Cellular Control Analysis (CCA) Metabolic Control Analysis Biochemical Systems Theory
advertisement

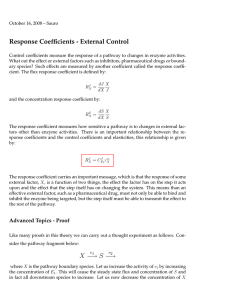
Cellular Control Analysis (CCA) Also called: Metabolic Control Analysis Biochemical Systems Theory Introduction to CCA It origins: Three groups, Edinburgh, Berlin and Michigan, simultaneously and independently developed almost an identical theory to describe the effects of perturbations in biochemical pathways. What is it? The approach aims to achieve a quantitative understand of the relationship between genotype and phenotype, to understand systems behavior in terms of the unit processes (enzymes) that make up biochemical pathways. Kacser H, Burns JA. 1973. The control of flux. Symposium of the Society for Experimental Biology 27, 65–104. (Reprinted in Biochemical Society Transactions 23, 341–366, 1995.) Cellular Control Analysis E1 X1 v1 S1 E2 v2 S2 E3 v3 X2 Steady state flux J Variables Parameters 1. Concentrations of Molecular Species 2. Fluxes 1. Enzyme Levels 2. Kinetics Constants 3. Boundary Species CCA investigates the relationship between the variables and parameters in a biochemical network.. Cellular Control Analysis E1 X1 v1 S1 E2 v2 S2 E3 v3 Steady state flux J At steady state: X2 A Thought Experiment Xo S1 S2 S3 v S4 S5 S6 X1 Xo and X1 fixed all reactions reversible, assume stable steady state. A Thought Experiment 50 % Xo S1 S2 S3 v What happens to the steady state? S4 S5 S6 X1 Xo and X1 fixed all reactions reversible, assume stable steady state. A Thought Experiment 50 % Xo S1 S2 S3 v S4 S5 S6 X1 What happens to the steady state? Xo and X1 fixed 1. Steady state flux goes down. 2. All metabolites upstream go up 3. All metabolites downstream do down all reactions reversible, assume stable steady state. Cellular Control Analysis E1 X1 v1 S1 E2 v2 S2 E3 v3 X2 Steady state flux J If we make a change, say to the levels if E2 (change its Vmax), we will observe a change in the steady state flux, J Let mean a small change, therefore to investigate the influence of changing E2 on the steady state flux J, we could measure the ratio Cellular Control Analysis E1 X1 v1 S1 E2 v2 S2 E3 v3 X2 Steady state flux J Unfortunately the ratio has two undesirable aspects: 1. The ratio depends on the size of the change that we make to E2 2. The ratio depends on the units that are used to measure E2 and J. Cellular Control Analysis E1 X1 v1 S1 E2 v2 S2 E3 v3 X2 Steady state flux J To avoid these issues, we scale the ratio to remove the units and we also use infinitesimal changes rather than small finite changes, as a result we get a new formula: Cellular Control Analysis Arithmetical interpretation: Cellular Control Analysis Geometrical interpretation: ~0.0 0.5 1.0 Cellular Control Analysis This is called the flux control coefficient Cellular Control Analysis Since changing the level of an enzyme activity can also change the metabolite levels, there is a similar control coefficient for the metabolites: This is called the concentration control coefficient Cellular Control Analysis Computing control coefficients using Jarnac p = defn cell … J1: S1 -> ….. end; println p.cc (<p.J1>, p.Vmax1); println p.cc (<p.S1>, p.Vmax1); Cellular Control Analysis Some characteristics of flux control coefficients. In a linear metabolic network, the value of any particular flux control coefficient is bounded between zero and one. This condition applies to a linear chain Cellular Control Analysis How can you measure control coefficients? 1. Changing gene expression and measuring the effect on the system. Cellular Control Analysis How can you measure control coefficients? 1. Changing gene expression and measuring the effect on the system. 2. Using inhibitors to change an enzyme’s activity and measuring the effect on the system. Cellular Control Analysis How can you measure control coefficients? 1. Changing gene expression and measuring the effect on the system. 2. Using inhibitors to change an enzyme’s activity and measuring the effect on the system. 3. Building a computer model and using the computer to compute the coefficients Cellular Control Analysis The Summation Theorems Cellular Control Analysis The Summation Theorems 1. The summation theorem implies that the enzymes of a pathway share the control of flux. 2. Changes in one control coefficient result in changes in other control coefficients. This means that a control coefficient is a system property and not an intrinsic property of the enzyme alone. Rate-Limiting Steps Where does the concept of the rate-limiting step fit into this picture? From CancerWeb: rate-limiting step The slowest step in a metabolic pathway. Is the rate-limiting step the step with a flux control coefficient of one? If so then there can only be one. OR Is the rate limiting step the step with the highest flux control coefficient? Rate-Limiting Steps Another complication: In branched and cyclic pathways, individual flux control coefficients can exceed 1.0, this means that some other steps may have flux control coefficients < -1.0. Does this mean that these steps are hyper-rate-limiting? Hyper-Rate-Limiting Steps Rate-Limiting Steps The answer is of course to drop the idea of the rate-limiting step and instead to quote, where possible, the value of the flux control coefficient which will give a quantitative measure of the limiting-ness of the step. Why they don’t exist Why rate-limiting steps don’t exist in metabolism. The flow through cellular networks does not behave like road traffic. The rate at which cars move in a traffic line is not a function of the number of cars in the line. Why they don’t exist The flow through cellular networks does not work like checkout lines. The rate at which customers move through the checkout line is not a function of the length of the line. Why they don’t exist The rate at which a substrate is catalyzed by an enzyme (reaction flow) is a function of the concentration of substrate. The “longer the substrate line” the faster the enzyme works Hexokinase http://mgl.scripps.edu/people/goodsell/pdb/p db50/pdb50_2.html Simple Example Xo R1 S R2 Xo R1 Flux Control on R1 Flux Control on R2 Product Insensitive ? ? Product Sensitive ? ? Simple Example Xo R1 S R2 Xo R1 Flux Control on R1 Flux Control on R2 Product Insensitive 1.0 0 Product Sensitive ? ? Simple Example Xo R1 S R2 Xo R1 Flux Control on R1 Flux Control on R2 Product Insensitive 1.0 0 Product Sensitive ~0.5 ~0.5 Cellular Control Analysis What about some real values? The following information was taken from a paper by S. Thomas et al, Biochemical Journal, 1997, 322, 119-127 Enzyme Glycolysis in tuber tissue of potato. Values computed using a combination of calculation and experimental work. Enzymes, metabolites and fluxes David A. Fell J Exp Botany, 56(410),267-272 Flux Control Coefficient PGM 0.029 PGI 0.139 PFK 0.132 Aldolase 0.0 TPI 0.0 GAPDH/PGK 0.001 enolase 0.005 PK 0.702 Sum 1.008 Cellular Control Analysis Outline of metabolism in Trypanosoma brucei http://bip.cnrs-mrs.fr/bip10/eise2.htm “Pharmacological Manipulation of Metabolism” http://bip.cnrs-mrs.fr/bip10/eise3.htm “Computer Simulation and Drug Design” http://homepage.mac.com/mfield/lab/Widgets.html Cellular Control Analysis Metabolic control analysis of glycolysis in trypanosomes as an approach to improve selectivity and effectiveness of drugs. Bakker et al. Molecular and Biochemical Parasitology 106 (2000) 1–10 Enzyme Flux Control Coefficient Glucose Transport 0.90 0.08 Hexokinase 0.02 0.05 Phosphofructokiase 0.00 0.01 Aldolase 0.02 0.28 GAPDH 0.02 0.23 Phosphoglycerate 0.02 0.15 Experimental and in Silico Pyruvate kinase 0.0 Analyses of Glycolytic Flux Control in Bloodstream G3P DH 0.02 Form Trypanosoma brucei, Sum 1.0 J. Biol. Chem., Vol. 280, Issue 31, 28306-28315, 35% increase over measured Vmax August 5, 2005 of Glucose Transporter 0.01 0.17 0.98 What Determines the Control Coefficients? Elasticities If the value of a control coefficient is a system property, what is the relationship of the control coefficients to the individual enzymes of the pathway? Somehow, all the enzymes contribute to the value of a given control coefficient, how can we describe this? In order to answer this question we must consider another type of coefficient, called the elasticity coefficient. Elasticities Michaelis-Menten Curve for an isolated enzyme Let us define the elasticity as: v S Elasticities Michaelis-Menten Curve for an isolated enzyme Note that substrate elasticities are positive and product elasticities are negative v S Elasticities Another way of looking at an Elasticity: We can use an elasticity to predict the change in the rate of a reaction given a change in the substrate concentration. Response at a Single Reaction Step Another way of looking at an Elasticity: Product inhibition term <0! In general if there are multiple changes happening around an enzyme we can simply sum each contribution using the appropriate elasticity. Response of a System E1 X1 v1 S1 E2 v2 S2 E3 v3 Increase E3: 1. v3 Increases 2. S2 Decreases 3. v2 Increases 4. S1 Decreases 5. v1 Increases X2 Response at a Single Reaction Step E1 X1 v1 S1 E2 v2 S2 E3 v3 X2 Response of a System E1 X1 v1 S1 E2 v2 S2 E3 v3 X2 Response of a System E1 X1 v1 S1 E2 v2 S2 E3 v3 X2 Response of a System E1 X1 v1 S1 E2 v2 S2 E3 v3 X2 Response of a System E1 X1 v1 S1 E2 v2 S2 E3 v3 X2 Response of a System E1 X1 S1 E2 S2 E3 X2 Extra term Response of a System E1 X1 S1 E2 S2 E3 X2 Response of a System E1 X1 S1 E2 S2 E3 X2 What happens as the feedback gets stronger and stronger? i.e Response of a System E1 X1 S1 E2 S2 E3 X2 Response of a System E1 X1 S1 E2 S2 E3 X2 What does this mean? Feedback has the following consequences: 1. All flux control moves down stream beyond the signal leaving little or no flux control upstream. In fact, the ‘controlled’ step has very little flux control. 2. The signal molecule is locked into homoeostasis Response of a System E1 X1 S1 E2 S2 E3 X2 What does this mean? The net effect of this is that feedback control creates a demand controlled network. That is, control over the flux through the pathway is determined largely by the demand for S2. Important examples is this include: 1. Glycolysis 2. Amino acid biosynthesis Response Coefficients Measureing the effect of external factors. Response Coefficients The effect that a perturbation has on a system depends on the target site and how the target site interacts with the rest of the network. Transfer Functions Linearize around steady state: Ingalls, B. P., A Frequency Domain Approach to Sensitivity Analysis of Biochemical Systems , Journal of Physical Chemistry B, 108 (2004) pp. 11431152. Relationship to Classical Control Theory: Transfer Functions This has the same structure as the linear state variable equations: Relationship to Classical Control Theory: Transfer Functions Response to step input Relationship to Classical Control Theory: Transfer Functions Response to sinusoidal input Frequency Input Relationship to Classical Control Theory: Transfer Functions Response to sinusoidal input Frequency Input Transfer Functions Frequency Response for Simple Gene Circuit Further Reading 185578047X, Ashgate Publishing 1996, David Fell 3527310789, Wiley-VCH, 2005, Kipp et al. Biochemical Systems Theory 0201067382, Addison Wesley Longman Publishing 1977, Michael Savageau. Response Coefficients Proof Increase all rates by the same factor, alpha Combining all local effects: Proof Response Coefficients Response Coefficients