Triplex metallohelices: new potent and selective compounds against colon cancer
advertisement

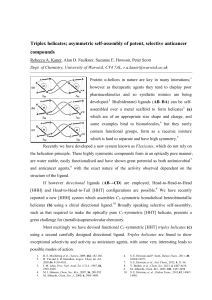
Triplex metallohelices: new potent and selective compounds against colon cancer R. A. Kaner,a,b A. D. Faulkner,a P. Gurnani,a S. E. Howson,a R. M. Phillips,c D. H. Simpson,a P. Scotta* a Department of Chemistry, University of Warwick, Coventry, CV4 7AL b Institute of Advanced Study, University of Warwick, Coventry, CV4 7HS c Department of Pharmacy, University of Huddersfield, Huddersfield, HD1 3DH There is a need to develop novel anticancer drugs which bypass multidrug resistance and exhibit high selectivity. Host defence peptide α-helices (a) help us to resist various diseases including cancers and infection,2 and there is a drive to develop synthetic functional mimics of these innate molecules.3 Metallohelices (e.g. helicates)4 being of a similar size and charge to α-helices,5 have risen to the fore.6 Unfortunately these metal complexes are unsophisticated in comparison to the natural systems,7 rarely contain functional groups and invariably have high symmetry.8 Our research lab developed new water compatible (a) Antibacterial α-helix of human systems known as flexicates (b), which are optically pure cathelicidin LL-37 of similar size manner, easily functionalised and have shown great potential as and charge density to a flexicate;1 (b) traditional symmetric flexicate [M2(AB'-B'A)3]; (c) low symmetry, triplex metallohelix. extremely potent and selective9 anticancer and antimicrobial agents. They are also found to cause significant changes to the cell cycle and induce early apoptosis in colon cancer.10 More recently we have established a novel asymmetric architecture, which employs directional ligands to form triplex metallohelices (c).11 These soluble, and stable compounds show exceptional anticancer activity. They are found to act on the cell cycle and induce apoptosis without damaging DNA. They appear to act similarly to the peptidic α-helices that they are designed to emulate, and are highly selective against cancer cells. These compounds take a significant step towards using metallohelix architectures as highly adaptable peptidomimetic systems in the fight against cancer.12 1. X. Li, et al., J. Am. Chem. Soc., 2006, 128, 5776-5785. 2. R. E. Moellering et al., Nature, 2009, 462, 182-188. 3. H. Yin and A. D. Hamilton, Angew. Chem. Int. Ed., 2005,44, 4130-4163. 4. J. M. Lehn, Proc. Natl. Acad. Sci. U.S.A., 1987, 84, 2565-2569. 5. M. J. Hannon, Chem. Soc. Rev., 2007, 36, 280-295. 6. R. A. Kaner and P. Scott, Future Med. Chem., 2015, 7, 1-4. 7. S. E. Howson and P. Scott, Dalton Trans., 2011, 40, 10268-10277. 8. M. Albrecht, Chem. Eur. J., 2000, 6, 3485-3489. 9. S. E. Howson, et al., Nat. Chem., 2012, 4, 31-36. 10. V. Brabec, et al., Chem. Sci., 2013, 4, 4407-4416. 11. A. D. Faulkner & R. A. Kaner, et. al.,Nat. Chem., 2014, 6, 797-803. 12. M. Albrect, Nat. Chem., 2014, 6, 761-762.