Student Research: Postgraduates Only
advertisement

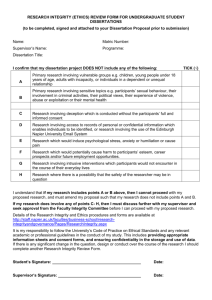
Student Research: Postgraduates Only The student(s) should complete Section 1, and then the supervisor should complete Section 2 of the electronic form. For Yes/No items, please delete the inappropriate response. Deception 3. Is there any deception involved? Yes No If yes, describe the deception and the reasons for its use: Ethical Approval Form Department of Psychology, University of Warwick1 SECTION 1 Name of investigator: Debriefing Project Title: 4. Supervisor: How will participants be debriefed (written or oral)? If they will not be debriefed, give reasons. Participants 1. Source of participants: Consent 2. Is prior informed consent to be obtained? Yes From participants? Yes No No From others? Yes Withdrawal from the investigation No 5. Describe the means of obtaining prior consent. If prior informed consent is not to be obtained, give reasons: Will participants be told explicitly that they are free to leave the study at any time without jeopardy? Yes No When and how will this be done? Will participants be explicitly informed of what the researcher’s role/status is? Yes No Will participants be told of the use to which data will be put (eg research publication, teaching purposes, media publication)? Yes No Confidentiality 6. Under the Data Protection Act information about a participant is confidential unless otherwise agreed in advance. Will confidentiality be guaranteed? Yes No If yes, what steps will be taken to ensure this? If no, what procedures will be taken in advance of obtaining consent (how will participants be warned?) 1 This form follows the British Psychological Society Code of Conduct, Ethical Principles & Guidelines (Leicester: British Psychological Society, 2000), pp. 1-11. It is based on a similar form used at University of Kent at Canterbury. Thanks to Julie Turner-Cobb for allowing us permission to use this. The form at Kent is based on one from Miami University, following APA principles. Thanks are due to Steve Hinkle for providing that. Protection of Participants 1 SECTION 2 7. Are the participants at risk of physical or psychological harm greater than encountered in ordinary life? Yes The following section should be completed by the Supervisor: No Does the project involve Delete as appropriate If yes, describe the nature of the risk and steps taken to minimise it: Clinical populations? Yes / No Children (under 16 years)? Yes / No Vulnerable adults (such as individuals with mental health problems, learning disabilities, prisoners, young offenders)? - if Yes, will CRB check(s) be needed? 8. Is the information gathered from the participants of a sensitive or personal nature? Yes No Yes / No Yes / No If yes, describe the procedures to be used for: Does the project involve the collection of material that could be considered of a sensitive personal, biographical, medical or psychological nature? Yes / No a) assuring confidentiality (see also 7): Does the project involve procedures that may be reasonably expected to upset or offend participants (e.g. presentation of unpleasant stimuli, arousal of emotion)? Yes / No Will the study be conducted on the internet? Yes / No Signature of Supervisor: ___________________________________ Date: ___________________ b) protecting participants from stress: The completed form (three hard copies, of which one has been signed, plus an electronic copy) should be submitted by the student with a project outline to the secretary of the Departmental Ethics Committee, Ursula Richards (Ursula.Richards@warwick.ac.uk). Observational Research 9. If observational research is to be conducted without prior consent, please describe the situations in which observations will take place and say how local cultural values and privacy of individuals will be taken into account. -------------------------------------------------------------------For Departmental Ethics Committee use only: Action Taken: I am familiar with the BPS’s ethical principles for human participants Yes No Signature of Researcher(s): Approved Approved with modifications or conditions noted below Action Deferred. Please supply additional information or clarification noted below Signed on behalf of the Departmental Ethics Committee: Date: ___________ Date: ________