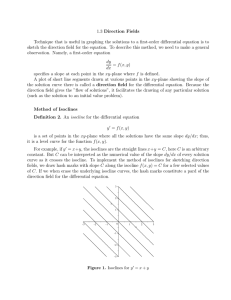
in Fisheries and Wildlife presented on Charles E. Warren
advertisement

AN ABSTRACT OF THE THESIS OF William Michael Booty for the degree of in Fisheries and Wildlife presented on Master of Science September 23, 1975 Title: A THEORY OF RESOURCE UTILIZATION Redacted for privacy Abstract approved: Charles E. Warren Resource utilization theory has lacked a method of explicitly defining the qualitative and quantitative interrelationships between populations of species in a community. Graphical isocline analysis as a method can be used to determine the effect of the environment, including the energy input rate on a community, with the kinds of dimensions of predator-prey interactions, competition, mutualism, commensalism, and amensalism. A nonlinear mathematical model of a community with an energy resource, a plant, two competing herbivores, and a carnivore is solved graphically in order to construct the prey and predator isoclines on successive predator-prey phase planes based on the density dependent responses of the populations. The equilibria on any of a series of predator-prey, competition, and diversity phase planes are functions of the commuhity' s energy input rate, the density of the highest predator in the trophic chain and the physical, chemical, and biological identities in the environment of the defined system. Different conditions were found where co-existence of competitors is both possible and not possible depending on the characteristic density dependent responses of organisms in the population. The community s global stability was analyzed and found to be strongly stable. Characteristic density dependent responses of the organisms promote strongly stable isodine configurations. A. Theory of Resource Utilization by William Michael Booty A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science June 1976 APPROVED: Redacted for privacy Professor of Fisheries and Wildlife in charge of major Redacted for privacy Head of Department of Fisheries and Wildlife Redacted for privacy Dean of Graduate Sjhool Date thesis is presented September 23, 1975 Typed by Opal Grossnicklaus for William Michael Booty ACKNOWLEDGMENT The writer wishes to extend his appreciation for the support of an EPA trainee ship grant awarded by the Environmental Protection Agency and the assistance and cooperation of certain people. Particu- lar thanks to a special person, Dr. Charles E. Warren, for his considerable time, direction, suggestions, and assistance in all phases of this work; to Dr. Paul Cull, Dr. James Hall, Dr. James Haefner and William Liss for their helpful suggestions; and finally to my wife Janie for her support and her willingness to proofread my numerous rough drafts and then type them and retype them. TABLE OF CONTENTS Pa e INTRODUCTION ................................. 1 PROCEDURE ................................... 3 ...................... .................... .................................. MATHEMATICAL FRAMEWORK 8 PREDATOR-PREY INTERACTIONS 12 COMPETITION 20 DIVERSITY 27 CONCLUSIONS 30 BIBLIOGRAPHY 32 LIST OF FIGURES Page 1. Schematic Representation of the Community 2. Development of Prey and Predator Isoclines on Prey-predator Phase Planes 3. Development of Competition Isoclines on the Competition Phase Plane 4. 3 7 Development of Prey Isoclines for Energy Resource and the Predator Isocline of the Plant #1 13 Family of Prey and Predator Isoclines on the R-P1, P1-H1 and H1-C1 Phase Planes 14 6. Development of Prey Isoclines for P1 16 7. Development of Predator Isoclines for H1 17 8. Development of Family of Competition Isoclines on Competition Phase Plane 22 Modification of H1 and H2 Competition Isoclines on H1-H2 Phase Plane due to H3 25 Development of Family of Herbivore Diversity Isoclines on Diversity Phase Plane 28 5. 9, 10, A THEORY OF RESOURCE UTILIZATION Resource utilization theory based on multispecies mathematical models has been inadequate in several important respects. It has limited equilibrium analysis to two-species models. Stability charac. teristics of the equilibrium have been determined only in the vicinity of equilibrium nodes, It has failed to show the expected increase in community stability with increased trophic complexity (May, 1973). It does not introduce in an appropriate way the impact of the corn- munity's energy resource on predator-prey interaction, competition, and diversity. It fails to include the important density dependent responses of organisms at the population level in the community's total response. And finally it has not established the important interrelationships between the different categories of species inter- action: commensalism, amensalism, mutualism or symbiosis, predator-prey phenomena, and competition. Extant graphical isocline analysis has provided a method of freeing resource utilization theory from some of the shortcomings of other modeling, but has failed so far to be useful in representation of multispecies systems. The discussion has been concerned mainly with the shape of the prey isocline and the stability of the equilibrium at the intersection of the prey and predator isoclines (Rosenzweig and MacArthur, 1963; May, 1972; Ro senzweig, 1973). Ro senzweig 2 (1973) expanded isocline analysis to a three-trophic level exploitative system on a three-dimensional phase space. Three-dimensional isoclines are difficult to visualize, thus limiting their usefulness. Expansion into the fourth dimension is graphically impossible. The purpose of this thesis is to analyze a small but comprehensive community model by a quantitatively and qualitatively explicit graphical method of isocline analysis not having the above shortcom- ings and use it as a basis of explanation of previously inadequately repre sented resource utilization phenomena. The community repre- sentation in Figure 1 has the kinds of dimensions necessary to include the most important community phenomena: the community' s energy resource, predator-prey interactions, competition, commensalism, amensalism, mutualism and diversity. 3 OM---TC + o M C C o M oMH Figure 1. Schematic representation of the simplest community matrix in an environment with the kinds of dimensions to analyze diversity, the five distinguishable categories of interaction between species, namely predator-prey interaction (+_), commensalism (-1-0), aniensalism (-0), competition (- -), and mutualjsm (-H-) and the affect of the energy input; rate on them. The sign in the direction of the arrow characterizes the effects of one species on the other as positive, neutral or negative depending on whether the species increase, remain unaffected or decrease due to the interaction. Competition between H1 and H2 occurs through the exploitation of a common prey (plants). Commensalism occurs between H1 and H3 and amensalism between C1 and C2. Mutualism occurs between H2 and H4 through exploitation of competing plant species (P1 and P2). The environment represents other communities and the physical and chemical environment interfacing with the community matrix. R is the energy resource, I the energy input rate, P the plants, H the herbivores and C the carnivores. Numerical subscripts denote different species on a given trophic level. Organisms on all trophic levels will contribute organic material (OM) utilized by decomposers (D). Decomposers will produce nutrients (N) utilized by the plants. (-I-) 4 PROCEDURE One solution to examining multispecies multi-pr edator -prey models by isocline analysis, without resorting to graphical repre- sentations having more than two dimensions, is to consider predator- prey interactions on successive, interconnected, two-dimensional phase planes in which the predator on one phase plane becomes the prey on the next successive phase plane (Fig. 2). Then graphical solutions of a special set of nonlinear differential equations permits construction of the prey and predator isoclines on each successive phase plane based on the characteristic density dependent responses of the populations. In this way the isoclines can be rigorously defined, a shortcoming that Rosenzweig (1969) began to remedy by mathematically defining his humped shaped isocline. The prey isocline on each phase plane has been found to be parameterized in terms of the com- munity' s energy input rate, the predator isocline in terms of the density of the highest predator in the trophic chain, the densities of competitors in the defined community, and amensalists, commensalists, and mutualists in the environment of the defined system. There- fore, the equilibrium and its stability on any of the two-dimensional phase planes is consistent with its equilibrium and stability in the x-dimensional hypervolume. These identities, as variables on the isoclines, generate any number of transitory equilibrium nodes which 5 Isoclines on Phase Planes Graphical Solutions ow I MedI R's Prey Isoclines dR/dt f(I) - f(P1) igh IOkhlkh 4 R Pred. Isoclines dP1/dt 5kh1 1 f(R)-f(H1) p 12345 R(k) 1 Plant Production at Different I's Prey Isoclines r 5 4ed1 C 4 .ow dP1/dtf(R)_f(H1%1) I H1s P red. Isoclines X 2 5k 1 :1 1 Herbivore No. 1 Production at Different l's H1's Prey Isoclines 4, Md Lo I dHlIdt=f(rh P1)-f(C, dli 1' ) 1 dC1/dtf(r,H1)-f(TC1,d.).+ , H1 LI C C1s Pred. Isoclines P1(k) High I \....3 Ok 3 2 12345 H(k \_tc I Figure 2. The prey and predator isoclines on prey-predator phase planes are derived and parameterized from the intersections of systems of gains and loss curves based on density depeudent responses of prey and predator in the differential equations. On the B-P1 phase plane, the energy resourc&s gain rates and in turn R's prey isoclines are parameterized by different energy input rates. On successive phase planes, the prey's production rates, gain rates and prey isoclines are identified by the energy input rate. The predator isoclines on any phase plane are parameterized by the density of the predator on the nect higher level, and ultimately by the predator in the highest level, Therefore the equilibriums between successive predator-prey relationships are parameterized by the energy input rate and indirectly by the TC's density. In the equations r is the recruitment rate and d is the nonpredatory loss rate. Ic is a density constant. define an area on the predator and prey phase plane limited only by the possible existence of predator and prey isoclines, Competition phase planes can be constructed from the predatorprey phase planes for predator populations competing for a common prey (Fig. 3), The competition isoclines have the same dimensions as the predator and prey isoclines and are parameterized accordingly, thus showing what processes lead to the co-existence or extinction of competing species. Diversity isoclines can be derived from equilibrium conditions on competition phase planes and are parameter ized by these conditions. 7 Competition Phase Plane Pred -Prey Phase Plane Equations K P 's MA H.1-' Prey I dP1/dt--f(R)-f(H1, d,H2 \210 'iine'. Hi's f(H) 2 2' Pred. dHl/dtf(rh, P1 )-f(C1dh lsoc1ine zk _\\ --U,'., H 's Competition 2, 0 1 + 2,1 kc1, kh2 Isoclines Z2 1 4.1 4,2 2 1 3 4 5 P1(k) LI L. I H, I Pi's L.Ij?&J s 42 H1 (kh) 2 f(H1) f(H1) H2s t Pred. dH2/dtf(rh,Pl)_f(cj,dh) L. I 123 45 '42/4 P dP1/dtrrf(R)_f(H1,dp1,H 1 to iociinr H21s + Competition Isoclines 1 1 2345 Pj(kpj) Figure 3. The isoclines on the competition phase plane are derived and parameterized from those on the predator-prey phase planes. Since the competing herbivores both utilize P1 for food, the differential equation for the plants will have a predation term for both H1 and H2 which results in a family of prey isoclines on the P1-H1 and P1-H2 phase planes parameterized by the energy input rates and the H2 and H1 densities respectively. The density dependent responses of recruitment and nonpredatory losses for each herbivore are a function of its competitor density and results in a family of predator isoclines for each carnivore density, parameterized by its competitor density. The competitor isodines for H2 on the H1-P1 phase plane and H1 on the H2-P1 phase plane is constructed on the H1-H2 competition phase plane from the intersections of the prey and predator isoclines and parameterized by the energy input rate and carnivore density. L. I, M. I and H. I are substituted for to Low I, Medium I and High I. MATHEMATICAL FRAMEWORK A community consisting of an energy resource (R.) and popula- tion densities (T..) on different trophic levels, utilizing the energy resource either directly or indirectly through predation, can be defined by the set of equations: n dRj/dt n 13 131 j=l j=l n T.. 13 j=1 1=1 31 =l n g.. ij (1) T1. n dT../dt = 13 I n Y l,1,L I ,3 T. 1+1,3 -d..T.. (2) 1J 13 where t is time, n0 is the total number of energy resource species, n is the total number of species on a trophic level, I is the trophic level, j is the species counter for trophic level i, I is the species counter for trophic level i-i, Ii is the energy input rate, r.. is the relative recruitment rate, g..1 is the relative growth rate of species j on trophic level I consuming prey species I, y..1 represents the yield of organism T.. per unit of prey T.11 consumed, and is the relative nonpredatory death rate. The relative growth rate g.. of a given species (T..) consuming the prey (T_1 can be I estimated from the empirical equation: M.1 T.11 IJL i-1,I,j +T. i-1,I (3) M.. is the maximum relative growth rate constant for species T.. '3 1J. consuming the prey (T.1) which can be developed under a particular operational condition when the prey concentration is so high that is independent of the prey density. the curvature of a plot of g The constant k.11. determines versus T11 and is numerically equal to the prey density at which g.. is half the value of M.. Assume for simplicity the maintenance ration of the prey is zero. I will use k.. as a convenient unit for representing the density of the population on trophic level T..., so that the general development of the relationships between populations on different trophic steps can be qualitative if k. is not defined and quantitative if it is defined. Equation 3) was originated by Monod (1942) on the basis of analysis of bacterial growth curves as a function of substrate concentration. The equation is equivalent to the Michaelis-Menton equation for enzyme kinetics. Figure 1 informally defines a community consisting of an energy resource and five populations on four trophic levels. This community in its environment can be formally defined with equations (1) and (2) by interpreting T11 as P1, T21 as FI[ T22 as F12, T31 as C1 and T41 as TC and making the appropriate substitutions. The resulting set of equations is dR/dt = I - g p1 P1/y (4) p1 10 MR Mh Pl±k:R dP1/dt= r Mh F 1 F 1 1 (5) P hi dH1/dt = rh Hlk [M p1 1 M rh H+ 2 k+P p1 M dC1/dt = rC1+ 1 (6) H1 1 H2+H k c12 1 M +H 1 k h21 H2 12 +H2 C1-d H2 (7) 2 2 M C .1 tc C k 1 21 1 1 (8) will hereafter equal kh and kh and kh 11 11 H2- 11 For simplicity kh C1-d + M cli H1 kh H C11 1 MP dH2/dt c H1- 21 1 2 respectively. The special set of equations is important because the energy input rate and TC densities as explicit variables and P2, H3, H4 and C2 as implicit variables define the community equilibrium in a field of community equilibria that are subject to change by dimensions within the system. This permits the community of x species in an x- dimensional space to be represented on a series of successive predator-prey phase planes in which the set of prey isoclines (dprey/dt =0) on every phase plane is parameter ized in terms of energy input rate, and the predator isoclines (dpredator/dt=0) are parameterized in terms of the predator's consumer density, which 11 is indirectly related to the density of TC. The prey and predator isoclines on the two-dimensional predator-prey phase planes are projections of x-dimensional community isoclines in the phase space. Likewise, a predator-prey equilibrium is a projection of a community equilibrium. Therefore the intersections of isoclines can be ex- amined for global stability of predator-prey interactions within the community framework without linearization as sumptions. Isocline equilibria and stability conditions on successive predator-prey and competition phase planes determine the equilibrium and stability of the community for a given set of environmental conditions. A changing environment results in a continuum of community equilibria, 12 PREDATOR-PREY INTERACTIONS Considering R as a prey or as a resource for F1, the prey isocline for B can be determined graphically from the gain-loss graph for R (Fig. 4A). The prey isocline for R rises at low densities of R, because the rate of consumption becomes so low that greater P density has little effect on the density of R (Fig. 5A). At higher densities of R, the prey isocline for R is independent of B because of the saturation growth rate of P1 (given a constant utilization coefficient) and will remain so until B at very high levels becomes inhibitory to P1 and the isocline starts to descend due to increased nonpredatory loss rate of P1, a condition not explicitly developed here. Considering P1 as a predator or utilizer of R, the predator isocline for P1 (Fig. 5A) can be determined graphically from the gain-loss graph for P1 (Fig. 4B). The predator isocline for P1 at zero H1 density has an abscissal intercept at its maintenance value of R. The predator isocline for P1 with an H1 identity has an ordinate intercept at the H1's maintenance ration of P1 on the R-P1 phase plane. The predator isocline approaches an asymptote at high P1 densities and low H1 densities, due to rapidly increasing nonpredatory loss rates of P1. This asymptote decreases as predatory loss rates increase with greater H1 densities. The predator and prey isoclines on the P1-H1 (Fig. 5B) and 13 A B '-4 dR/dt = I - g1P1/1 Gain--- dPildt zLP1+gp IF 1_g1Hl//%t1.j_1P1 Gain---- '-4 5k1 '-4 04 Loss p.. ii 0 0 0 C,, 0) Okh1 3 kh1 j r -I High '-4 04 5khl J5'cr 0.. bO U '-4 04 0 Ca, VS 0 04 Med. Low '4 0) f Figure 4. 4 0 VS 04 S Energy Resource, R(kr) Plant, P1(kp1) The prey isoclines for the energy resource at different energy input rates are constructed on the R-P1phase plane from the graphica' solution of equation (1) in (A). The equilibrium between R and P1 are determined from the intersections of the gain curve at low, medium and high energy input rates with the plant consumption loss rate curves at different plant densities. The energy input rate is ndependent of any dimension in the system and therefore constant with respect to the energy resource. Adding resource species for the plants would result in a family of gain curves for each input rate. The plant's functional and numerical utilization r esponse is Holl ing type 2 predation re sponse. The predation isocline for the plants at different H1 densities is constructed on the R-P1phase plane from the graphical solution of equation (2) in (B). The densities at which the plants are at equilibrium with the H1 at different R is determined from the intersections of the gain curves at different energy resource levels with the loss rate curves at different herbivore no, 1 densities. The total gain response for the plants is a summation of recruitment (assumed to be zero) and production. Production is a direct function of the amount of plants available to the herbivore, consequently the family of straight line production responses at different resource levels. The total loss rate curve is a summation of nonpredatory plant losses and predation at different H1 densities. The time track on the R-P1 phase plane from some instable point back to equilibrium can be constructed from the R-P1 gain loss graphs by solving for the resultant vector from the R1 and P1 component vectors. If at lkr and 3k1 for a system at equilibrium at high I and 3khr the magnitude of the R component vector in (A) is the difference between the gain curve at high I and the loss curve at 3kp1. The magnitude of the P1 component vector in (B) is the difference between gain c4rve at lkr and 3khj. The resultant vector constructed from the R and P1 component vectors (in Fig. 5) establishes where the next sequence of component vectors originates and eventually returns to equilibrium at high I and 3khi. The vectors are numbered in sequence. A B C T 5 5 LOW I '-4 4 Cr u'-4 '-4 2k 5) 0 tc CS 1 1 5kt 1 i Energy Resource, R(kr) Figure 5 1 2 4 3 Plant, P (k 'p1 ) 5 1 2 3 4 No. 1 Herbivore, H (kh 5 The family of resource and utilization isoclines in Figures A, B and '' express the density relationship between the resource and utilizer on successive trophic steps as determined from the graphical solution of equation (1) and (2) for the appropriate i. The family of resource isoclines has either iow, medium or high energy input rate as an identity on each phase plane, The family of predator isoclines has different predator densities as the identity. Time tracks show how the perturbed system returns to equilibrium for different system identities, The time tracks can be approximated by connecting the end of one resultant vector to the tail of the next in sequence. The resultant vectors are constructed from the horizontal--R component vector--derived from Figure 4A and from the vertical--P1 component vector--derived from Figure 4B in A. The shorter the vector length the more accurate the approximation is to the actual instantaneous change of the time track. I 15 H1-C1 (Fig. 5C) phase planes are constructed from the graphicaE solution of equation (2), which has gains due to recruitment and production and losses due to predation and other factors (Fig. 1). Recruitment is a summation of density dependent reproduction and immigration, and it represents the density dependent rate at which biomass is added to the population from all other sources except production. The reproduction rate usually rises to a peak and then declines as population density increases (Ricker, 1954; Guiland, Immigration rate is taken to be inversely related to population 1967). density. The recruitment response will take on different shapes with changing community and environmental conditions resulting in systems of recruitment curves (dashed lines as in Fig. 6C and 7C) and a consequent shift in the isocline. The consumer production rate at different consumer densities is decomposed into the asymptotic growth response of the consumer population with respect to its prey density, and the density of the consumer population with respect to the prey as defined by the prey isocline (Warren, 1971). For example, P1's production curve (Fig. 6D) is developed from the prey isocline for R on the R-P1 phase plane and P1' s growth response as defined by equation (3). The consumer production rate will increase rapidly as the consumer density increases, owing to high growth rate at high prey densities (low con- sumer densities), then peak and decline as the growth rate decreases 16 A '-I 0.4 '-4 0.4 -1 0.. 0 P(K ) P1 c Recruitment Total Gains o Production .HihI IA\ frLow I .Low I P1 P1 F E Totaflosses Predatio by Hi 1>. P1 G Nonprdatory \ H Predation by H2 5kr4kr kh t,/4kr P1 Figure 6. The resource isoclines for the plants are constructed on the P1-H1 phase plane from the graphical solution of equation (2) translated for T1 = P1, T21 H1, and T22 = H2 above. The densities at which P1 are at equilibrium with l-I are determined from the intersections in A of the total gain rate curves (B) at low, medium and high energy input rates with the total loss rate curves (E) at different H1 densities. The total plant gains are a summation of recruitment (C) and production (D). Plant recruitment is zero but could be represented by a family of curves, as represented by dashed lines, due to competition from other plants, energy input rate, habitat or any other phenomena for the energy resource on the R-P1 phase plane, and the plant's growth response with respect to the energy resource levels. A family of different production curves exist for each I for every condition that modifies the plant's growth response. The total plant losses are a summation of predation by H1 (F) and the nonpredatory losses (C). The predation's terms meas ure the consumption rate of plants at different herbivore's densities. Alternative prey could modify the predation response with increasing H1 densities. The nonpr edatory loss response of the plants can also be represented by a family of curves which could result from competition, inhibitory effects of high energy resource levels (i. e. 4Kr) or other physical, chemical, or biological phenomena. Introduction of a competitor (H2) would result in an additional set of predation responses (H) at each H2 density which, if added to each total loss rate curve, would create a family of total loss rates for each H1 density. 17 dH1/dt=rH1+g1H1_g1C1/Yc1_dh1Hl Oke + 2kclbk1 '-4 4' C £.1 1 2 3 4 5 Hl(kh C B Total Gains Recruitment D Production k :: 5khHF:kI2 Total Losses Predation Nonpredatory 4ky2kci h2 Cl H Figure 7. H1 H1 The predation isoclines for H1 are constructed on the P-H1 phase plane from the graphical solution of equation (2). The densities at which H1 is at equilibrium with P1 and C1 are determined from the intersections in (A) of the total gain rate curves (B) at different plant densities with the total loss rate curves (E) at different carnivore densities. The total gain rate curves are a summation of recrui tment (C) and production (D) at different plant densities. Families of recruitment curves can exist due to different competitor densities as shown by the dashed lines in (C) as well as other phenomena such as habitat, predation or energy resources. Production is a family of straight lines due to increasing growth rates at higher plant densities with the horizontal curve representing the H1 mainterance ration of plants. The total losses (E) are a summation of predation (F) nonpredatory losses (G). The carnivor&s consumption rate increases as Ci increases. The addition of an alternative prey such as H2 for the carnivore could change its consumption response as a function of H1. H1s nonpredatory loss rate is also sensitive to competition and increasing H2 can shift the loss rate curve to the left, increasing the loss rates of lower H1 densities, (C). at low prey densities (high consumer densities) (Fig. 6B). Since I is the identity on the prey isocline of P1 on the R-P1 phase plane, the production rate for P1, and in turn the prey isocline of P1, has I as its identity. Similarly I parameterizes the set of prey isoclines on all successive prey-predator phase planes. The characteristic functional response of a predator to increas- ing prey (resource) density is an initial rapid rise in the case of invertebrate species (Fig. 4B and 6F) or a response lag for vertebrate species (Fig. 7F) before leveling off at high prey density (1-lolling, 1959). Predation together with exponentially increasing nonpredatory losses (Fig. 6G and 7G) comprise the loss curve at different H1 densities (Fig. 6E) or at different C1 densities (Fig. 7E). The nonpredatory losses can be represented by families of curves (dashed lines in Fig. 6G and 7G) each with its own identity due to habitat, community, and environmental conditions, which lead to new iso- dines as additional identities are introduced. In this way the community equilibrium is defined for a given I, TC, and other community environmental conditions. The equilibrial C1 density is determined from the intersection of the prey isocline at a given I with a predator isocline at a given TC density (Fig. 5C). This equilibrial C1 density, together with I, spe- cifies the equilibrial H1 density in Figure 5B which in turn determines the equilibrium on the R-P1 phase plane. The time tracks in Figure 5 '9 tend toward the equilibrium nodes for different I' s and TC densities. Since TC and C2 both have the same negative effect, C2 could be substituted for TC in a qualitative sense. The equilibrium due to the configurations of the predator and prey isocline is very stable, approaching in most cases the most stable configuration: a vertical prey isocline and horizontal predator isocline. The time track returning from an instable point to equilib- rium can be rigorously defined from the gain-loss graphs as in Figures 6 and 7. This approach is a substantial improvement over matrix analysis of community stability for multi-species models, which can only characterize the system as stable or instable in the neighborhood of the equilibrium node. The community as defined by Figures 4-7 from equations (4)-(8) has strongly stable predator-prey equilibriums on successive phase planes and therefore has strong, global, stable community equilibria. The system is at equilibrium for a given I and TC and/or C2 density(ies). In a strongly stable community, the community' s time track follows the community equilibrium as the values of the isocline identities change as a function of the community' s environment. I 20 COMPETITION Classical competition theory based on the Lotka-Volterra competition equations has been inadequate in several important respects. It is based solely on the assumption that the system follows a logistic response through time. It does not introduce in any way interrelationships between competition and predator -prey phenomena. It fails to include density-dependent responses of organisms at the population level in the competition response. And finally it does not establish a biological basis for the competition coefficients. Conversely the competing herbivores in the community outlined in Figure 1 are competing for P1 as food and being preyed upon by C1. Isocline analysis provides a qualitative (if k is not defined) and quantitative (if k is defined) method of analysis that permits the interactions of competitors to be explicitly defined in terms of their responses to predators and prey in their environment. Competition is dimensionalized by adding H2 to the system. I-J and H2 will compete for the same resource (P1) and have the same population of C1 as a predator (Fig. 1). With the population of utilizing P1 as resource, an additional loss term is added in the construction of P11s gain-loss graph (Fig. 6H). Summing the preda- tory losses due to H2 density with P1's loss response at different H1 densities results in a family of P1 loss curves at different H2 21 densities, each at a given H1 density (not shown). Equilibrium between H1 and P1 is now a function of I, C1 and H2. As the H2 den- sity increases, the prey isocline of P1 shifts to the left, each new prey isocline with an H2 density as an identity in addition to I (Fig. 8B). The shifting isoclines are not parallel but shaped as a function of the added loss response due to H2. The predator isocline for H1 shifts downward (Fig. 8B) as competition affects the recruitment or nonpredatory loss rate of H1 (dashed lines, Fig. 7C and 7G). The equilibrium and stability of the two herbivore species can be examined by constructing the H1-H2 competition phase plane. The herbivore isoclines for H and H2 competing for the same P1 at a particular land cjdensit are constructed from the H1-P1 and H2-P1 phase planes by using the plant dimension as the common value at particular I' s and C1 densities (Fig. 8A). The H1 isocline has an intercept on the H1 axis which corresponds to its density on the phase plane (Fig. 8B) for a zero H2 density. The H2 isocline is con- structed similarly from the H2-P1 phase plane (Fig. 8C). Both isoclines will have 1 and C1 density as identities. Inter sections of isoclines of the two herbivore species will be equilibria. I and C1 as variables create a continuum of H1-H2 equilibria bounded only of the extreme competition isoclines of H1 and H2. Examination of the competitive ability of populations requires characterization of the gain and loss rates for competitors of different 22 A 5 - '0k High I "3 s 0kC Lowl 1 ....-2 i Righ 1.High -1 4kc 1Low \\ok1 \\ LowI'.,. 1 2 4 3 H1(kh) LowI Med I High I 420') S 4 i' 420 ' 420 ft°_\- 5 . 0,0 C', tlY\\\ y'2,0 kc,kh2 : 4 kh kh 2 . -R 5) - Z 12345 Plant, P1(k) C') 0 z 1 kh 12345 Plant, P1(k) Figure 8. The family of competition isoclines (A) compares how r selected (H1) and k selected (H2) competitors coexist at different H1, H2, C1and I from the intersections of the prey isocline and predator isoclines in the P1-H1 (B) and P1-H2 (C) phase planes respectively. The competition isoclines have energy input rate of the prey isocline and carnivore density of the predator isocline as identities. Solid lines denote' dH1/dt=0 and broken lines dH2/dt=0 in A. Addition of a competitor (H2) to the system results in a family of prey isoclines and in some cases a family of predator isoclines on the Pi-Hi and P1-H2 phase planes each at a different H2 or H1 density respectively. The densities that each herbivore can maintain at a given energy input rate, plant density and carnivore density decrease as the competitor's density increases. When conditions exist such that H1 cannot maintain itself under increasing competition from H2, the predator isoclines no longer have an intercept on the herbivore axis, but intercept the plant's axis at higher and higher densities as H2 density increases (B). When the prey and predator isoclines no longer intersect, l-I can no longer coexist with H2 and the H1 isocline will intercept the H2 axis in A on the H2-P phase plane, H1 shifts the predator and prey isoclines but does not change their stable character because of the r and k nature of H1 and H2 respectively. There is a continuum of competition isoclines due to changing system dimensions which creates a field of equilibrium nodes bounded only by the extreme isoclines. The time tracks converge on the only equilibrium nodes shown. S 23 types. MacArthur and Wilson (1967) and Pianka (1970, 1972) classify populations as r and k strategists on the basis of the relationships between their instantaneous rates of increase and population densities. These relationships characterize their competitive ability at different levels of intra- and inter-specific competition. The impact on the different characteristic rate terms as weLl as the I and C1 dimensions on the equilibrium and stability of r and k selected herbivores can be analyzed by letting H1 be the r strategist and H2 the k strategist and selecting rate terms accordingly. A successful competitor or k-selected organism puts a high amount of energy into its individual progeny and produces less of them. The r-selected organism expends less energy on each progeny and produces more. The r strategist has a higher maximum recruit- ment rate than the k strategist but its recruitment rate will peak and return to zero at a lower population density than the k strategist. A k-selected species, having greater competitive ability, has a nonpredatory loss rate much less sensitive to increasing population densities than an r-selected species. Competitors that reduce their resource availability in much the same manner as other members of their own population respond not only to their own density, but also to the density of the competitor. As H11s food density and recruitment rate decreases and the nonpredatory loss rate increases due to high densities of H2, H1 24 cannot compete and will become extinct on the H1-H2 phase plane (Fig. 8A). The predator isocline at some low C1 density (not neces- sarily zero) and the prey isocline at some low I no longer intersect. As C1 density increases and the population densities of H1 and H2 are reduced, the r-selected H1 can coexist with H2. Increasing the I shifts the resource isocline to the right on the H2-P1 phase plane which means that H2 can exist at higher competitor and lower C1 densities. In contrast, H2, the k-selected herbivore, is able to survive at all densities of H1 and C1. Predators on one particular trophic level may promote community stability (Hutchinson, Paine, 1966; Hall, Cooper and Werner, 1970; 1961; and Connell, 1971). Comtnensalism (+0), the I-T1-J-13 interaction, is another category of species interaction in the environment of the community defined in Figure 1. H3 can have a positive affect on H1 by either increasing its gain rates (rh or g h1 ) and/or decreas.ing its loss rates (g c1 /y c1 1 of dh ). The H1 isocline (solid line) in Figure 9 would respond to an 1 increased gain rate or lower nonpredatory loss rate by shifting H1 to higher densities in relation to H2. If the commensal interaction de- creases the conversion ratio (g h1 'h ) of plants to H , 1 1 then both the H1 and H2 isoclines would shift in the favor of H1rs density. Com- mensal interrelationships can also be adapted to organisms from other trophic levels. Mutual (++) interactions with the organismic environment could be handled similarly. 25 A B Low latO, 2, 4kh @ Ok 3 ------.--..-----.-o 2 124@Ok @4kci 1 1 o24 @ O 2 H1 (kh) Figure 9. Ok H1(kh) The H1-H2 competition isoclines on the H1-H2 phase plane (Fig. 8A) are modified to show the effects of a commensal (+0) interaction between H3 and H1 for low I (A) and high 1(B) at 0, 2 and Solid lines denote dH1/dt+0 and broken lines 3 dH2/dt=O. 26 An indirect mutualistic bond also exists between H2 and H4 as shown by the addition of H4 and P2 to the environment of the defined community (Fig. 1). Increase in H4 density lessens the negative effect of P2 on P1 and then increases the production rate of H2. Explicit examination of the H2-H4 interaction would lead to a mutual phase plane constructed similarly to the competition phase plane. 27 DIVERSITY The explanation of species diversity has been largely empirical and quite inadequate. A theoretical basis has not been established to dimensionalize species diversity in terms of the way species interact, the energy input rate and the physical, chemical and biological environment. Isocline analysis as developed for a system with perfect competition (system lacks prey switching dimension) can be used as the theoretical basis for qualitative and quantitative analysis of diversity by expanding the competition isocline. The family of diversity isoclines (Fig. 1OA) is parameterized in terms of carnivore predation, energy input rate and the commensal herbivore (H3). A.s suggested by the productivity hypothesis (Connell and Orias, 1964; Margelef, 1969), which states that greater produc- tion results in greater stability, the diversity index (Fig. lOB) increases with increasing I which, with all things equal, results in greater production rates (Fig. 6B). The hypothesis that predation increases diversity is illustrated with the upward shifting isoclines to higher levels of diversity as the carnivore density (predation pressures) increases for given H3 densities. The amensal relation- ship between C1 and C2 decreases the diversity by reducing predation. Diversity increases with increasing H3 densities because the commensal interaction of H1 and H3 improves the competitive A 13 Low I High I A 4k1 Q 4k 0. 5 5 h3 2kh3 0.4 4 kh3 2k 1 3 0.3 n3 4) 0.2 2 4) 0. 1 )kh3 p Low Herbivore Species - I Figure 10. Med High Energy Input Rate (I) The family of herbivore diversity isoclines in terms of the herbivores' density (A) and diversity index (B) are parameterized by the energy input rate and the magnitude of carnivore predation. The diversity isoclines in A are determined from the equilibria on the competition phase plane in Figure 9. The diversity index was calculated at equilibria of H1,H2 and the commensal H3 by assuming khl = kh2 =kh3, by the Shannon-Weaver equation, log .. where H = E H1. Z9 performance of H1, the r-selected herbivore. The addition or omission of species as it occurs in succession can now be added as a new dimension to resource utilization theory. A change in the number of herbivores in the community matrix cre- ates families of isoclines as the addition of H3 did on the diversity phase plane in Figure 10. CD CONCLUSIONS Productivity theory (Brocksen, Davis and Warren, 1970; Warren, 1971) supplied the impetus and direction for the develop- ment of a resource utilization theory. This theory postulated that families of density relationships between successive predator and prey relationships along pathways of energy and material transfer are functions of the physical, chemical and biological environment. It further suggested that relationship at one trophic step can be parameterized in terms of variables at previous steps. Therefore the problem has been not so much what to lookfor but how to get there. Resource utilization theory as proposed appears to satisfy all of the criteria for the theory of productivity. The density relation- ships between the predator and prey are families of predator-prey isoclines, the prey isocline being a function of the dimensions that add energy and materials to the community along the pathway to the prey isocline. The predator isocline is a function of the top consumer density in the community and other dimensions that affect the density relationship between predator and prey down to the predator isocline. The intersections of the isoclines in the family of successive predator prey relationships establish the community equilibrium. The iso- dine identities as variables generate families of predator and prey 31 isoclines limited only by their possible existence. Competition, rnutualism, amensalism and commensalism are integrally related to predator-prey phenomena and cannot be considered independently. Community stability theory does not have to be restricted to neighborhood analysis and confining approximations, but can be analyzed anywhere within the framework of the community model. The density dependent processes of species appear to make predator- prey interactions strongly stable. Only after introduction of a more efficient competitor does an instable predator-prey relationship exist. Greater community model complexity does not reduce its strongly stable condition. Introduction of optimal foraging theory as another dimension could provide added stability for populations under competi- tive stress by switching predation pressures to the other competitor, and in effect providing a refugium for soon-to-be extinct competitors. The framework may now exist for examining other dimensions of diversity in addition to the supported predation and productivity hypothe sis. 32 BIBLIOGRAPHY Analysis of trophic processes on the basis of density-dependent functions, Pages 468-498. In J. H. Steele (Editor), Marine Food Chains. University of California Press, Berkeley. xvii + 603 pp. Brocksen, R. W., G. E. Davis, C. E. Warren. 1970. Connell, 3. H. 1971. On the role of natural enemies in preventing competitive exclusion in some marine animals and in the rain forest trees. Pages 289-312. In Dynamics of populations. Wageningen, Centre for agricultural publishing and documen- tation x 412 pp. Connell, 3. H. and E. Orias. 1964. The ecological regulation of species diversity. American Naturalist 98:399-414. Gulland, 3. A. 1967. The effects of fishing on the production and catches of fish. Pages 399-415. In S. D. Gerking (Editor), The Biological Basis of Freshwater Fish Production. Blackwell Scientific Publications, Oxford. Xiv + 455 pp. 1970. An experimental approach to the production dynamics and structure of freshwater animal communities. Limnological Oceanog- Hall, D. 3., W. E. Cooper and E. E. Werner. raphy 15:839-928. Holling, C. S. 1959. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. The Canadian Entomologist 91:293-320. Hutchinson, G. E. 1961. The paradox of the plankton. American Naturalist 95:137-145. MacArthur, R. H. and E. 0. Wilson. 1967. The theory of island biogeography. Princeton University Press, Princeton. x+ 203 pp. Margalef, R. 1969. Diversity and stability: a practical proposal and a model of inter-dependence. In Diversity and Stability in Ecological Systems. pages 25-3 7. Brookhaven, Symposium Biology. 250 pp. 33 May, R. M. 1972. Limit cycles in predator-prey communities. Science 97:209-233. May, R. M. 1973. Stability and complexity in model ecosystems. Princeton University Press, Princeton. v + 235 pp. Monod. Recherches sur Ia Croissance de cultures Bacteri- 1942, ennes. I-Ierxnann and Cie, Paris. 235 pp. Paine, R. T. Foodweb complexity and species diversity. The American Naturalist 100: 65-75. Pianka, E. R. 1966. 1970. On r and k selection, The American Naturalist 104: 592-597. Pianka, E. R. 1972. r and k selection or b and d se1ection American Naturalist 106:581-588. The Ricker, W. F. 1954. Stock and recruitment. Journal Fisheries Research Board of Canada 11:559-623. Rosenzweig, M. L. and R. H. MacArthur. 1963. Graphical representation and stability conditions of predator-prey interactions. The American Naturalist 97: 209-223. Rosenzweig, M. L. 1969. Why the prey curve has a hump. The American Naturalist 103:81-87. Rosenzweig, M. L. 1973. Exploitation in three trophic levels. The American Naturalist 107:275-294. Warren, C. E. Biology and water pollution control. W. B. Saunders Company, Philadelphia, xvi + 434 pp. 1971.