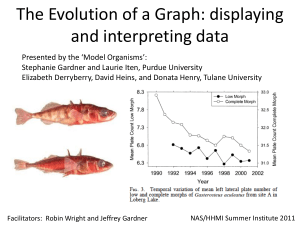
Wataru Koketsu for the degree of Master of Science in... Title: Environmental Correlates of Parasitism in Introduced Threespine Stickleback
advertisement

AN ABSTRACT OF THE THESIS OF Wataru Koketsu for the degree of Master of Science in Fisheries Science Presented on April 19, 2004. Title: Environmental Correlates of Parasitism in Introduced Threespine Stickleback (Gasterosteus aculeatus) in the Upper Deschutes River Basin, Oregon Redacted for Privacy Abstract Approved: W. Daniel Edge The Pseudophyllidean tapeworm, Schistocephalus solidus, is a parasite that requires both copepods and fish, mainly the threespine stickleback (Gasterosteus aculeatus) as intermediate hosts, and birds as its final host to complete its life cycle. A rapid increase in the abundance of both the non-native stickleback and the tapeworm in the Upper and Middle Deschutes Basin began in the late 1990s. To determine environmental factors that correlate with higher infection levels, fish and water quality data were collected at 77 sites during the summer of 2003 using a stratified random sampling design. The sites at which stickleback were infected with the parasite were concentrated both in the Crane Prairie and Wickiup reservoirs area. Multiple regression and logistic regression analyses showed mean size of stickleback, catch-perunit-effort, and water flow were significantly related to parasite prevalence (the percentage of hosts that are infected with the parasite) and mean infection intensity (mean number of parasite per fish). These occurrences were likely associated with human-altered habitat. A differential equation model was developed to characterize the parasite distribution among the host populations. This equation may serve to quantify and compare the impact of the parasite in host populations among basins or among different parasite species. © Copyright by Wataru Koketsu April 19, 2004 All Rights Reserved Environmental Correlates of Parasitism in Introduced Threespine Stickleback (Gasterosteus aculeatus) in the Upper Deschutes River Basin, Oregon By Wataru Koketsu A THESIS Submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science Presented April 19, 2004 Commencement June 2004 Master of Science thesis of Wataru Koketsu presented on April 19, 2004. APPROVED: Redacted for Privacy Major Professor, representin4'isheries Science Redacted for Privacy Head of the Department of Fisheri-and Wildlife Redacted for Privacy Dean oft ate School I understand that my thesis will become part of the permanent collection of Oregon State University Libraries. My signature below authorizes release of my thesis to any reader upon request. Redacted for Privacy Wataru Koketsu, Author ACKNOWLEDGEMENTS I wish to express sincere appreciation to W. Daniel Edge for his guidance and assistance with the preparation of this manuscript. Without his unwavering help and patience, I could not have completed this thesis. I would also like to thank Philippe A. Rossignol who always encouraged and motivated me, Jerri L. Bartholomew who provided me with plenty of concrete and practical input, and Ikuo Ninomiya who gave me many solutions and wisdom regarding both my research and academic life. I would like to thank Barbara Shields and William Liss for providing me with my study design and the initial concept. I would like to thank Phil Larsen, David Peck and Yonghai Li for their kindness and sharp methodology and analyses skills. I would like to thank Jaya Lapham and Victoria Cronin for their great assist and encouragement with my field work. In addition, I would like to thank Jay Bowerman for his hospitality and cooperation throughout my entire field season. I would also like to express deep appreciation to my family and relatives for their financial and mental support, and I would like to thank all of my friends including Todd Miller, John Seigle, Cory Overton, Troy Guy, Ted Hart, Luis Francisco, and Chichang Liu for their support. Especially, I deeply appreciate Ichiro Misumi and Yusuke Koseki, both talented young researchers, who provided overall support of my academic life. I would like to thank Howard and Jeannine Horton, and Mary Concer who have been surrogate parents for me here in the U.S. I sincerely appreciate the assistance of Soshichiro Nakagawa, Robert E. Witters, Yasuaki Ninomiya, and Lori Emmons; my life in the U.S. could not have begun without their initiative. I deeply appreciate Tsunehal Hibino for his 20 years of help and encouragement as my best friend. Whenever I faced difficulty, he was the first person for me to talk to. Finally, I would like to announce that I fulfilled my promise to Tsuya Hioki, my grandmother who went to heaven in May 2003, to acquire a Masters degree in the United States of America. TABLE OF CONTENTS Page GENERAL INTRODUCTION Exotic Species Stickleback Case Study Stickleback Tapeworm Research Significance 4 6 INTRODUCTION 7 1 1 2 3 MATERIALS AND METHODS Study Area Fish Sampling and Measurement of Environmental Factors Data Analyses ..10 10 13 ..15 RESULTS Fish and Parasite Distribution Environmental Correlates of Parasitism Distribution of Parasite in Host Differential Equation Models 16 . ..16 24 ... .29 31 DISCUSSION 33 REFERENCES 42 APPENDIX 49 LIST OF FIGURES Figure Page Life cycle of the tapeworm, Schistocephalus solidus 5 Map of study area in the Upper Deschutes Basin, central Oregon. The number in parenthesis indicates the elevation 11 Photos of study sites representing various habitat conditions in the Upper Desehutes Basin, central Oregon, July - August 2003: a) Cultus River, a tributary of Crane Prairie Reservoir; b) Crane Prairie Reservoir resort area; c) connection between Crane Prairie reservoir and Wickiup Reservoir; d) Sunriver residence area near golf course; e) Mainstem of Upper Deschutes River, Little Lava Island area; f) Mirror Pond area in Bend. Photos by Wataru Koketsu . ..12 Plot layout used to collect fish samples and environmental data for study of parasites in threespine stickleback in the Upper Deschutes Basin, central Oregon, July August 2003 14 Study area indicating distribution of threespine stickleback and S. solidus infections in the Upper Desehutes Basin, central Oregon, July - August 2003 Length distribution of (a) all stickleback (n 4,208), (b) infected stickleback (n = 549), (c) stickleback at non-infected sites (n = 2,259) and (d) stickleback at infected sites (n 1,949), Upper Deschutes Basin, central Oregon, July - August 2003 . .20 Relationship between catch-per-unit-effort and mean fork length of fish measured at 62 sites (a), and relationship between prevalence of infection and mean intensity of S. solidus (b) measured at 27 sites, Upper Desehutes Basin, central Oregon, July - August 2003 .. 22 Mean number of S. solidus per threespine stickleback (11 549) sorted by total fork length of threespine stickleback, Upper Deschutes Basin, central Oregon, July - August 2003 23 LIST OF FIGURES (continued) Figure Page Relationship between mean parasite intensity of S. solidus in threespine stickleback and environmental factors measured at 62 sites, Upper Deschutes Basin, central Oregon, July - August 2003 25 Relationship between mean parasite intensity of S. solidus in threespine stickleback and environmental factors measured at 62 sites, Upper Deschutes Basin, central Oregon, July - August 2003 26 10. Distribution of S. solidus in three spine stickleback with normal scale (a) and with log scale (b), Upper Deschutes Basin, central Oregon, July - August 2003 ..30 LIST OF TABLES Table Summary of threespine stickleback, parasite and environmental parameters collected at 62 sites, Upper Deschutes Basin, central Oregon, July - August 2003 Page 18 Ordinary least squares multiple regression statistics for independent variables predicting mean parasite intensity in threespine stickleback, Upper Deschutes Basin, central Oregon, July - August 2003 .26 Logistic regression statistics for independent variables predicting parasite prevalence in threespine stickleback, Upper Deschutes Basin, central Oregon, July - August 2003 28 Environmental Correlates of Parasitism in Introduced Threespine Stickleback (Gasterosteus aculeatus) in the Upper Deschutes River Basin, Oregon GENERAL INTRODUCTION Exotic Species The infestation of exotic species because of human influences has been massive (Li etal. 1999). According to the U.S. Congress (1993), within the last [00 years, over 4,500 exotic species have become established in the continental U.S. alone, and the worldwide explosion of fish diseases has been unprecedented during this period. The U.S. may have the largest number of fish introductions of any country in the world. Nearly 200 native species and at least 70 fishes from other continents have been introduced and become established in new ecosystems (Dextrase and Coscarelli 1999). At the same time, aquatic ecosystems in the world have been and are being altered at an alarming speed (Dudgeon 1992). These changes are caused by various interactive factors, but most ecosystems have experienced habitat alteration, overharvesting, and introductions of exotic species. Exotic species in particular may significantly affect native species through competition, predation, hybridization, and the introduction of new parasites and diseases (Harvey and Matthews 1999). Unfortunately, ecologists and natural resource managers cannot predict the outcome of the addition of exotic species to aquatic ecosystems (Lodge 1993). Since European colonization of North America, most nonindigenous introductions have been caused by human activities. These introductions include not only species that arrived from outside of North America, but also species native to North America introduced to waters outside their historical ranges. Coho salmon 2 (Oncorhynchus kisutch) is an example of the latter. Native of the Pacific Coast from northern California to Alaska, this species was introduced into the Great Lakes as early as the 1930s (Benson and Boydstun 1999). Moyle and Sato (1991 from Harvey and Matthews 1999) noted that the establishment of exotic species often is associated with past or ongoing habitat alterations. They also suggest the linear, interconnected nature of streams, the mobility of many aquatic species, and the possibility of accidental or illegal introductions make likely the infestation of exotic species even in the most closely managed stream preserves (Moyle and Sato 1991 from Harvey and Matthews 1999, Moyle and Light I 996a, b). By the time biologists become concerned about the presence of an alien species, it is often well established in its new habitat, and its source and natural history are either unknown or poorly understood (Li et al. 1999). The importance of studying exotic organisms is in understanding their possible effects on native organisms and the surrounding physical environment. In response, a large effort has been undertaken to document the history and monitor the distribution of aquatic introductions in North America (Benson and Boydstun 1999). Li et al. (1999: 431) point out that "the greatest need is for a management framework to assess the following issues: (1) the potential impact of the alien species upon the receiving community, (2) the ecological interactions for which understanding is most critical, and (3) strategies that guide management policy to minimize potential deleterious effects of ecological change." Stickleback Case Study In the Upper Deschutes River Basin, Oregon, the introduction of an exotic host and parasitic disease has occurred and may be still proceeding today. The threespine stickleback (Gasterosteus aculeatus) is believed to have been introduced from west of the Cascade Range as bait for recreational fishing or as released pets within last two decades, and has since spread to the Upper Deschutes River Basin. In addition, the 3 parasitic tapeworm, Schistocephalus solidus, has been observed in the body cavity of stickleback at multiple sites in the Deschutes Basin in Oregon. This parasite requires both copepods and fish, as intermediate hosts, and birds as its final host to complete its life cycle (Hopkins and Smyth 1951, Arme and Owen 1967). The introduction of stickleback into the Upper Deschutes River Basin has resulted in a rapid increase in the abundance of both the stickleback and the tapeworm since middle 1990s. In the case of parasites that require multiple intermediate hosts, the alteration of community structure directly affects the broader ecosystems via food webs because the amount of primary production, phyto/zooplankton community or mortality, and population dynamics of host predators are all linked by the parasite (Anderson and May 1982, Kennedy et al. 1986, Kennedy and Fitch 1990, Huxham et al. 1996, Marcogliese 2002). The colonization of stickleback may affect the habitat or resource competition with other species (e.g., juvenile salmonids, sculpins). Increases of parasitized stickleback population may also affect the survival of fish-eating birds or other wildlife (Hopkins and Smyth 1951, Arme and Owen 1967). These alterations of community structure, triggered by an exotic host, may induce parasite outbreaks in host populations if optimal environmental conditions occur. Stickleback The worldwide distribution of the threespine stickleback is in boreal and temperate regions of the northern hemisphere where it inhabits coastal marine waters, brackish water, and a wide array of freshwater habitats (Bell and Foster 1994). In the Pacific Basin, their habitat ranges from Baja California, Mexico, northward along the coast of North America, across the Bering Strait and southwest along the coast of mainland Asia and Japan to the southwest coast of Korea (Wootton 1984a). Unlike central Europe, populations in Asia and North America do not appear to penetrate significantly into interior fresh waters. 4 The normal body size of the stickleback is about 5 cm from the tip of the snout to the end of the vertebral column (Hubbs and Lagler 1970 from Bell and Foster 1994), but length ranges up to 11 cm (Bell and Foster 1994). In the Upper Deschutes River Basin in Oregon, the maximum size was approximately 7 cm and 4 g. Stickleback have been observed in various lentic habitats over a range of sizes. For example, Bell and Baumgartner (1984) reported a population from a 260-m2 spring pooi, and at the other extreme, Hubbs and Lagler (1970 from Bell and Foster 1994) reported stickleback from the 1,968-km2 Lake Ontario, North America. Similarly, stickleback can use various lacustrine habitats and resources (Bell and Foster 1994). When male sticklebacks become reproductive, their eyes and body typically change to a blue tinge, and the ventral surface of the head and trunk becomes red (Wootton 1 984b, Reimchen 1989). The color change of reproductive females is less pronounced, but it is biologically significant (Rowland et al. 1991), and reproductive females are distinguished by the distension of the pale abdomen containing a massive brood of eggs (Bell and Foster 1994). The threespine stickleback is also well known for a wide array of conspicuous, highly ritualized behaviors. Studies of stickleback aggression, territoriality, courtship, and male parental behavior have contributed to our understanding of basic is sues in animal behavior (Rowland et al. 1991). Tapeworm The life cycle of the S. solidus tapeworm was unknown until 1919, when Nybelin experimentally infected a copepod (Cyclops spp.) with coracidia (Hopkins and Smyth 1951). Hopkins and Smyth (1951) and Clarke (1954) summarized these historical findings of multiple hosts and revealed the lifecycle of S. solidus in more detail. A free-swimming larva, a coracidium, hatches out after development and is eaten by a copepod. Development up to the procercoid stage occurs in the body cavity of the crustacean, and when the crustacean is swallowed by a stickleback, growth and 5 development of procercoid continue in the body cavity of the fish to the plerocercoid stage. Two species of sticklebacks, the threespine and the ninespine (Pungitius pungitius), are the usual hosts at this stage. When the stickleback is eaten by a bird, the worm sexually matures in the avian gut (adult stage) and produces eggs. An estimated 20,000 eggs could be expected from average-sized worms. The eggs pass out into the water with the feces (Cooper 1918 from Clarke 1954, Hopkins and Smyth 1951, LoBue and Bell 1993) (Fig. 1). Schistocephalus solidus appears to occur normally in species Fish-eating bird (adult stage) Paratenic host Eggs (coracidium stage) Threespine stickleback (plerocercoid stage) Copepod (procercoid stage) Figure 1. Life cycle of the tapeworm, Schistocephalus solidus. 6 of the stickleback family, and its occurrence in other secondary hosts is probably accidental and rarely happens (Hopkins and S myth 1951). Five species of Cyclops and 40 species of birds have been reported as potential first and final host respectively (Hopkins and Smyth 1951, Clarke 1954, Anne and Owen 1967). Infection of paratenic host, which is an accidental host and rarely occurs, may include larger fish or mammals which forage on stickleback. Research Significance In the Deschutes Basin, Oregon, stickleback have not been studied and are not being managed because they are a nongarne species. Thus, little research has been conducted dealing with both the stickleback and tapeworm from a viewpoint of ecological impacts. Specifically, the distribution of parasitized stickleback is largely unknown. One goal of this study was to determine the environmental conditions where stickleback and tapeworms occur. I started this research by gathering basic information about stickleback and tapeworm occurrences and their associated habitat characteristics, such as water quality, in the Upper Deschutes River system. My research question is whether there are correlations between parasitism and environmental parameters. I collected fish and environmental data at 77 sites along 90 km of streams, tributaries and lakes in the summer of 2003. The goals of this research are to (1) determine the distribution of stickleback and its parasite, (2) predict the sites that likely contain parasites, and (3) seek an alternative indicator to evaluate the degree of parasite infection. 7 INTRODUCTION Emergent parasitic pathogens, caused by the introduction of exotic species, have triggered new and complicated ecological interactions in many ecosystems (Sakanari and Moser 1990, Marcogliese and Cone 1997). Introduced hosts or parasites can establish new associations in which the host-parasite interactions may lead to increased host resistance and increased parasite virulence (Anderson and May 1982). Parasites are known to have the capacity to regulate host populations, and may be important determinants of community structure because they are typically considered as top predators within food webs, although their host (and the parasites) may fall prey to predators (Dubois et al. 1996, Marcogliese and Cone 1997). Introduction of exotic hosts often cause changes in populations of native species, and the parasites of exotic species may colonize the new region if they are capable. Transmission into another species of hosts is also possible. Alterations of host's habitat or feeding behavior by a parasite normally enhance further opportunity for transmission (Apanius and Schad 1994). For example, mate selection, reproductive success and host survival may each be affected by parasitism (LoBue and Bell 1993, Reimchen and Nosil 2001, Hems and Baker 2003). Especially in the case of parasites that require multiple intermediate hosts, the alteration of host's behavior or population structure affects the broader ecosystem via food webs (i.e., the amount of primary production, phyto/zooplankton community, or mammal and bird survival and density) (Anderson and May 1982, Kennedy et al. 1986, Kennedy and Fitch 1990, Huxham et al. 1996, Marcogliese 2002). In addition to endogenous factors, exogenous factors such as environmental conditions may induce parasitism and alter community structure because they play important roles in susceptibility of hosts and for habitat selection of both host and parasite (Whittier and Hughes 1998, Sandell et al. 2001, Malcogliese 2002). Water pollution, as a result of pesticides, metal cations and paper mill effluents, may change the population structure of zooplankton and effect humoral and cellular immune 8 functions with the consequent impairment of fish health and diseases resistance (Maule et al. 1989, Kuris et al. 1991, Marcogliese et al. 1992, Dunier 1996). In addition, some environmental factors, like conductivity or pH, may be associated with both parasite and host abundance, or prevalence of parasite infection (Marcogliese 1995, Marcogliese and Cone 1996, Sandell et al. 2001). Some researchers consider parasite communities to be variable and those local factors, chance of colonization and extinction events promote the distinctiveness of parasite communities (Kennedy et al. 1989; Kennedy and Fitch 1990; Dubois et al. 1996; Marcogliese etal. 1992; Marcogliese 2001, 2002). Human mediated disturbance on local and landscape scales have also been implicated for rapid increases in parasites (Barker et al. 1996, Marcogliese 2001). Introduction of parasites can substantially alter basic food web properties, including connectance, chain length and proportions of top and basal species, and can allow the testing of specific hypotheses related to food-web dynamics. Fish-parasite dynamics may also affect copepod communities (Marcogliese and Esch 1989, Mareogliese and Cone 1997). The threespine stickleback, Gasterosteus aculeatus, was believed to have been introduced to the Upper Deschutes River Basin from west of the Cascade Range as bait for recreational fishing or as released pets in the 1 980s and has since spread to the basin (Jay Bowerman, Sunriver Nature Center, personal communication). This species is widely distributed in coastal ranges in North America, and are tolerant of a wide range of temperatures but prefer cool temperatures (Bell and Foster 1994). The Pseudophyllidean tapeworm, Schistocephalus solidus, is a parasite that requires both copepods and fish, as intermediate hosts, and birds as its final host to complete its life cycle (Hopkins and Smyth 1951, Arme and Owen 1967). Although the tapeworm naturally occurs in rivers or lakes, the infection intensity for fish and birds is normally low under equilibrium conditions (Clarke 1954). Prior to the introduction of the threespine stickleback, the Upper Deschutes Basin might not have contained a suitable intermediate host for the tapeworm, whereas the cyst of the tapeworm had already 9 been carried into the basin by birds. However, the introduction of stickleback into the Upper Deschutes River Basin has resulted in a rapid increase in the abundance of both the stickleback and the tapeworm since the middle 1 990s. This study was designed to examine patterns of occurrence of S. solidus of stickleback in freshwater habitats of the Upper Deschutes River and adjacent systems. I sought to determine the distribution of stickleback and parasites throughout the region and to establish if there were relationships between the prevalence and intensity of infection of stickleback and environmental factors. Additionally, I attempted to evaluate the impact of parasite burden on fish populations by building differential equation models. During three weeks beginning the end of July 2003, I collected information on the abundance of stickleback, tapeworm and on water quality variables at 77 sites using a stratified random sampling program based on a modified Environmental Monitoring Assessment Program (Peck et al. 2003). 10 MATERIALS AND METHODS Study Area The study area is located in central Oregon in the Upper and Middle Deschutes Basin. Seventy-seven potential sampling sites were distributed from Little Lava Lake (elevation 1,444 m) to Cline Falls (elevation Deschutes near Redmond (elevation 865 m) located on the Middle 913 m), Oregon (Fig. 2). These sites were selected based on land use, road access, and river conditions with enough gradients of targeted environmental factors at sites which have various habitat characteristics (Fig. 3). I collected samples from 77 of these possible sites over 3 weeks starting 30 July 2003 when there was no snowmelt and little precipitation. All sampling sites were selected using a stratified random sampling design based on the U.S. Environmental Protection Agency's Environmental Monitoring Assessment Program (EMAP) (Peck et al. 2003) and sampling order was determined by block-randomized sampling. The limitations of the stratified random sample were that sites must be physically accessible, on public land, and 20 minutes walk from the road. The EMAP is a synoptic survey designed to answer questions about the numbers, spatial extent, and ecological condition of surface water resources at a regional scale (Larsen and Christine 1993). The sampled plots are representative of the range of conditions in all but the inaccessible sites in the Upper Deschutes River because of the probabilitybased design used to select them (Larsen and Christine 1993, Whittier and Hughes 1998, Peck et al. 2003). Oregon Cline Falls (865 m) . Redmond (913 m) Turn al 0 (970 m) Tumalo Creek Bend (1,106 m) Little Lava Lake (1,444 m) Sunriver (1,271 m) Desch utes River Cultus Lake (1,423 m) Crane Prairie Reservoir (1,355 m) Paulina Creek . La Pine Wickiup 1 Reservoir (1,322 m) (1,290 m) Little Deschutes River Paulina Lake (1,930 m) 0 10km Figure 2. Map of study area in the Upper Deschutes Basin, central Oregon. The number in parenthesis indicates the elevation. 12 (d) Figure 3. Photos of study sites representing various habitat conditions in the Upper Deschutes Basin, central Oregon, July August 2003: a) Cultus River, a tributary of Crane Prairie Reservoir; b) Crane Prairie Reservoir resort area; c) connection between Crane Prairie reservoir and Wickiup Reservoir; d) Sunriver residence area near golf course; e) mainstern of Upper Deschutes River, Little Lava Island area; f) Mirror Pond area in Bend. Photos by Wataru Koketsu. 13 Fish Sampling and Measurement of Environmental Factors Fish were collected and environmental parameters were measured at each sample site. At each site, a 15 m X 1 m plot was defined along the littoral and riparian line. Fish were collected by dip nets to obtain a range of fish sizes using a fishing effort of 30 minutes per site within the 15 m X 1 m plot. The field sampling objective was to collect a representative sample of the fish assemblage at each plot. All stickleback were packed in ice immediately after capture. Length and weight were measured, and the body cavity of each fish was opened and plerocercoids were removed, counted, and weighed. Plerocercoids were identified according to Hopkins and Smyth (1951) and Clarke (1954). At each station, three replicates were taken for all environmental data by dividing the plot into three 5 m X 1 m subplots (Fig. 4). Except for fish collections, water flow and maximum water temperature, all environmental parameters were measured at the center of each subplot. Maximum water temperature in a day was measured by high-low thennal recorder. I measured minimum temperature between 05:30 h and 08:30 h with a YSI 85 dissolved oxygen/conductivity meter. Water flow was measured three times at the center of the plot. I timed a porous plastic ball (diameter =4 cm) flowing 10 m and calculated the flow velocity. For plots with very low flows, this distance was shortened to 5 m. Dissolved oxygen, conductivity and water temperature were measured with a YSI 85 dissolved oxygen/conductivity meter. Water level was measured with a wooden ruler; depths ?120 cm were recorded as 120 cm. Cover of submerged macrophytes and substrate, i.e., mud and sand substrate ratio, were visually estimated according to Peck et al. (2003). 14 Dissolved oxygen Conductivity Minimum temperature Water depth Vegetation Substrate / / Fish collection Water flow Maximum temperature / water stream bank Figure 4. Plot layout used to collect fish samples and environmental data for study of parasites in threespine stickleback in the Upper Deschutes Basin, central Oregon, July - August 2003. 15 Data Analyses Because 2.2 cm sticklebacks were the smallest fish infected with plerocercoids, I only used fish ?2.2 cm for my analysis. Prevalence and mean intensity were calculated for each site. I defined "prevalence" as the percentage of hosts with parasite infection (%) and "mean intensity" as the mean number of parasites per host. Significant correlations of environmental variables with the prevalence and mean intensity of infection of stickleback were identified by regression analysis. Ordinary least squares (OLS) multiple regression was used to determine if any of the environmental variables could account for variation in mean intensity. All multiple regression models were analyzed for multi-collinearlity to ensure that the assumptions of the OLS technique were met (Ramsey and Schafer 1997). For stepwise variable selection, a significance level of 0.05 was used as the criterion for allowing a variable to enter the model, and 0.05 was used as the criterion for retaining a variable in the model. Variables were tested for normality and log transformed when required. Significance was defined as P <0.01. To determine correlates of parasite prevalence, logistic regression was used because the response variable is the probability of parasite infection among host populations. I used chi-square for the rich model to test for lack- of-fitness. Three observations were eliminated because they were outliers in residual plots. A full model was constructed and model selection was conducted by drop in deviance test (Ramsey and Schafer 1997). Statistical analyses were conducted using SPLUS (Statsci 2000) and SAS version 8.2 (SAS 2003). In the past, many parameters have been created to evaluate parasite influence on individual host or host populations (e.g., prevalence, mean intensity, Parasite Index [Pennycuick 1971], and truncated negative binomial analysis [Brass 1958, Crofton 1971, Lanciani and Boyett 1980]). To evaluate parasite distribution in host population among basins, differential equations were developed based on the host frequency parasite per host relationship using a log transform of the y-axis. 16 RESULTS Fish and Parasite Distribution Between 30 July and 17 August 2003, I collected fish and environmental data at 77 sites. Stickleback were observed and captured at 62 sites, resulting in a total capture of 4,208 fish. A total at 549 fish from 27 sites were infected with S. solidus. My research objectives were 1) to determine the distribution of both stickleback and tapeworm throughout the study area, and 2) to characterize the variation of environmental factors between sites. I collected stickleback throughout most of the study area (Fig. 5). However, stickleback habitat was patchy and locally distributed, resulting in 15 sites where stickleback were absent. The 27 sites where infected fish were collected were mostly concentrated upstream of Crane Prairie and Wickiup reservoirs, located near the origin of Deschutes River (Fig. 5). Sites with infected fish were generally characterized as lower elevations with pool habitats, submerged macrophytes and muddy substrates. Most of the sites in mainstream of the Deschutes River did not have parasites except for the Bend area. Other infected sites in mainstream of the Deschutes River were detected sporadically. No fish with parasites were collected at either Paulina Lake or Paulina Creek. Prevalence and mean intensity varied throughout the study area (Table 1). I collected a total of 1,463 fish at the 27 sites with infected fish; 549 of the fish had a total of 1,464 worms. Among infected fish (n = 549), mean number of parasite per host was 2.667 worms/fish (SD fish (n 2.197). Among all fish captured at the sites with infected 1,463), mean number of parasite per host was 1.001 worms/fish (SD = 1.865). Mean intensity per site was 0.620 worms/fish (SD site was 22.4 % (SD = 37.6). 1.224) and mean prevalence per 17 Upper Deschutes River x stickleback with parasite I (27) Redmond stickleback without parasite (35) 0 stickleback absent (15) Tumalo Creek Bend Sunriver Crane Prairie Reservoir Paulina Lake La Pine Wickiup Reservoir 10km 0 I I I Figure 5. Study area indicating distribution of threespine stickleback and S. solidus infections in the Upper Deschutes Basin, central Oregon, July - August 2003. 18 Table 1. Summary of threespine stickleback, parasite and environmental parameters collected at 62 sites, Upper Deschutes Basin, central Oregon, July - August 2003. Variable Mean Range SD CVa Total fish captured at each site 67.9 1 -483 69.9 103.0 Number of fish 2.2 cm 48.7 39.5 81.1 Number of fish infected Prevalence (%) Mean intensity (mean # of worms/fish) Mean fish size (fork length, cm) Catch-per-unit-effort (fish/br) Dissolved Oxygen (mg/I) Conductivity (ps/cm) Water flow (mis) Water depth (cm) Minimum temperature (°C) Maximum temperature (°C) Submerged macrophytes cover ratio (%) b Substrate cover ratio (%) b 8.9 17.2 194.6 0.6 l -237 0- 72 0-100 0.0-5.7 3.26 1.90-5.00 0.82 25.3 173.5 2-966 190.9 110.0 7.063 2.51 - 10.73 1.477 20.9 82.4 28.2-530.0 117.6 142.7 0.222 0.000 - 0.859 0.203 91.7 42.9 1.8- 120.0 28.5 66.4 a b Coefficient of variation (SD/mean X 100). Categorized according to Peck et al, (2003). 22.4 37.6 168.1 1.2 197.4 16.4 5.2- 24.3 4.0 24.5 20.0 10.0-35.6 4.8 23.8 56.4 0.0-87.5 0.0-87.5 32.9 58.3 28.8 39.8 72.4 19 The reservoir area encompasses various watershed characteristics (i.e., two large reservoirs with large daily temperature fluctuations, many tributaries originating from cold springs, lakes, dams, etc., resulting in large variations in environmental data), whereas the Deschutes River mainstem had less variation because of its relatively constant water environment. The highest and second highest dissolved oxygen levels were recorded at Quinn River and Cultus River, respectively; both are tributaries of Crane Prairie Reservoir. Conductivity was high at Paulina Lake, South Twin Lake and Sunriver area. Water temperature was high in the reservoir and Sunriver areas. Compared with the reservoir areas, the mainstream of the Deschutes River had relatively higher dissolved oxygen levels, lower conductivity levels, lower water temperature and higher water flows. Most of these parameters were stable and had less variation than tributaries, reservoirs and lakes. During dissections, I found that sexually mature female and male stickleback and gravid females tended not to be infected with S. solidus, even in areas with high infection prevalence. The size structure of all stickleback collected suggested two age classes (Fig. 6a), with a high frequency of young-of-the-year stickleback. In contrast, most of the infected fish were more than one year old (Fig. 6b). The length distribution at sites without infected fish had a uni-model distribution (Fig. 6c), whereas the sites with infected fish had a bi-model length distribution (Fig. 6d). 20 10 (a) All fish (n = 4,208) 8 0 .0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 Total fork length (cm) 10 (b) All infected fish (n = 549) U- .jhØ5,0 !"& 0 ;. 1.0 Total fork length (cm) 10 (c) Fish at non-infected sites (n = 2,259) IbihIllIhIl JLn 0 1.5 1.0 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 Total fork length (cm) 10 (d) Fish at infected sites (n = 1,949) 0 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 Total fork length (cm) Figure 6. Length distribution of(a) all stickleback (n 4,208), (b) infected stickleback (n = 549), (c) stickleback at non-infected sites (n = 2,259) and (d) stickleback at infected sites (n = 1,949), Upper Deschutes Basin, central Oregon, July - August 2003. 21 The reservoir areas had relatively large mean fork length and CPUE, and Deschutes River mainstem had relatively small mean fork length and CPUE except for the Bend area. There was no significant linear relationship between mean fork length and CPUE (partial r 0.270, P 0.034). The sites with larger mean fork length had lower CPUE, whereas the sites with smaller mean fork length had a wide range of CPUE (Fig. 7a). The sites with higher prevalence tended to have a higher level of mean intensity. Prevalence and mean intensity were correlated (partial r 0.901, P < 0.001) and were highest at the sites in Crane Prairie Reservoir (Fig. 7b). Mean number of parasite per host was plotted against fish fork length to characterize the relationship between mean parasite intensity and host size, which resulted in a curvelinear or exponential curve relationship (Fig. 8). Intensity appeared to be most pronounced around 5.0-cm size fish, and either reached equilibrium or gradually declined with larger fork length. 22 900 - 1000 S (a) 800 - S 700- n = 62 600- r20.073 500- P=0.034 D 400l:L o 300- S S S - 200 100 0 0.0 1.0 . S 5 2.0 0 4.0 3.0 6.0 5.0 Arage fork length (cm) . 100 S S 90 80 S . S 70 60 50 n= 27 40 30 20 0.812 P< 0.001 10 0 0.0 1.0 2.0 3.0 4.0 5.0 6.0 Mean intensity (worms/fish) Figure 7. Relationship between catch-per-unit-effort and mean fork length of fish measured at 62 sites (a), and relationship between prevalence of infection and mean intensity of S. solidus (b) measured at 27 sites, Upper Deschutes Basin, central Oregon, July - August 2003. 23 5.0 4.5 I. 4.0 -C (1) I 3.5 3.0 I. S 2.5 I I 2.0 . C S S S I S 1.5 1.0 - S 5.5.5. .5 I 0.5 0.0 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 Total fork length (cm) Figure 8. Mean number of S. solidus per threespine stickleback (n 549) sorted by total fork length of threespine stickleback, Upper Deschutes Basin, central Oregon, July August 2003. 24 Environmental Correlates of Parasitism My second objective was to determine environmental factors that are correlated with parasitism. Firstly, I checked correlations between mean parasite intensity and each single variable. There were no significant linear relationships between mean parasite intensity and any single environmental parameter (partial r < 0.69, F> 0.001). However, there may be an optimal range or threshold of parasitism for dissolved oxygen level and water temperature (Fig. 9a, 9b). I used OLS multiple regression analysis to determine variables that influenced mean intensity and used both-ward stepwise model selection to build my models. Before analysis, water depth and minimum water temperature were omitted from the full model because of interactions, and mud/sand substrate cover ratio was omitted because it was highly skewed. Mean fork length, CPUE, conductivity and water flow were significantly correlated with mean parasite intensity (Table 2). Mean parasite intensity was positively related to mean fish size and CPUE, and negatively correlated with conductivity and water flow. Mean parasite intensity was not related to dissolved oxygen level (partial r = 0.002, P minimum (partial r 0.299, P 0.9896), water depth (partial r = 0.401, P = 0.0012), 0.0181) or maximum daily temperature (partial r 0.334, P = 0.008), submerged macrophytes cover ratio (partial r = 0.389, P = 0.0018) or mud/sand substrate cover ratio (partial r = 0.229, P 0.074). The OLS multiple regression model that best describes mean intensity of tapeworms in stickleback was log e mean intensity -10.96 + 2.72 mean fork length (cm) + 0.520 log e CPUE (fish/hr) - 1.54 loge conductivity (ts/cm) - 0.55 log e water flow (mis) (r2 0.0001). 0.6537, P < 25 6.0 6.0 5.0 r=0.477 4.0 P< 0.001 5.0 .. 3.0 S . C U) I . 0.020 P= 0.273 C .. 3.0 CU (U 2.0 2.0 I. I 1.0 0.0 (U 1.0 2.0 3.0 4.0 Fvan fork length (Cm) 5.0 6.0 6.0 6.0 5.0 5.0 500 600 0.061 P=0.053 4.0 (0 C 41) (U 3.0 C I I C (U 41) 200 300 400 Conductivity (ps/cm) 100 0 C ' S 0.0 00 4 I 1.0 I (U 2.0 I I . 1.0 I 1.0 0.0 I 0.0 0 200 400 600 800 1000 0.0 0.2 0.4 CF'(JE (fish/hour) 6.0 6.0 5,0 ,2 4.0 P= 0.990 0.000 0.8 1.0 . 2 5.0 (U 41) (U 3.0 I I I 3.0 . C (U (U I IS 2.0 I I. 1.0 0.161 P= 0.001 . (U C 2.0 0.6 Water flow (rn's) 1.0 0.0 00 0. 0I0 5-4 0.0 2.0 4.0 6.0 8.0 Dissolved oxygen (n/l) 10.0 12.0 0 20 40 111-4 60 80 100 Water depth (cm) 120 140 Figure 9a. Relationship between mean parasite intensity of S. solidus in threespine stickleback and environmental factors measured at 62 sites, Upper Deschutes Basin, central Oregon, July - August 2003. 26 6.0 5.0 - 6.0 . 0.090 P=0.018 . r2 = 0.152 5.0 P= 0.002 . C a, 3.0 1 S C Ce a, S 2.0 S 1.0 . I 5 10 S 1.0 I . 0.0 . 2.0 . 15 : 0.0.. 20 30 25 35 40 0.0 0.2 0.4 Mninumw ater teirçerature (°c) 6.0 5.0 - 0.8 6.0 = 0.052 P=0.008 S P=0.074 5.0 p4.0 S . Ce C . 3.0 S S C . Ce 2.0 2.0 . S 1.0 2 . I 1.0 ¶ 0.0 0 5 10 . ..... 15 25 30 35 40 Maxinijmw ater terrperature (cc) .. ! 0.0 20 1.0 Vegetation (%) . r=0.112 0.6 0.0 0.2 0.4 0.6 0.8 1.0 Fvtid/sand substrate (%) Figure 9b. Relationship between mean parasite intensity of S. solidus in threespine stickleback and environmental factors measured at 62 sites, Upper Deschutes Basin, central Oregon, July - August 2003. Table 2. Ordinary least squares multiple regression statistics for independent variables predicting mean parasite intensity in threespine stickleback, Upper Desehutes Basin, central Oregon, July - August 2003. Variable Mean fork length (cm) Catch-per-unit-effort (fishlhr) b Conductivity Qis/cm) b Water flow (mis) b Coefficient Sum of Squares a 2.718 307.409 38.203 0.0000 0.520 -1.543 7.842 0.0011 -0.554 83.680 0.0000 P-value a 0.0851 aSum of squares and probability for each variable when entered into the model after all other variables. bNatural log (loge) transformed. 27 Logistic regression analysis indicated that mean fork length, CPUE, dissolved oxygen level, conductivity, water flow, maximum daily water temperature, and mud/sand substrate cover ratio were significantly correlated with prevalence (Table 3). The odds that a stickleback will be infected by a parasite decrease with mean fork length and increase with CPUE, dissolved oxygen level and mud/sand substrate cover ratio (Table 3). The odds of infection decrease by an estimated 99.9 % per centimeter for the mean fork length, increase by an estimated 2.0 % per number of fish/h for the CPIJE, 5.93 x io % per mgIL of dissolved oxygen, 1.63 X l0 % per rn/s for water flow, 101.4 % per degree for maximum daily water temperature and 5.3 )< l0' % per cover ratio of mud/sand substrate. The logistic regression model that best describes prevalence of tapeworms in stickleback was logit (ir) = - 29.640 - 11.63 mean fork length (cm) + 0.026 CPUE (number of fish / h) + 3.94 dissolved oxygen (mg / L) + 0.024 Conductivity (ps/cm) + 9.759 water flow (mi's) + 0.038 Water depth (cm) + 0.6701 highest water temperature in a day (°C) + 29.01 mud/sand substrate cover ratio (%) + square terms (P < 0.00 1). Although the results of both regression analyses were slightly different, two endogenous factors, mean fork length and CPUE, and the one exogenous factor, water flow, were common parameters for both analyses. Endogenous factors of fish size (age) and abundance, rather than exogenous factors, appear to better explain mean parasite intensity and prevalence of infection. 28 Table 3. Logistic regression statistics for independent variables predicting parasite prevalence in threespine stickleback, Upper Deschutes Basin, central Oregon, July - August 2003. Variable Coefficient z-statistic Intercept -29.640 -11.630 -2.76 1 -3.116 0.0038 0.026 6.502 0 Meanforklength(cm) Catch-per-unit-effort (fish/br) Dissolved Oxygen (mg/i) Conductivity (.ts/cm) Water flow (mIs) Water depth (cm) Maximum temperature (°C) Substrate cover ratio (%) b Squared mean fork length Squared Catch-per-unit-effort Squared dissolved oxygen Squared conductivity Squared water flow Squared Maximum temperature Squared substrate cover ratio P-value a 3.941 7.63 5 0 0.024 2.424 0,0099 9.759 2.795 0.0056 0.038 3.338 0.0003 0.670 3.319 0.0004 29.008 1.148 0.0031 2.679 4.849 0 -0.00002 -5.586 0 -0.287 -6.659 0 -0.00007 -2.468 0.0007 -50.945 -7.155 0 -0.013 -2.834 0.0027 -23.348 -1.3 18 0.0025 d.f.=43 Residual Deviance = 60.9 ap - value was calculated by drop in deviance test except for intercept. Values less than are indicated as zero. 10 bCategorized and measured according to Peck etal. (2003). 29 Distribution of Parasite in Host To clarifr the distribution of S. solidus in the threespine stickleback population, the frequency of the host population (n = 549) was plotted against the number of parasites that hosts contain (Fig. 1 Oa). The maximum number of worms per fish was 15 and the mean number of worms per fish was 1.001 (SD = 1.865). Most of the host population was aggregated at the one worm infection level and frequency declined substantially after one or two worms; few hosts contained 210 worms. I fitted observed data to a negative binomial distribution curve; the observed relationship closely approximated a negative binomial distribution (2' 2 = 7.975, P < 0.01). To characterize the bar graph, I plotted the number of parasites per host on the x-axis, and the number of host on the y-axis (Fig. lob). The y-axis was log scaled and O for parasite load was omitted to focus only on fish with parasites. If as the number of parasites per host increases, the mortality of the host also increases, this relationship can be modeled with an exponential function: Nae (1) Where N: the number of host n : the number of parasite per host a : constant number b : constant number I found a significant relationship between number of hosts (log scale) and the number of parasites per host (N= 225.16 e 0393ui, [r2= 0.9326, P <0.01]). The slope of the line (b = 0.393) represents the degree of utility that the parasite could make of the host population or the degree of the host capacity for containing parasites. 30 250 (a) Host 200 :n=549 Parasite: n = 1,464 150 100 50 0 IIIII1I.l____l 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Number of parasite per host 1000 C) (b) Y= 225.16 e°39 C.) C,) .2 r2=O.9326 100 U) 0 9- 0 10 C) E 2 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Number of parasite per host Figure 10. Distribution of S. solidus in threespine stickleback with normal scale (a) and with log scale (b), Upper Deschutes Basin, central Oregon, July - August 2003. 31 Differential Equation Models An alternative model was developed to express the characteristics of parasite distribution in a basin-wide host population and to evaluate the potential of the parasite to induce host mortality. Based on the host frequency - parasite per host relationship and its trend line (Fig. 1 Oa, b), differential equations were built using log transform of the y-axis to obtain an alternative model. This model can be used for comparison of infection level of host population in a large scale habitat compared to evaluations from a small scale habitat such as mean parasite intensity or prevalence of infection in local populations. I transformed equation (1) into a differential equation, N ae - dn b bN then divided both sides by time dt, dt dt and the per capita rate of change is: 1 dN -b N di' and solved for b. dn di' 32 Ndt/ (5) dt The term "parasite intensity" is defined as the number of worms per host. The term - in equation (5) indicates the per capita host frequency reduction, and is the per capita parasite intensity increment. The "per capita" concept is often used for population growth models in population ecology (Gotelli 2001). Therefore, equation (5) shows that the ratio of the per capita host frequency reduction to the per capita parasite intensity increment is constant. Because the ratio of numerator to denominator is constant, if dt increases, dN Ndt decreases at a regular rate and vice versa. For the Deschutes Basin in August 2003, this ratio was 0.393 (N= 225.16 e 0.393 ? 0.9326, thus, b 0.393). We might be able to compare b among multiple reaches or basins to evaluate the risk of the parasite to the host. Furthermore, if I multiply both sides by n, equation (6) is available. ldN //ldn ---=nb Ndt/ ndt This equation represents the ratio of words, when n is large, the ratio of ldN ldn - Ndt -- to -ndt ldN - Ndt - regulation of the parasites intensity on both (6) . is in proportion to n. In other ldn ndt to -- also becomes large, i.e. the - -Ndt and --- ndt becomes stronger. 33 DISCUSSION Threespine stickleback are distributed throughout the Upper Deschutes River system, but the sites with parasites are concentrated at high-elevation reservoirs sites and infection level was correlated primarily with endogenous factors of the host. Both the mean parasite intensity and prevalence of tapeworm infection were related to mean fork length of stickleback, catch-per-unit-effort, and water flow indicating the parasite may have a preferred host size, host density, and physical enviromnent. An alternative model may be used to express the characteristic of parasite distribution in basin-wide host population whereas the mean parasite intensity and prevalence of infection are 'used to evaluate the degree of infection among local populations. In its native habitat, the threespine stickleback does not tolerate high-gradient streams, and rarely occurs in fluvial streams more than a few hundred meters above sea level (Bell 1982). However, stickleback have successfiully colonized the Upper Deschutes River Basin and acclimated to the systems elevation, water flow, and water temperatures. This unexpected acclimation may be a key event for infestation of the exotic host and its parasite population in the system. I predicted that low dissolved oxygen level, high conductivity level, low water flow, high water temperature, and larger abundance of submerged macrophytes and mud/sand substrate at sites would result in a higher parasite prevalence or mean intensity. As water temperature increases, the activity and metabolism of fish increases and fish have greater appetites and digestion ability (Guderley 1 994 Moyle and Light 1 996a, 1 996b), which may increase consumption of copepods and increase the speed of intestine wall penetration by the procercoid (Clarke 1954, LoBue and Bell 1993). Lower dissolved oxygen is stressful for fishes and weakens their immune system (Maule et al. 1989), which may make fish more susceptible to parasitism. When water flow decreases, parasite prevalence and intensity should increase because coracidia, copepods, and stickleback prefer slow or standing water (Clark 1954), facilitating the 34 consumption of coracidia by copepods and of copepods by stickleback. My prediction for water flow was supported by my analyses, but my other predictions of environmental correlates were not. In my study, parasitism in the threespine stickleback was more related to the endogenous factors of fish size and CPUE rather than exogenous parameters. Catch-per-unit-effort is an index of population density. As population density increases, the per capita fish size decreases because of decreasing food and resource availability. In my results, this appeared to occur at approximately half of the sites (i.e., the larger the fish length, the lower the CPUB [Fig. 7aJ). However, some sites had both smaller fish length and lower CPUE; most of these sites were located in the mainstem of Deschutes River. Larger fish can use a wider range of flow conditions whereas smaller fish live in only slow or zero-flow habitat. Because many factors can alter water quality, it is unlikely that generalizing the interaction between water quality and parasitism is possible (Marcogliese 1992, Khan et al. 1994, Lafferty and Kuris 1999). Likewise, I found only indirect relationships between parasitism and environmental factors. Other studies have also attempted to identify relationships between parasite infection in fish and environmental factors. Sandell et al. (2001) tried to identify environmental correlates for whirling disease in salmonids caused by a myxozoan parasite. Of four water quality measurements, only conductivity was correlated with infection prevalence (Sandell et al. 2001). Vincent (2002) demonstrated that as water volume increases, the intensity of microparasitic infection of trout decreases. He hypothesized that as water volume increases the parasite becomes diluted, decreasing the chance of "hitting" and attaching to the host (Vincent 2002). This conclusion may be applicable in my study because both copepods and stickleback prefer lower water flow areas as their habitat (Bell and Foster 1994, Marcogliese 2001). In such habitats, the abundance of both copepods and procercoid may be greater, and stickleback will have greater opportunity to forage on copepods, resulting in higher infectious by S. solidus. As Vincent (1998, 2002) 35 suggested, a comparison study between in-situ and in-vitro experiments might provide more conclusive results. Lafferty and Kuris (1999) suggest that altered water quality may improve conditions for parasites if host density increases. Eutrophication and thermal effluent often raise rates of parasitism because the associated increased productivity can increase the abundance of intermediate hosts (Lafferty and Kuris 1999). This may be the case in the reservoir areas. The Upper Deschutes River Basin is highly developed and altered, containing numerous reservoirs, dams, and agricultural diversions, often resulting in large fluctuations of daily water temperatures or algal blooms. These combined effects of high temperatures or high nutrient concentrations increase pH and decrease dissolved oxygen levels via metabolic activity of algae, and can create stressful conditions for fish (Giannico and Heider 2001). The sites in Crane Prairie Reservoir, Sunriver residence, and Bend areas where most parasitism occurred were typical of conditions containing these human modifications, resulting in substantially higher water temperatures. In fact, of 11 sites having ?20 °C minimum daily water temperature, 10 contained parasites with relatively higher prevalence and mean intensity (Fig 9b). Likewise, of 9 sites with ?25 °C maximum daily water temperature, 8 contained parasites with relatively higher prevalence and mean intensity (Fig 9b). These sites were all in either Crane Prairie Reservoir or the Sunriver residence area. The procercoid stage of S. solidus and copepods both prefer human-altered areas with higher primary production and standing water (Hopkins and S myth 1951, Arme and Owen 1967, Marcogliese et al. 1992). Although stickleback are able to colonize these habitats, they prefer cooler temperatures, and thus may be stressed to a certain extent by unsuitable water quality and climate, making them more susceptible to parasitism (Bell and Foster 1994, Guderley 1994). These three biological conditions, preferred procercoid and copepod habitat, and stressed stickleback may have influenced parasite occurrence. 36 Based on the relative importance of biotic and abiotic factors, a series of six predictions regarding fish invasions in California streams and estuaries were developed by Moyle and Light (1996b). Three of their predictions may applicable to the introduction of stickleback in the Upper Deschutes River Basin (Moyle and Light 1996b: 1667, 1668, 1669): "In streams, the most successful invaders will be those adapted to the local hydrologic regime"; "In aquatic systems with high level of human disturbance, a much wider range of species can invade than in systems with low levels of human disturbance"; and "Successful invasions in aquatic systems are most likely to occur when native assemblages of organisms have been temporarily disrupted or depleted". Their study also suggests that the match between introduced species and the hydrologic regime is the most important factor for the success of colonization, and they conclude that biotic interactions seem to play a smaller role than abiotic factors except during the early stages of introduction. However, this is only partially consistent with my results. The local hydrologic regime in the Upper Descbutes River Basin has strong seasonal patterns; low flows in the rainless summer and higher flows in winter and spring in response to higher precipitation and snow melt. In the reservoirs, large fluctuations of water level occur because of diversions for irrigation. These hydrologic regimes do not match with stickleback's favored habitat (Bell 1982, Bell and Baumgartner 1984, Bell and Foster 1994). Wild runs of native salmonids in the Deschutes River, kokanee (Oncorhynchus. nerka) and chinook (0. tshawytscha) salmon, steelhead (anadromous rainbow trout, 0. mykiss), and bull trout (Salvelinus confluentus), had been eliminated or their populations decreased because of the barriers of hydroelectric projects or agricultural diversions (Sollid et al. 2002). The most important factors for stickleback introduction in the Upper Deschutes River Basin may be its high capacity for physiological acclimation and low biota resistance, especially from native and wild salmonids. Theoretical considerations of the evolution of host-parasite associations include three models proposed by Holmes (1983 from Sakanari and Moser 1990): the mutual 37 aggression model, the prudent parasite model, and the incipient mutualism model. In addition, Sakanari and Moser (1990) suggested an alternative model as 1-sided evolution based on increased host resistance. The type of ho st-parasite relationship depends on the nature of the system's equilibrium. Because I have no evidence to support or to refute whether emergent parasitism in the Upper Deschutes has reached equilibrium or not, I am not able to conclude which type of host-parasite associations applies to my study. However, stickleback have a three-year lifespan, were introduced more than 20 years ago, and were distributed throughout the Upper Deschutes. Also, infection of S. solidus may not directly make stickleback's physiological condition lehal (Pennycuick 1971). My study system may be less likely to represent mutual aggression or incipient mutualism models and more likely to represent the prudent parasite or 1-sided evolution models. My results represent a cross-sectional study in a single river system and they should not be extrapolated outside of the study area or time. To generalize my results, longitudinal studies in multiple river systems are needed. Monitoring fish migration (daily, monthly, yearly) to determine the time and specific location of parasite infection would be necessary for more accurate prediction of prevalence and mean intensity, and to expand a risk assessment to other fish and wildlife. I collected healthy post spawning stickleback in the heaviest infection sites. During dissection, I noticed that post-spawning females and male stickleback and gravid females tend to not be infected with S. solidus, even in areas with extremely high prevalence. For example, the Quinn River Campground site in Crane Prairie Reservoir is one of the heaviest infected sites with 71 out of 79 fish infected. Uninfectecl fish in this site were four post-spawning sticklebacks, ?5 .5 cm fork length, and four juvenile sticklebacks, all 2.3 cm. I suggest two hypothetical reasons for this tendency: (1) natural mortality of parasite -- fish were infected once, but parasite died before the fish were captured; and (2) dominance against parasite infection -- dominant fish were not infected by parasites and were not consumed by predators. In their 38 experimental study, Milinski and Bakker (1990) demonstrated that because the ciliate parasite Ichthyophthirius multfihiis infects stickleback and causes less coloration, female stickleback use male coloration in mate choice and therefore avoid parasitized males. If this result is applicable for S. solidus, uninfected males would tend to have higher reproductive success. Additionally, parasite infection generally alters host behavior, making them more susceptible to predation by the next host, possibly enhancing parasite transmission (Milinski 1984, Apanius and Schad 1994). Thus, parasitized fish are more likely eaten by predators before a physiological condition reaches a lethal stage (Pennycuick 1971). Stickleback mortality induced by S. solidus may be primarily caused by predation, not physiological deterioration. These reports may explain my observation that uninfected stickleback have higher mating success and live longer. I did not identify gender of stickleback during dissection; a comparison study of parasitism between genders may also increase understanding of reproductive success influenced by parasite infection. My study has a number of limitations. My study encompasses 19 days at a total of 77 sites. The sampling period was short to reduce seasonal influences on data. Also, although I standardized my daily schedule, time of fish collection and parameter measurement varied among sites. Stickleback migration, behavior, and susceptibility might vary with time of day. Additional field crews could conduct sampling within shorter time periods in a day. Sample size of fish varied from 0 to 250 fishes per site. Electro-fishing may have allowed me to increase sample sizes at each site and to determine the population densities. Ordinary linear regression requires sufficient variance for the explanatory variables (Ramsey and Schafer 1997). In my study, visual estimates for submerged macrophytes and mud/sand substrate cover had low variance and were skewed, inducing lack of fit and decreased statistical significance. To avoid this problem, more detailed classification or quantitative measurement might be useful for these two parameters. Because half of my sites contained no infected fish, it would be useful to focus sampling on areas with parasite infection if the goal were to 39 maximize fish collected. Reservoirs areas, that seem to contain the source of parasite populations, would be preferable sample sites for obtaining larger sample sizes of infected fish. Also, OLS regression analysis assumes that sample units are independent regardless of the size of the study area (Ramsey and Schafer 1997). Biological interactions among sites, such as stickleback migration, should be eliminated to obtain low biased samples. Biological parameters rather than environmental factors seemed to have stronger relationships with parasitic infection. Survey for zooplankton community structure or of stickleback predators (e.g., birds) would be helpful for future study (Marcogliese et al. 1992). Although mating choice of parasitized males has been researched (Milinski and Bakker 1990), clarifying the relationship between susceptibility of pre-spawning stickleback and post-spawning stickleback, or the relationship between parasitic infection and spawning ability would increase our understanding of intermediate host - parasite dynamics. My study focused on a macro perspective, the spatial distribution and host - parasite population. A micro perspective of the infection, the physiological mechanism of stressor to the host and parasite and how they compromised by each other, may also be an important topic. Many parameters have been used to evaluate infection level of macro/micro parasites, bacteria, virus or other diseases. However, estimating infectious degree (the "force of infection") has been a problem that preoccupies researchers in most epidemiological sub-disciplines (Smith 1994). Incidence, prevalence, and attack rate are relatively easy and convenient parameters (Smith 1994), but they work only in limited cases and require many assumptions, hence, are often poor indicators. Mean intensity, parasite index, condition factor and truncated negative binomial analysis may represent a more accurate characterization of infectious events in the host population (Arnie and Owen 1967, Crofton 1971, Pennycuick 1971, Meakins 1974, May and Anderson 1979). The negative binomial analysis is a useful tool for estimating host mortality induced by a parasite. When parasites are more highly aggregated than free- 40 living organisms, their distribution can be estimated by the negative binomial distribution (Crofton 1971). According to Burgett et al. (1990: 1424), "Briefly, the negative binomial distribution belongs to a family of functions that describe either aggregation (negative binomial), randomness (Poisson), or repulsion (positive binomial)". In my study, the frequency distribution of stickleback can be described statistically by the negative binomial distribution. Although S. solidusassociated mortality may be detected by truncation of the negative binomial (Crofton 1971, Lanciani and Boyett 1980), I could only test lack-of-fitness and did not compute the truncated analysis because of the complexity and not being able to obtain the program. In this study, I compared the degree of parasitism among sites using prevalence and mean intensity. The benefit of my alternative model is its parsimonious model structure and the durability of the characteristics expressed. Because this model explains the availability of tapeworm infection among stickleback populations within this particular basin, I assume that the slope, b, would be similar regardless of when and where the samples were collected from within Upper Deschutes Basin. Because of the similarity of the curve, the slope may also be similar regardless of the sample size. Only the y-intercept will be changed by sample size. Data from another river system might have a different value for b because of a difference in ability of the parasite to kill the host or the fish's physiological tolerance for parasitism. We need to be careful when we compute mortality from this technique because fish behavior is changed by parasitism, and mortality from predators (i.e., birds, mammals or other fish) also increases (Pennycuick 1971, Apanius and Schad 1994, Medley 1994). Thus, the computed degree of parasitism represents physiological impact of the parasite, parasite load, and physical impact of predatory animals. Parasites are important components of food webs and should no longer be neglected. Parasites can regulate host populations and can be keystone species 41 (Marcogliese and Cone 1996, 1997). In this study, introduction of stickleback made the ecosystem more complex and both stickleback and tapeworm may not have reached equilibrium conditions yet. Thus, it remains uncertain whether the tapeworm is regulating the stickleback population. I suggest that risk assessments with longitudinal observations are needed to better define the risk to the ecosystem from this introduced fish. 42 REFERENCES Anderson, R.M., and May, R.M. 1982. Coevolution of hosts and parasites. Parasitology 85: 41 1-426. Apanius, V., and Schad, G.A. 1994. Parasitic and Infectious Diseases. Epidemiology and Ecology: Host behavior and the flow of parasites through host populations. Chapter 10, pp1 15-128. Scott, M.E., and Smith, G. (eds.). Academic Press, San Diego, California. Arme, C., and Owen, R.W. 1967. Infections of the three-spined stickleback, Gasterosteus aculeatus L., with the plerocercoid larvae of Schistocephalus solidus (Muller, 1776), with special reference to pathological effects. J. Parasitol. 57: 301-3 14. Barker, D.E., Marcogliese, D.J., and Cone, D.K. 1996. On the distribution and abundance of eel parasites in Nova Scotia: local versus regional patterns. J. Parasitol. 82: 697-701. Bell, M.A. 1982. Melanism in a high elevation population of Gasterosteus aculeatus. Copeia 4: 829-835. Bell, M.A., and Baumgartner, J.V. 1984. An unusual population of Gasterosteus aculeatus from Boston, Massachusetts. Copeia 1:258-262. Bell, M.A., and Foster, S.A. 1994. The evolutionary biology of the threesipine stickleback. Introduction to the evolutionary biology of the threespine stickleback. Chapter 1, ppl-27. Bell, M.A., and Foster, S.A. (eds.). Oxford University Press, Oxford. Benson, A.J., and Boydstun, C.P. 1999. Nonindigenous Freshwater Organisms Vectors, Biology, and Impacts. Documenting over a century of aquatic introductions in U.S. Chapter 1, ppl-32. Claudi, R. and J. H. Leach (eds.). Lewis Publishers, Boca Raton, Florida. Brass, W. 1958. Simplified methods of fitting the truncated negative binomial distribution. Biometrika 45: 59-68. Burgett, M.D., Rossignol, P.A., and Kitprasert, C. 1990. A model of dispersion and regulation of brood mite (Tropilaelaps clareae) parasitism on the giant honeybee '(Apis drsata). Can. J. Zool. 68: 1423-1427. 43 Clarke, A.S. 1954. Studies on the life cycle of the pseudophyllidean cestode Schistocephalus solidus. Proc. Zool. Soc. Lond. 124: 257-302. Cooper, A.R. 1918. North American pseudophyllidean cestodes from fishes. Ill. Biol. Monogr. 4: 1-243. Crofton, H.D. 1971. A quantitative approach to parasitism. Parasitology 62: 179-193. Dextrase A.J., and Coscarelli, M.A. 1999. Nonindigenous Freshwater Organisms Vectors, Biology, and Impacts. Intentional introductions of nonindigenous freshwater organisms in North America. Chapter 4, pp6l-98. Claudi, R. and J. H. Leach (eds.). Lewis Publishers, Boca Raton, Florida. Dubois, N., Marcogliese, D.J., and Magnan, P. 1996. Effects of the introduction of white sucker, Catostomus commersoni, on the parasite fauna of brook trout, Salvelinusfontinalis. Can. J. Zoo!. 74: 1304-1312. Dudgeon, D. 1992. Endangered ecosystems: a review of the conservation status of tropical Asian rivers. Hydrobiologia 248: 167-191. Dunier, M. 1996. Water pollution and immunosuppression of freshwater fish, Ital. J. Zoo!. 63: 303-309. Giannico, G. and Heider, C. 2001. An Assessment ofNatural Resource, Economic, Social, and Institutional Issues with a Focus on the Upper Kiamath Basin: Coho salmon and water management in the Kiamath Basin: Water Allocation in the Kiamath Reclamation Project, 2001. Chapter 6, pp 119-151. Oregon State University - University of California, Corvallis. Gotelli, N. J. 2001. A primer of ecology third edition. Logistic population growth. Chapter 2, pp25-48. Gotel!i, N.J. (eds.). Sinauer Associates. Sunder!and, Massachusetts. Guderley, H.E. 1994. The evolutionary biology of the threesipine stickleback. Physiological ecology and evolution of the three spine stickleback. Chapter 4, pp85-1 13. Bell, M.A., and Foster, S.A. (eds.). Oxford University Press, Oxford. Harvey, B.C. and Matthews, M. 1999. Nonindigenous Freshwater Organisms - Vectors, Biology, and Impacts. Success and failure of nonindigenous aquatic species in stream systems: case studies from California and Hawaii. Chapter 29, pp6l-98. Claudi, R. and J. H. Leach (eds.). Lewis Publishers, Boca Raton, Florida. 44 Hems, D.C. and Baker, J.A. 2003. Reduction of egg size in natural populations of threespine stickleback infected with a cestode macroparasite. J. Parasitol. 89: 16. Holmes, J.C. 1983. Evolutionary relationship between parasitic heirninthes and their hosts. In coevolution, ppl6l-185. D.J. Futuyma and M. Slatkin (eds.). Sinauer Associates, Sunderland, Massachusetts. Hopkins, C.A., and Smyth, J.D. 1951. Notes on the morphology and life history of Schistocephalus solidus. Parasitology 41: 283-291. Hubbs, C.L., and Lagler, K.F. 1970. Fishes of the Great Lake region. University of Michigan Press. Ann Arbor. Huxham, M., Beaney, S., and Raffaelli, D. 1996. Do parasites reduce the chances of triangulation in a real food web? Oikos 76: 284-300. Kennedy, C.R., Bates, R.M., and Brown, A.F. 1989. Discontinuous distributions of the fish acanthocephalans Pomphorhynchus laevis and Acanthocephalus anguillae in Britain and Ireland: a hypothesis. J. Fish Biol. 34: 607-6 19. Kennedy, C.R., and Fitch, D.J. 1990. Colonization, larval survival and epidemiology of the nematode Anguillicola crassus, parasitic in the eel, Anguilla anguilla, in Britain. J. Fish Biol. 36: 117-131. Kennedy, C.R., Laffoley, D.A., Bishop, G., Jones, P., and Taylor, M. 1986. Communities of parasites of freshwater fish of Jersey, Channel Is lands. J. Fish Biol. 29: 2 15-226. Khan, R.A., Barker, D.E., Ryan, K.W., and Hooper, R.G. 1994. Influence of crude oil and pulp and paper mill effluent on mixed infections of Trichodina cottidari urn and T saintjohnsi (Ciliophora) parasitizing Myxocephalus octodecemspinosus and M Scorpius. Can. J. Zool. 72: 247-251. Kuris, A.M., Blau, S.F., Paul, A.J., Shields, J.D., and Wickham, D.E. 1991. Infestation by brood symbionts and their impact on egg mortality of the red king crab, Paralithodes camtschatica, in Alaska: Geographic and temporal variation. Can. J. Fish. Aquat. Sci. 48: 559-568. Lafferty. K.D., and Kuris, A.M. 1999. How environmental stress affects the impacts of parasites. Limnol. Oceanogr. 44: 925-931. 45 Lanciani, C.A., and Boyett, J.M. 1980. Demonstrating parasitic water mite-induced mortality in natural host populations. Parasitology 81: 465-475. Larsen, D.P., and Christine, S.J. editors. 1993, Environmental Monitoring Assessment Program - surface waters 1991 pilot report: U.S. Environmental Protection Agency, EPAI62O/R-93/003, Corvallis, Oregon. Li, H.W., Rossignol, P.A., and Castillo, G. 1999. Nonindigenous Freshwater Organisms - Vectors, Biology, and Impacts. Risk analysis of species introduction. Insights from qualitative modeling. Chapter 30, pp43 1-447. In Claudi, R. and J. H. Leach (eds.). Lewis Publishers, Boca Raton, Florida. LoBue, C.P., and Bell, M.A. 1993. Phenotypic manipulation by the cestode parasite Schistocephalus solidus of its intermediate host, Gasterosteus aculeatus, the threespine stickleback. Am. Nat. 142: 725-735. Lodge, M. D. 1993. Biological invasions: lessons for ecology. Tree 8: 133-137. Marcogliese, D.J. 1992. Metazoan parasites of sticklebacks on Sable Island, Northwest Atlantic Ocean: biogeographic considerations. J. Fish Biol. 41: 399-407. Marcogliese, D.J. 1995. Comparison of parasites of mummichogs and sticklebacks from brackish and freshwater ponds on Sable Island, Nova Scotia. Am. Midl. Nat. 13: 333-343. Marcogliese, D.J. 2001. Implications of climate change for parasitism of animals in the aquatic environment. Can. J. Zool. 79: 1331-1352. Marcogliese, D.J. 2002. Food webs and the transmission of parasites to marine fish. Parasitology 124: S83-S99. Marcogliese, D.J,, and Cone, D.K. 1996. On the distribution and abundance of eel parasites in Nova Scotia: Influence of pH. J. Parasitol. 82: 389-399. Marcogliese, D.J., and Cone, D.K. 1997. Food webs: a plea for parasites. Tree 12: 320325. Marcogliese, D.J., and Esch, G.W. 1989. Alterations in seasonal dynamics of Bothriocephalus acheilognathi in a North Carolina cooling reservoir over a seven - year period. J. Parasitol. 75: 378-3 82. 46 Marcogliese, D.J., Esch, G.W., and Dimock Jr., R.V. 1992. Alterations in zooplankton community structure after selenium-induced replacement of a fish community: a natural whole-lake experiment. Hydrobiologia 242: 19-32. Maule, A.G., Tripp, R.A., Kaattari, S.L., and Schreck, C.B. 1989. Stress alters immune function and disease resistance in Chinook salmon (Oncorhynchus tshawytscha). J. Endocrinol. 120: 135-142. May, M.R., and Anderson, R.M. 1979. Population biology of infectious diseases: Part II. Nature 280: 455-461. Meakins, H.R. 1974. A quantitative approach to the effects of the plerocercoid of Schistocephalus solidus Muller 1776 on the ovarian maturation of the threespined stickleback Gasterosteus aculeatus. L.Z.Parasitenk 44: 73-79. Medley, G.F. 1994. Parasitic and Infectious Diseases. Epidemiology and Ecology. Chemotherapy. Chapter 12, ppl4l-157. Scott, M.E., and Smith, G. (eds.). Academic Press, San Diego, California. Milinski, M. 1984. Parasites determine a predator's optimal feeding strategy. Behav. Ecol. Sociobiol. 15: 35-37. Milinski, M., and Bakker, T.C.M. 1990. Female stickleback use male coloration in mate choice and hence avoid parasitized males. Nature 344: 330-333. Moyle, P.B., and Light, T. I 996a. Biological invasions of fresh water: Empirical rules and assembly theory. Biol. Conserv. 78:149-161. Moyle, P.B., and Light, T. 1996b. Fish invasions in California: Do abiotic factors determine success? Ecology 77:1666-1670. Moyle, P.B., and Sato, G.M. 1991. Native fish management in the American West: On the design of preserves to protect native fishes, in Battle Against Extinction. Minckley, W.L. and Deacon, J.E., (eds.). University of Arizona Press, Tucson, AZ. Peck, V.D., Lazorchak, J.M. and Klernm, D.J. 2003. Environmental monitoring and assessment program - surface waters western pilot study: field operation manual for wadeable streams: Initial site procedures. Water chemistry. Stream discharge. Physical habitat characterization. pp4.l - 7.8 in US Environmental Protection Agency, EPA/620/R-97/00 1, Washington, D.C. 47 Pennycuick, L. 1971. Quantitative effects of three species of parasites on a population of three-spined stickleback, Gasterosteus aculatus. J. Zool. (Lond.) 165: 143162. Ramsey, F.L., and Schafer, D.W. 1997. The Statistical Sleuth: A course in methods of data analysis. Mode! checking and refinement. Chapter 11, pp291-324. Ramsey, F.L., and Schafer D.W. (eds.). Duxbury Press, Belmont, California. Reimchen, T.E. 1989. Loss of nuptial color in threespine sticklebacks (Gasterosteus aculeatus). Evolution 43:450-460. Reimchen, T.E., and Nosil, P. 2001. Ecological causes of sex-biased parasitism in threespine stickleback. Biol. J. Linn. Soc. 73: 51-63. Rowland, W.J., Baube, C., and Horan, T. 1991. Signalling of sexual receptivity by pigmentation pattern in female sticklebacks. Anim. Behav. 42: 243-249. Sakanari, A.J., and Moser, M. 1990. Adaptation of an introduced host to an indigenous parasite. J. Parasitol. 76: 420-423. Sandell, A.T., Lorz, H.V., Stevens, D.G., and Bartholomew, J.L. 2001. Dynamics of Myxobolus cerebralis in the Lostine River, Oregon: Implications for resident and anadromous salmonids. J. Aquat. Anim Health 13: 142-150. SAS. 2003. SAS user's guide. version 8.2. SAS Institute. Cary, North Carolina. Smith, G. 1994. Parasitic and Infectious Diseases: Ecological epidemiology and standard measures of disease occurrence. Chapter 6, p65-7 1. Epidemiology and ecology. Scott, M.E., and Smith, 0. (eds.). Academic Press, San Diego, California. Sollid, S.A., Lorz, H.V., Stevens, D.G., and Bartholomew, J.L. 2002. Relative susceptibility of selected Deschutes River, Oregon, salmonid species to experimentally induced infection by Myxobolus cerebralis. Am. Fish. Soc. Symposium 29: 117-124. Statsci. 2000. S-PLUS Statistical software, MathSoft, Inc., Seattle, Washington. U.S. Congress. 1993. Harmful non-indigenous species in the United Status. Summary, Issues and Options. Chapter 1, ppl -51. Office of Technology Assessment. OTA-F-565. U.S. Government Printing Office. Washington, DC. 48 Vincent, E.R. 1998. The relationship of time, temperature, and fish life histories to whirling disease infections. Proceedings. Whirling disease symposium. Research in progress. February 19-21, 1998. pp3l-32. Prepared by the Whirling disease foundation. Bozeman, Montana. Vincent, E.R. 2002. Effect of water flows on infection rates in rainbow trout. Proceedings. Whirling disease symposium. Putting a fresh spin on whirling disease. February 13-15, 2002. pp43-44. Prepared by the Whirling disease foundation. Bozeman, Montana. Whittier, T.R., and Hughes, R.M. 1998. Evaluation of fish species tolerances to environmental stressors in lakes in the Northeastern United States. N. Am. J. Fish. Manag. 18: 236-252. Wootton, R.J. 1984a. A Functional Biology of Sticklebacks. Spatial distribution. Chpater 2, pp4-19. Wootton, R.J. (eds.). University of California Press. Berkeley and Los Angeles, California. Wootton, R.J. 1984b. A Functional Biology ofSticklebacicy. Reproduction. Chapter 7, pplO3-154. Wootton, R.J. (eds.). University of California Press. Berkeley and Los Angeles, California. 49 APPENDIX Dataset collected in the Upper Deschutes Basin, central Oregon from July - August 2003. Number ID Site name Fish Captured Mean Intensity (paras eel I CP cow meadow a 54 1.333 2 3 CPresorta 4 5 6 CP resortc CPdam a CPdam b 44 77 15 7 CP dam gaging 8 9 Southtwinlakea 2.475 2.754 2.071 3.633 3.000 1.609 1.375 0.000 0.000 0.377 0.015 0.000 0.000 2.158 0.208 0.177 2.117 5.708 3.910 1.458 3.000 0.667 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.037 0.000 0.000 0.000 10 11 12 13 14 15 16 17 18 19 CP resort b Bigrivercamp Bigriverboatlaunch SunriverLodge Sunriveraspine lake Sunriver humming bird Sunrivermarina CP cow meadow b Cultus river CP quinn a CP quinn b CP rock boat 20 CP rock camp 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 South twin lake b Bull bend camp Wyeth boat launch Lapinebigtree La pine dayuse camp Paulina lake a Paulina lake b Paulina lake c Paulina lake d Burgess rd. south Burgess rd. north Recriatjon rd. south Recriation rd. north Threft shop south Threft shop nouth 31 12 69 32 40 36 54 67 231 39 19 24 483 79 24 67 61 1 23 45 37 68 180 87 106 22 96 56 65 25 71 ) Prevalence ( o) Mean fork length (an) 100.0 60.0 4.757 4.307 3.822 4.547 4.446 4.367 3.436 96.9 0.0 0.0 24.5 4.141 2.465 2.219 4.043 1.5 0.0 0.0 100.0 12.5 3.452 2.914 3.003 4,284 4.533 12.2 93.5 100.0 98.5 96.6 100.0 60.0 2.211 74.1 75.0 76.9 85.7 93.3 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 3.7 0.0 0.0 0.0 4.619 4.750 4.460 4.030 5.000 2.504 3.020 2.173 2.809 2.503 2.322 2.828 3.200 2.602 2.352 2.498 3.072 2.593 r) ( 810 88 231 60 124 36 276 64 141 154 108 134 462 78 38 48 966 158 48 134 122 2 46 90 74 204 540 174 212 44 192 112 130 50 213 Dissolved Minimum Maximum Submerged Oxygen Conductwty Water flow Water depth temperature temperature macrophytos (l.is rn) s) (cm) (mg/I) C) (CC) (%) C 8.777 5.503 7.587 7.677 6.207 6.240 5.587 6.827 4.377 6.737 2.507 4.760 2.613 7,337 8.597 10.093 7.750 10.727 6.570 6.470 6.827 7.730 7.523 7.567 7.743 7.517 7.510 7.227 5.333 6.333 6.267 6.423 6.450 6.723 6.670 28.5 35.4 46.8 46.2 46.2 46.9 46.8 135.6 48.1 48.0 146.9 121.3 124.2 50.0 28.2 38.3 43.4 33.0 42.3 44.1 135.5 47.3 47.2 46.4 46.9 514.0 507.0 514.7 530.0 30.9 30.9 33.0 33.0 48.2 48.2 0.655 0.000 0.000 0.000 0.000 0.085 0.300 0.000 0.131 0.229 0.013 0.000 0.016 0.268 0.494 0.196 0.000 0.184 0.000 0.000 0.000 0.457 0.337 0.443 0.527 0.000 0.000 0.000 0.000 0.518 0.389 0.257 0.323 0.375 0.395 29.3 1.8 9.0 12.2 10.0 15.0 52.7 29.7 17.0 20.3 63.0 29.3 48.7 18.0 9.0 20.7 19.0 17.0 7.7 8.3 27.3 76.0 52.3 89.3 75.3 13.0 29.3 26.3 10.3 78.0 44.7 81.7 36.0 120.0 47.7 9.90 12.77 22.43 22.40 23.33 24.17 24.27 23.20 15.60 16.57 20.53 21.77 20.50 15.80 9.53 5.17 19.43 5.23 19.63 21.57 22.93 15.57 15.40 15.00 15.07 17.30 17.07 17.17 17.73 18.10 18.00 18.47 18.50 16.73 16.60 18.0 35.6 28.9 29.4 27.0 27.0 26.7 30.0 22.0 16.7 25.0 26.7 26.7 16.0 14.0 10.0 24.0 12.8 22.0 16.7 23.0 17.2 21.1 15.0 14.4 21.0 20.5 20.0 18.0 20.6 21.1 20.6 21.1 18.0 20.0 0.083 0.117 0.033 0.225 0.392 0.000 0.675 0.775 0.875 0.775 0.775 0.875 0.875 0.775 0.000 0.083 0.875 0.017 0.775 0.000 0.017 0.567 0.875 0.358 0.567 0.033 0.875 0.875 0.575 0.183 0.775 0.567 0.667 0.875 0.875 Mud/sand substrate (%) 0.575 0,875 0.875 0.875 0.875 0.875 0.575 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.575 0.000 0.875 0.875 0.575 0.575 0.875 0.875 0.667 0.875 0.875 Dataset collected in the Unner Deschutes Basin. central Oregon from July - August 2003. (continuecfl Number ID 36 37 site name Tumalo a Tumalo b 38 Tumalo 39 101 c 9 Tumalod 31 40 Tumalo e 41 Tumalof 18 42 43 Clinefallssouth 24 Mirror pond north 26 44 45 46 Mirrorpondsouth 59 64 47 Bend mall north 39 Old mill bidge north Old mill bidge south 6 48 Bend mall south 49 Lava island north 139 150 103 38 50 Lava island middle 34 51 Lavaislandsouth 51 52 53 54 55 Dillon falls north 23 85 52 Dillonfallsn, Dillonfallssouth Benham falls north 56 Benham falls middle 57 Benhamfallssouth 63 61 North davis west 96 115 63 103 84 175 62 Northdaviseast 17 58 Cardinal land middle 59 Cardinal land south 60 Cardinal land extra Mean Maximum number Prevalence Mean Dissolved Conducteity Water flow Water depth Cared 67.9 483 Minimumnumber 1 Standard Deviation Coefficient Variance 69.919 103.018 0.000 0.000 0.000 0.000 0.000 0.000 0.042 0.000 0.123 0.083 0.000 0.034 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.056 0.000 0.000 0.013 0.000 0.022 0.000 0.620 5.708 0.000 1.224 197.384 0.0 0.0 0.0 0.0 0.0 0.0 4.2 0.0 12.3 6,7 0.0 1.1 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 5.6 0.0 0.0 1.3 0.0 2.2 0.0 22.39 100.000 0.000 37.643 168.120 3.382 2.654 3.756 3.629 4.128 3.850 3.579 3.712 3.446 3.822 2.366 2.429 2.629 3.305 3.232 3.167 3.122 2.651 3.340 3.070 2.205 2.142 2.556 2.603 2.327 2.688 1.894 3.258 5.000 1.894 0.824 25.283 78 202 18 62 36 12 48 52 118 128 278 600 412 76 68 102 46 170 104 126 192 230 126 206 168 700 34 173.5 966.000 2.000 190.896 110.047 7.833 7.543 7.783 7.893 7.907 8.007 7.647 8.003 7.437 7.703 8.240 7.513 7.773 7.873 7.950 8.000 7.997 8.063 8.100 7.760 7.617 7.587 7,533 6.773 7.240 3.560 3.800 7.063 10.727 2.507 1.477 20.915 49.1 48.7 48.8 47.9 47.8 47.8 51.6 50.4 50.3 50.4 50.4 49.5 49.8 49.3 49.4 49.3 48.9 48.9 48.6 49.0 48.9 48.7 48.1 48.2 48.1 34.2 34.2 82.41 530.000 28.200 117.598 142.693 0.273 0.025 0.546 0.172 0.400 0.462 0.181 0.442 0.038 0.411 0.275 0.341 0.362 0.337 0.859 0.378 0.289 0.269 0.330 0.195 0.000 0.254 0.022 0.146 0.147 0.000 0.000 0.222 0.859 0.000 0.203 91.723 26.0 65.7 23.7 37.7 39.3 16.0 50.7 29.7 85.3 80.7 60.3 46.3 30.7 54.7 56.3 88.7 120.0 85.3 28.0 75.0 70.7 53.3 15.0 28.3 31.7 47.7 67.7 42.89 120.000 1.800 28.502 66.448 Minimum Medmum Submerged temperature temperature macrophytos 16.27 15.97 15.97 15.60 15.63 15.63 18.03 16.20 16.13 16.13 16.40 15.37 15.47 14.90 14.90 14.90 14.47 14.50 14.53 14.97 14.77 14.57 14.30 14.20 14.43 8.73 8.80 16.358 24.270 5.170 4.011 24.520 20.5 23.0 21.0 22.0 20.6 20.0 24.0 17.8 18.3 19.5 20.0 18.1 18.1 16.1 15.8 15.8 15.8 16.1 15.8 18.3 18.3 18.3 17.8 17,8 17.8 12.2 13.3 19.98 35.600 10.000 4.764 23.844 0.358 0.775 0.292 0.183 0.358 0.183 0.875 0.358 0.875 0.875 0.875 0.875 0.567 0.667 0.250 0.000 0.467 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.575 0.875 0.564 0.875 0.000 0.329 58.319 Mud/sand substrate 0.050 0.875 0.000 0.117 0.117 0.050 0.875 0.000 0.875 0.875 0.875 0.875 0.775 0.250 0.050 0.575 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.875 0.724 0.875 0.000 0.288 39.777