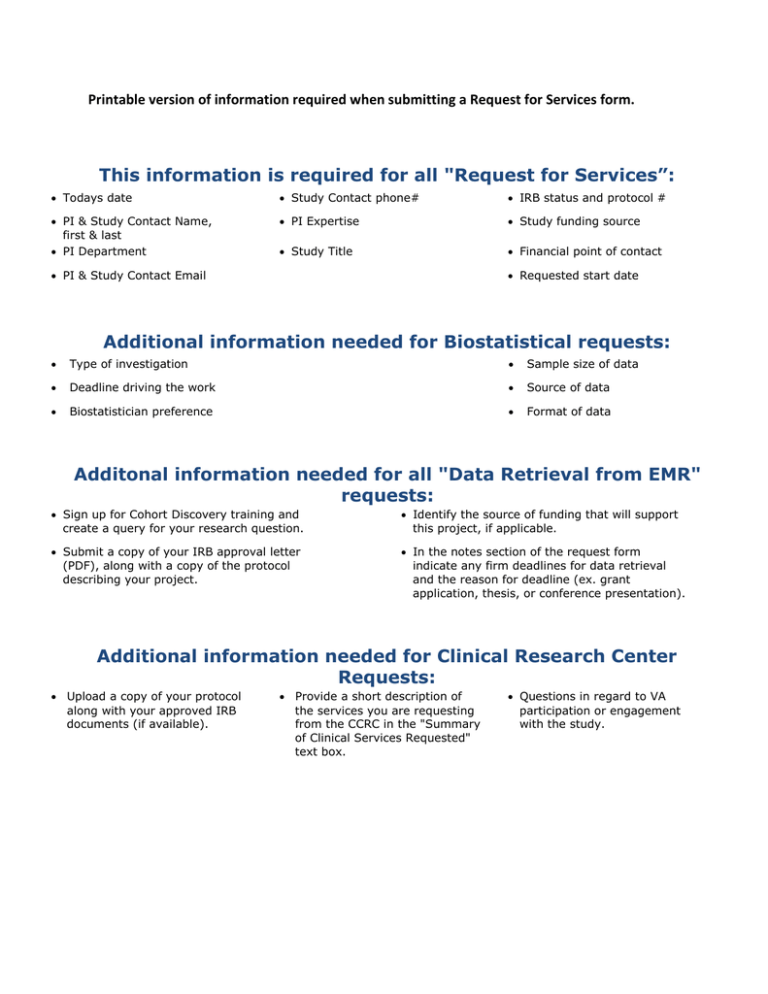
Printable version of information required when submitting a Request for Services form. This information is required for all "Request for Services”: • Todays date • Study Contact phone# • IRB status and protocol # • PI & Study Contact Name, first & last • PI Department • PI Expertise • Study funding source • Study Title • Financial point of contact • PI & Study Contact Email • Requested start date Additional information needed for Biostatistical requests: • Type of investigation • Sample size of data • Deadline driving the work • Source of data • Biostatistician preference • Format of data Additonal information needed for all "Data Retrieval from EMR" requests: • Sign up for Cohort Discovery training and create a query for your research question. • Identify the source of funding that will support this project, if applicable. • Submit a copy of your IRB approval letter (PDF), along with a copy of the protocol describing your project. • In the notes section of the request form indicate any firm deadlines for data retrieval and the reason for deadline (ex. grant application, thesis, or conference presentation). Additional information needed for Clinical Research Center Requests: • Upload a copy of your protocol along with your approved IRB documents (if available). • Provide a short description of the services you are requesting from the CCRC in the "Summary of Clinical Services Requested" text box. • Questions in regard to VA participation or engagement with the study.