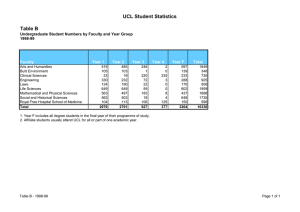
Basic Life Sciences 1 UCL SCHOOL OF LIFE AND MEDICAL SCIENCES
advertisement

Basic Life Sciences UCL SCHOOL OF LIFE AND MEDICAL SCIENCES Creating knowledge, achieving impact 1 PREFACE The UCL School of Life and Medical Sciences is one of the world’s largest and most prestigious aggregations of academics in medical, brain, life and population health sciences. Our performance in the UK’s last Research Assessment Exercise was outstanding. We headed the UK’s performance table in biomedicine and life sciences with more than 200 researchers meriting the highest rating of 4*, some 25 per cent ahead of our closest rival. Moreover, when quality and volume were combined in what the Times Higher Education called ‘research power’ the School again headed the rankings, with a score some 24 per cent ahead of its nearest competitor. In part because of UCL’s size and organisational complexity, the scale of the School’s achievements is not always apparent. This publication, one of five, seeks to address this. Our reorganisation in August 2011, with the creation of four new Faculties, has been designed to create a more coherent structure, of which the Faculty of Life Sciences, headed by the Dean, Professor Mary Collins, is a clear example. But the School’s restructuring has also placed great emphasis on cross-Faculty interactions and interdisciplinary research – and indeed on interactions with UCL departments outside the School. Such interdisciplinary endeavour is promoted through ‘Domains’, inclusive strategically led fluid networks. This approach allows us to connect all our activities related to fundamental research, promoting collaboration and the sharing of expertise, platforms and resources. Professor Michael Duchen and Dr Paola Oliveri are chairs of the Basic Life Sciences Domain. UCL is acutely aware that scientific advance of real relevance to society is not only aided by an interdisciplinary approach but also through collaborative strategic alliances with other researchintensive institutions with complementary strengths. Our founding partner status in the new Francis Crick Institute engages us in what will be the European powerhouse of biomedical research expertise. Our links with our London Academic Health Science Centre partners also include our joint engagement together with the Medical Research Council in a new imaging company, Imanova, and our commitment to the London Life Sciences Concordat. Our growing collaboration with our Bloomsbury neighbours, the London School of Hygiene and Tropical Medicine, is fuelling exciting developments in genetic epidemiology and pathogen research. The breadth and quality of our research creates almost unique opportunities. Our recent merger with the School of Pharmacy, a stimulating addition to the Faculty of Life Sciences, adds to our capacity in drug development, formulation and adoption. Our highly productive links to the health service, through UCL Partners, provides access to unmatched clinical expertise and large patient groups. We are fortunate to be partners in three National Institute for Health Research (NIHR) Biomedical Research Centres and a new NIHR Biomedical Research Unit in dementia. The School’s academic environment is one in which intellectual curiosity can prosper, while a high priority is also given to the practical application of knowledge to improve health and quality of life. This can take many forms, including commercialisation of new products as well as developing and informing health and social policy, and engaging with important stakeholders, including the public. This publication, one of five (see right), showcases some of the outstanding fundamental research being carried out within the School and with collaborators across UCL and our partners in London, nationally and internationally. It is impossible to be comprehensive, but the stories give a flavour of the breadth, quality and impact of the School’s research in this area. Looking forward, our aims are to enhance and expand our research to ensure we remain a global leader, and to see more people benefit from the groundbreaking research being carried out across the School. Sir John Tooke Vice-Provost (Health) 1 Basic Life Sciences: ‘Discovery’ research, from molecules to ecosystems. 2 Translation and Experimental Medicine: Driving translation to benefit patients’ health and well-being. 3 Neuroscience and Mental Health: The science of the brain and nervous system, from synapse to social interactions. 4 Population Health: Protecting and improving the health of populations, UK and globally. 5 Education: Innovative practice across the educational life course. CONTENTS Overview: The roots of discovery 2 UCL supports groundbreaking curiosity-led research across all biological scales. Section 1: Molecular basis of life 4 Exploring the structure and function of molecules and molecular complexes fundamental to life. Feature: The great and the good: Nobel laureates and other key figures in UCL’s life science history 10 Section 2: The cellular world 12 Understanding the processes that control a cell and contribute to disease. Feature: CoMPLEX: A model for interdisciplinary research 22 Section 3: Building tissues 24 Dissecting the mechanisms by which tissues and organs develop. 32 Section 4: All systems go Systems-based approaches, from the cell to the brain – and beyond. Feature: Genes, culture and human behaviour 38 Section 5: Origins: Genes and evolution 40 Using genetics to understand evolutionary relationships and human biology. UCL institutes, support services, partners, funding and sponsors. 46 BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 1 BASIC LIFE SCIENCE UCL supports groundbreaking curiosity-led research across all biological scales. Dorsal view of the zebrafish brain. Research in the UCL School of Life and Medical Sciences has the potential to improve human health and well-being. There is an oft-cited danger that, in an understandable drive to translate research into practical benefits, the well-spring of discovery is neglected. At UCL, fundamental curiosity-led research remains a high priority and a core activity. into the structure and function of large multiprotein complexes. Core structural biological techniques – X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy and electron microscopy – are being combined with other biophysical techniques and biochemical characterisation to provide an integrated view of molecular function. There are many ways to categorise such activities. To avoid disciplinary pigeonholing, in this publication we have chosen to focus on questions of scale. Obviously, even this approach is arbitrary – a satisfactory understanding of biological phenomena often requires integration across multiple biological scales. The cell remains at the heart of fundamental research. As well as being the basic building block of organisms, abnormalities in cellular function underlie numerous disease processes. And manipulating the activities of cells is increasingly offering new therapeutic opportunities. At the molecular level, structural biology provides important insight into the function of biological molecules. As technologies improve, the size of structures that can be studied continues to increase, with insight now being gained 2 While the basic function of many cellular structures have been determined, how they are regulated is often less clear. The dynamics of cellular processes, and their responses to external stimuli, are therefore important areas of study. Similarly, cellular mechanisms of disease often The cell remains at the heart of fundamental research. As well as being the basic building block of organisms, abnormalities in cellular function underlie numerous disease processes. remain poorly understood. In recent years, for example, it has become apparent that mitochondrial abnormalities play an important role in Parkinson’s disease yet details remain obscure. Central to many questions is how the fate of cells is decided. Once the factors controlling cell fate are better understood, it will be considerably easier to intervene to alter cell fate for therapeutic ends. Genetic approaches, particularly genome-wide association studies, have generated a long list of genes potentially involved in disease processes, but rarely is much known about their functional roles. Additional work is normally needed to explore their biological function and how they might be contributing to disease. In particular, work in model BASIC LIFE SCIENCES UCL School of Life and Medical Sciences organisms is crucial if gene function is to be understood in the context of living dynamic processes. As well as laboratory mice, zebrafish are an extremely valuable tool, and UCL houses one of the largest zebrafish facilities in Europe. Furthermore, cells do not operate in isolation but as part of integrated wholes. Developmental biology obviously depends on the coordinated behaviour of cells. In addition, many physiological processes can only be understood in terms of the interactions between multiple cells and tissues. Biology is adopting an increasingly systemsdriven, integrative approach – at levels as varied as the genetic programmes that drive cell behaviour to the physiological systems that regulate eating. Part of the bacterial type IV secretion system. Scanning electron micrograph of the surface of a zebrafish embryo. Frequently these integrative approaches need to embrace mathematic and computational methodologies. Much biological research now includes collaborations across the mathematical and computational sciences. Neuroscience* is one obvious area where such approaches have gone hand in hand with experimental studies, but such crossdisciplinary interactions are now increasingly common. UCL established CoMPLEX with the specific aim of developing researchers able to move fluidly between the physical and life sciences. Indeed, much UCL life science research crosses traditional disciplinary barriers. As well as mathematics and computational input, chemists have a critical role to play in developing agents to explore biological function, while nanotechnology provides a wealth of new opportunities to explore macromolecular and cellular Fibres of secreted von Willebrand factor. function. One of the most important of areas, imaging, draws all these areas together, creating tools that can provide unprecedented views of living biological processes (see right). While discovery remains at the heart of basic life science research, it is informed by dialogue with clinicians and medical scientists, and alert to opportunities for translation. The communication is two-way, with medically important genes or processes providing an intellectual challenge for basic researchers, and the insight generated through fundamental studies providing inspiration for new therapies. * Neuroscience makes an important contribution to UCL’s basic life sciences research. Given the extent of these activities, they are covered in a separate publication (Neuroscience and Mental Health). SEEING AND BELIEVING The ability to understand biological processes is being greatly enhanced by new imaging techniques. Across different scales, imaging technologies are proving central to the gathering of new information about biological processes. At the molecular level, X-ray crystallography, NMR spectroscopy and electron microscopy and tomography can provide atomic-level detail of individual protein structures and multiprotein complexes. Combined with advanced computer graphics, this insight can be transformed into dynamic representations of biological processes. Pinpointing the precise location of molecules and ions in living cells can provide dazzling views of dynamic biological processes, such as calcium currents. Biochemical reactions can also be visualised, for example to explore mitochondrial function. Many groups at UCL have developed great expertise in microscopy techniques, and technical innovations continue to push back the frontiers of visualisation. In neuroscience, two-photon microscopy and other technologies are providing more information about neural function in living tissue (see companion volume on Neuroscience and Mental Health). With further developments such as the brightness and stability of quantum dots, the specificity of single-molecule tagging and super-resolution microscopy, the ability grows to gather still more detailed information from living cells and tissues. At higher levels of organisation, a variety of non-invasive techniques are providing unique insight into biological function and disease mechanisms. UCL’s Centre for Advanced Biological Imaging is a world-leading core centre of expertise in imaging in living systems, supporting work across multiple departments. With technologies such as magnetic resonance imaging and nuclear imaging, ultrasound, bioluminescence and fluorescence imaging, it has particular strengths in tissue- and organism-level imaging in model organisms. Technical accomplishments such as the remarkable analysis of living heart function after treatment to promote heart muscle cell repair again illustrate the power of new technologies to visualise dynamic biological processes. As well as supporting basic discovery, the Centre also makes an important contribution to early translational studies. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 3 SECTION 1 MOLECULAR BASIS OF LIFE Structural biology is providing insight into ever-larger complexes, while other techniques are shedding light on key molecules inside the cell and those signalling between them. A bacterial transmembrane complex generating adhesive filaments (pili). POPULATION HEALTH School of Life and Medical Sciences 4 Changes to the TRIMCyp restriction factor alter interactions at its virus-binding site. Structural biology has provided a view of biomolecules way beyond the resolution afforded by light microscopy. Increasingly, structural techniques are being applied to large protein structures and, combined with information from other biophysical technologies and sophisticated computer graphics, provide a glimpse of dynamic biological processes at a molecular level. Visualising nano-machines Professor Gabriel Waksman and colleagues at UCL and Birkbeck College are focusing on two bacterial ‘nano-machines’ that control the movement of proteins across the inner and outer cell walls of Gram-negative bacteria such as E. coli. One of these complexes generates the long sticky filaments, pili, that bacteria use to attach to host cells (see page 7). The second is one of a range of mechanisms bacteria use to secrete materials or Increasingly, structural techniques are being applied to large protein structures and, combined with information from other biophysical technologies and sophisticated computer graphics, provide a glimpse of dynamic biological processes at a molecular level. transfer them to target cells. This type IV secretion system is of particular medical interest, as one of its roles is to transfer plasmids containing antibiotic resistance genes. As a model, Professor Waksman’s group has used the type IV system of E. coli – a huge multiprotein complex that stretches methods of structure determination to the limit. A combination of cryo-electron microscopy of the core of the complex1 and X-ray crystallography of the outer membrane region2 has revealed two layers forming pores in the inner and outer membrane. Unexpectedly, one of the proteins in the complex spans both layers – the only protein known to span both inner and outer bacterial membranes. The structures provide clues to how the system may operate. Moreover, by identifying key points of interaction between protein subunits, they also reveal a host of regions that could be targeted by small chemicals, to inhibit the structure’s function. Professor Waksman has worked with groups in Sweden and the USA on agents that block pilus formation3,4 and a similar strategy could be applied to the type IV secretion apparatus. 1 Fronzes R et al. Structure of a type IV secretion system core complex. Science. 2009;323(5911):266–8. 2 Chandran V et al. Structure of the outer membrane complex of a type IV secretion system. Nature. 2009;462(7276):1011–5. 3 Chorell E et al. Design and synthesis of C-2 substituted thiazolo and dihydrothiazolo ring-fused 2-pyridones: pilicides with increased antivirulence activity. J Med Chem. 2010;53(15):5690–5. 4 Pinkner JS et al. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc Natl Acad Sci USA. 2006;103(47):17897–902. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 5 Structure of the core complex of the bacterial type IV secretion apparatus. All living organisms can be categorised into three domains – eukaryotes, bacteria and archaea. The latter may superficially look like bacteria, but their genetics and biochemistry align them more closely with eukaryotes. Professor Finn Werner has taken advantage of archaeal peculiarities to gain insight into the molecular mechanisms of RNA polymerase (see page 8). The findings also shed intriguing light on the possible evolutionary origins of this most fundamental of living processes. All living things need to read (transcribe) sequence information from DNA into RNA, a task accomplished by RNA polymerases. Indeed, the core proteins involved in this process are conserved in eukaryotes, bacteria and archaea. But there are also significant differences between the three groups, raising questions about how the forerunner of all living organisms – the ‘last universal common ancestor’, or LUCA – transcribed its RNA. 6 Notably, points out Professor Werner, alongside RNA polymerase, only one other critical protein (known as Spt5, SPT5 and NusG) is conserved across the three groups, and hence is likely to have been present in LUCA. Surprisingly, Spt5 is involved not in initiation – binding of RNA polymerase to DNA – but in locomotion of the enzyme along the DNA template. The proteins responsible for initiation are related in archaea and eukaryotes, but completely different in bacteria. The simplest explanation is that LUCA lacked initiation factors, which evolved independently in bacteria and in the lineage that later split to give rise to the archaea and eukaryotes5. If this is true, it implies that initiation was initially a passive process, and regulation was to begin with based on control of elongation steps. Possibly, RNA polymerase originally bound to AT-rich regions of DNA, where strands of DNA are naturally easier to separate (notably, bacterial and eukaryotic initiation Present in the membranes of most if not all cells, ion channels are critical to numerous cellular functions and are also important targets for many therapeutic agents. sites, although dissimilar, are both AT-rich). Spt5 may have had a general role during elongation, helping RNA polymerase through DNA sequences that slowed its progress. Indeed, Spt5 may have allowed RNA polymerases to transcribe longer genes, ultimately leading to larger genomes. No open and shut case Among the most widely studied macromoleular structures are ion channels. Present in the membranes of most if not all cells, ion channels are critical to numerous cellular functions and are also important targets for many therapeutic agents. Professor Annette Dolphin has studied the mode of action and properties of calcium channels which, among other things, are the targets of the gabapentin class of painkilling drugs (see page 7). BASIC LIFE SCIENCES UCL School of Life and Medical Sciences Surprisingly, detailed work on their mechanism of action suggests that these drugs interfere not with channel activity directly but with trafficking to the plasma membrane. A similar regulatory process has been seen by Dr Josef Kittler in his work on GABA receptors (see page 18). Channel function – and their role in numerous disease states – is a core area of neuroscience research (see companion volume on Neuroscience and Mental Health). Defence molecules To coordinate cellular responses, the body produces countless signalling molecules. Again, these are common targets for chemical interventions. 5 Werner F, Grohmann D. Evolution of multisubunit RNA polymerases in the three domains of life. Nature Rev Microbiol. 2011;9(2):85–98. A multi-subunit complex generates filamentous pili. Elevated levels of 2 1 (green) in rat spinal cord. BUILDING REGULATIONS BACK TO THE SURFACE Stunning structures of multiprotein complexes have revealed how E. coli synthesises its adhesion-promoting pili. Drugs used to treat pain associated with nerve damage have a highly unusual mode of action. Bacteria such as E. coli attach to host cells through long sticky filaments known as pili. The pili of E. coli that infect the urinary system consist of an adhesive tip composed of three types of pilin protein attached to a long filament made up of a polymeric chain of a fourth filament protein. Using X-ray crystallography, Professor Gabriel Waksman and colleagues at UCL and Birkbeck College have generated a remarkable view of the pilus being synthesised and threaded through the bacterial cell wall. Previous work had identified the core components of this extrusion system. At its heart lies an ‘usher’ protein, FimD, a large barrel-shaped protein spanning the bacterium’s outer membrane. Also critical is a ‘chaperone’, FimC, which sits in the space between the outer and inner membranes, picks up new subunits posted through the inner membrane and delivers them to the FimD usher. Professor Waksman’s latest study solved the structure of the FimD usher bound to the terminal pilin subunit, FimH, and its associated chaperone, FimC. On its own, access to the inside of the FimD usher is blocked by a molecular ‘plug’. When FimC and FimH dock, however, this plug hinges open, enabling FimH to enter the core of the FimD barrel. Furthermore, FimH is positioned in such a way to promote binding and attachment of the next pilus subunit (FimG), delivered by the FimC chaperone. This arrangement depends on a second, previously unsuspected FimC-binding site on the FimD usher. Although not directly visualised, binding of a new chaperonebound pilin subunit is presumed to position it for attachment to the base of the growing pilus. It is then translocated to the other chaperone-binding site, freeing up the first binding site for another chaperone-bound subunit. In this way, the pilus is extended from its base in a stepwise fashion, subunit by subunit. As well as revealing a likely mode of action for a remarkable bacterial ‘nano-machine’, the study may also have practical spinoffs. The structures highlight the key areas that could be targeted to disrupt pilus formation and prevention attachment to cells lining the urinary system, thereby preventing infection by E. coli. Ion channels control the flux of ions across cell membranes, playing important roles in numerous physiological processes, not least nerve function. They are a popular target for drug development, the goal generally being to modulate ion flows in ways that are therapeutically beneficial. However, Professor Annette Dolphin has found that one class of agents, gabapentin and its relatives, act in an entirely different way. Professor Dolphin has worked extensively on calcium channels, which are critical to nerve function and many other cellular processes. As their name suggests, voltage-gated calcium channels open and close in response to changes in voltage across the cell membrane. They consist of an 1 subunit, which forms the actual pore, an intracellular subunit and a membrane-bound but predominantly extracellular 2 subunit. Gabapentin was originally developed as an analogue of the neurotransmitter GABA (gamma-aminobutyric acid) but it soon became clear that it did not bind to GABA receptors. In fact, evidence began to accumulate that its effects were mediated through interactions with the 2 subunit of calcium channels. Colleagues at Pfizer, in collaboration with Professor Dolphin, provided the first convincing evidence in animal models that this was indeed the case. Even so, gabapentin’s mode of action remained unclear, with conflicting reports of its effects on ion currents. However, the drug takes several days to have an effect and does not inhibit acute pain, leading Professor Dolphin to suggest that it might work by affecting ion channel numbers at the cell surface rather than by directly modulating channel function. Indeed, gabapentin’s site of action was found to be within the cell, and it reduced the number of ion channels at the cell surface. More detailed analysis revealed that the critical step was not the production and delivery of new ion channels but the recycling of existing calcium channels through endosomal pathways. The studies therefore highlight an entirely novel way in which agents can influence the action of ion channels involved in pain. Remaut H et al. Fiber formation across the bacterial outer membrane by the chaperone/usher pathway. Cell. 2008;133(4):640–52. Field MJ et al. Identification of the alpha2-delta-1 subunit of voltagedependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci USA. 2006;103(46):17537–42. Phan G et al. Crystal structure of the FimD usher bound to its cognate FimC-FimH substrate. Nature. 2011;474(7349):49–53. Hendrich J et al. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci USA. 2008;105(9):3628–33. Bauer CS et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci. 2009;29(13):4076–88. Tran-Van-Minh A, Dolphin AC. The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J Neurosci. 2010;30(38):12856–67. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 7 Different forms of TRIMCyp differ in their affinity for HIV. NEAT AND TRIM Retroviruses and the cells they prey upon are locked in a constant evolutionary battle. Lentiviruses, the family of retroviruses that include HIV, are extremely fussy in the species they infect. Species specificity depends on components of the innate immune response known as restriction factors, particularly the TRIM family of proteins. As Professor Greg Towers and colleagues have discovered, TRIM proteins and lentiviruses have been waging an ongoing evolutionary battle lasting millions of years. Professor Towers has focused on the TRIM5 restriction factor. The tip of TRIM5 includes a domain that binds the surface coat of lentiviruses, intercepting invading viruses and targeting them for destruction. The specificity of TRIM5 binding to viral coat proteins dictates which viruses can and cannot establish infections. Remarkably, in a new world monkey, the owl monkey, the virusbinding domain of TRIM5 has been replaced by a host protein, cyclophilin. The protein is still functional, and now targets cyclophilinbinding viruses. Even more remarkably, Professor Towers and colleagues subsequently found that old world monkeys, Rhesus macaques, also have a ‘TRIMCyp’ fusion – but it differs significantly from the owl monkey version. Hence the two seem to have evolved entirely independently. The sequence of the TRIMCyp gene differs across the macaque genus, and Professor Towers has enlisted the help of structural biologists at the University of Cambridge to investigate the consequences of these differences. Sequence variants influencing binding specificity typically affect amino acid residues that make contact with the virus. In TRIMCyp, however, key changes lie some distance from virus-binding sites – but the changes affect a charged residue and trigger a cascade of conformational shifts, ultimately altering the virus-binding site. Mapping TRIMCyp sequences onto the macaque family tree suggested that a single amino acid change appeared in the cyclophilin domain early in evolution, probably providing protection against multiple lentiviruses. Later, other amino acid changes enhanced recognition of specific viruses, but narrowed the range of viruses that could be recognised. Oddly though, present-day macaques are not known to harbour lentiviruses. Possibly, the relevant macaque virus has not yet been identified, or antiviral responses were so effective that the virus was vanquished. A further evolutionary conundrum is the independent appearance of two TRIMCyp fusions. Their existence hints at the importance of cyclophilin to lentivirus infection. Cyclophilin is part of a complex at the nuclear pore that appears to control traffic into the nucleus, which HIV may hijack. In its absence, HIV shows markedly different patterns of integration into the host genome. Wilson SJ et al. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc Natl Acad Sci USA. 2008;105(9):3557-62. Price AJ et al. Active site remodeling switches HIV specificity of antiretroviral TRIMCyp. Nat Struct Mol Biol. 2009;16(10):1036–42. Ylinen LM et al. Conformational adaptation of Asian macaque TRIMCyp directs lineage specific antiviral activity. PLoS Pathog. 2010;6(8):e1001062. Ocwieja KE et al. HIV integration targeting: a pathway involving Transportin-3 and the nuclear pore protein RanBP2. PLoS Pathog. 2011;7(3):e1001313. 8 BASIC LIFE SCIENCES UCL School of Life and Medical Sciences Factors compete for binding to RNA polymerase. EXTREME ANSWERS Work on enzymes found only at hydrothermal vents has revealed an elegant mechanism controlling a key event in transcription. RNA polymerases, enzymes that read DNA sequence information into RNA, are huge multisubunit complexes with many associated factors regulating their behaviour. Because of their size and complexity, their detailed mechanisms of action have been difficult to study. By developing a novel in vitro system based on heat-resistant archaeal proteins, Professor Finn Werner has been able to gain unparalleled insights into a critical step in transcription. Archaea are the third kingdom of life, alongside bacteria and eukaryotes. Although single-celled and superficially similar to bacteria, their genetics and biochemistry hint at a closer relationship to eukaryotes. Archaea include many exotic forms of life, including the ‘extremophiles’ – organisms that thrive in some of the most challenging environments on Earth. Using an archaeal strain living around ‘black smoker’ hydrothermal vents, Professor Werner has been able to generate recombinant proteins that reconstituted a functioning in vitro system – something that has so far proven impossible with eukaryotic RNA polymerases. The crucial factor seems to be adaptation of the archaeal enzymes to high temperature – they form highly stable structures and refold efficiently in the test tube. With this system, Professor Werner has been able to engineer fluorescent tags into the archaeal proteins, and then explore interactions between different regulatory proteins and RNA polymerase during its initial binding to DNA and as it begins to move along the DNA template. Of particular interest was a critical transcription factor, TFE, which helps to separate DNA strands during the initiation of transcription. TFE appeared to bind to a previously identified ‘clamp’ region on RNA polymerase, but so too did a second factor, Spt4/5. Indeed, the two factors appeared to compete for binding to the clamp. At initiation, TFE wins the battle, promoting binding of RNA polymerase to the promoter of the DNA template and separation of DNA strands. Then, however, conformational changes in the complex subtly alter binding affinities, enabling Spt4/5 to get the upper hand. This lifts the handbrake imposed by TFE, enabling the enzyme to travel along the DNA template more efficiently. Transcription machineries are highly conserved across all living systems. Homologues of the archaeal proteins are also found in eukaryotes. Hence the mechanisms discovered in archaea are likely to be relevant to eukaryotes, and the unique in vitro system developed by Professor Werner should continue to provide general insights into this fundamental biological process. Grohmann D et al. The initiation factor TFE and the elongation factor Spt4/5 compete for the RNAP clamp during transcription initiation and elongation. Mol Cell. 2011;43(2):263–74. Professor Derek Gilroy has explored responses to one of the most commonly used agents, aspirin, revealing individual variation in responses that could have significant implications for inflammatory and defence responses (see right). Studies of the human genome are revealing sites where human evolution has been shaped by contact with pathogens. Across the animal world more generally, evidence can be found of an ongoing battle between viruses and their hosts. The constant vying for supremacy, suggests Professor Greg Towers, is an example of the Red Queen hypothesis – the evolutionary theory named after the Alice Through the Looking Glass character who declared, “It takes all the running you can do, to keep in the same place.” Professor Towers’ interest was sparked by the observation that particular lentiviruses (the class of retroviruses that includes HIV) can only infect cells from certain species. The answer lies in ‘restriction factors’ – components of the host’s innate immune system that prevent viruses becoming established in a cell. Classic restriction factors include TRIM5 proteins, which bind to viral coat proteins and target both themselves and their attachments for digestion within the cell. Professor Towers has gathered considerable evidence of the evolutionary interplay between lentiviruses and their host cells. Suggestive evidence comes from signs of ‘positive selection’ in the genome – genetic changes that appear to have been actively selected for in evolution. The importance of such changes can be confirmed by functional studies, which compare the effects of different sequences – in viral or host proteins – on the efficiency of infection. Sure enough, even minute changes at sites in TRIM5 can dramatically affect the specificity of infection. Furthermore, structural studies can provide mechanistic explanations for the changes seen (see page 8). To date, no chemical agent has managed to eradicate HIV from the body – small reservoirs always survive. The only exception is a single patient who was being treated for a blood cancer and received a bone marrow transplant. Fortunately, a matched donor was available who carried a genetic variant in a co-receptor required for HIV infection, rendering his cells essentially resistant to HIV. Encouragingly, the recipient’s blood cells are not being infected by HIV. Inspired by this case, in collaboration with Professor Waseem Qasim, Professor Towers is pursuing the idea of gene therapy for HIV patients. The idea is that T cells would be collected from patients and engineered so that they would be resistant to HIV infection, before being returned to the patient. This novel approach holds particular promise for young people infected with HIV, who otherwise face the prospect of a lifetime on powerful antiretroviral drugs. Professor Derek Gilroy. RESOLVE TO DO BETTER People differ markedly in their response to inflammation. The classic signs of inflammation – redness, swelling and so on – usually disappear of their own accord fairly quickly. It used to be thought that this was a passive process in which inflammatory processes gradually faded away, but it is now clear that inflammatory responses are more actively curtailed. And Professor Derek Gilroy and colleagues have found that people differ markedly in their ability to terminate inflammatory reactions. The discovery arose out of Professor Gilroy’s interest in lowdose aspirin’s effects on inflammation. High-dose aspirin has well-known anti-inflammatory properties, while much lower doses protect against cardiovascular disease. Although low-dose aspirin was known to trigger production of lipids with inflammationresolving powers, it was unclear whether it was anti-inflammatory in practice. To answer this question, Professor Gilroy turned to an unusual model – skin blistering cause by a toxic extract of the Spanish fly (confusingly, a type of beetle). Cantharidin, historically used as an aphrodisiac (albeit a potentially lethal one), and more recently to treat warts, generates fluid-filled blisters when applied to the skin. The blisters show all the hallmarks of classic inflammation. And low-dose aspirin did indeed turn out to have anti-inflammatory properties (though through a different mechanism from high-dose treatment). Curiously, though, only about 60 per cent of people responded to low-dose aspirin. Indeed, people fell into two clear classes – ‘early resolvers’, in whom inflammation cleared within a few days, and ‘delayed resolvers’ whose blisters persisted much longer. A key difference between the two groups was a lipid-like mediator, 15-epi-LxA4. In early resolvers, 15-epi-LxA4 levels started low and rose as inflammation cleared, while in delayed resolvers it started high but then fell away. Low-dose aspirin triggered 15-epi-LxA4 production, but only in early resolvers. As for mechanisms, 15-epi-LxA4 is generated by the enzyme COX-2 after it has been chemically modified (acetylated) by aspirin. COX-2 levels increase during inflammation so, in the presence of aspirin, more acetylated enzyme is generated, levels of 15-epi-LxA4 rise, and inflammation is brought under control. Recently, small amounts of 15-epi-LxA4 have been identified even in the absence of aspirin, but it remains unclear where they come from. The results suggest that natural variation affecting 15-epi-LxA4 pathways could influence responses to infections and inflammatory responses. They also highlight a potentially important pathway that needs to be considered in the design of anti-inflammatory agents. Morris T et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183(3):2089–96. Morris T et al. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc Natl Acad Sci USA. 2010;107(19):8842–7. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 9 UCL has housed some of the UK’s most pre-eminent life science and medical researchers, who set a benchmark against which current research must be judged. Below left: Bernard Katz. Below right: Archibald Vivian Hill. THE GREAT AND THE GOOD Neuroscience is a particularly strong discipline at UCL, and it is building on notable foundations. A pivotal figure was Sir Bernard Katz, noted for his pioneering work on the synapse. The rise of Nazism in the 1930s saw a stream of talented Jewish researchers from Germany and other European countries seek refuge in the UK. Among them was a young medic, Bernard Katz, who arrived at UCL in 1935 to study under Archibald Vivian Hill – Hill co-founded the Academic Assistance Council, which helped hundreds of academics escape Nazi persecution. Following a period in Australia, Katz returned to UCL after the war, remaining until his retirement. Katz was interested in events at the synapse, particularly acetylcholine signalling at the neuromuscular junction. His key discovery was that the release of neurotransmitter was ‘quantal’ – it always increased in discrete steps. Now known to be due to the release of neurotransmitters from uniform secretory vesicles, the discovery earned Katz a Nobel Prize in 1970. One of Katz’s students 10 was Bert Sakmann, who with Erwin Neher went on to win a Nobel Prize for the development of patch clamping. Katz’s mentor, Archibald Vivian Hill, had himself been awarded a Nobel Prize, in 1922 for his work on the biophysics of muscle contraction. He joined UCL in 1923, taking up a position of Professor of Physiology from yet another giant of research – Ernest Starling, perhaps best known for his eponymous ‘law of the heart’. Starling also showed that secretin stimulates secretion from the pancreas, and was the first to use the term ‘hormone’. Among Katz’s many collaborations was with Andrew Huxley, who joined UCL in 1960. With Alan Hodgkin, Huxley was responsible for one of the most outstanding scientific discoveries of the past century. Working primarily with the squid giant axon, and combining experimental studies with theoretical work, they not only recorded action potentials but also generated computational models to explain their origins. Their studies led them to propose the existence of ion channels, years before they were actually isolated. As well as receiving a Nobel Prize in 1963 (with Hodgkin and John Carew Eccles), Huxley served as President of the Royal Society and received many other plaudits. Huxley and Hodgkin’s seminal contributions owed a sizeable debt to John Zachary (J Z) Young, who discovered and pioneered work on the squid giant axon. Although he never received the Nobel Prize, he was awarded the Linnean Society’s Gold Medal and delivered the BBC’s 1950 Reith lectures (on ‘doubt and certainty in science’). One of J Z Young’s early BASIC LIFE SCIENCES UCL School of Life and Medical Sciences collaborators was a young Peter Medawar, who spent most of the 1950s at UCL before becoming Director of the National Institute for Medical Research at Mill Hill in 1962. Another Nobel Prize winner (in 1960), Medawar is perhaps as well known for the exceptional quality of his writing as for his landmark studies in immunological tolerance and transplantation. In Darwin’s footsteps One of the most colourful figures in 20th-century science, J B S Haldane, spent the bulk of his career at UCL. With R A Fisher (who was also at UCL for almost a decade from 1933) and the American Sewall Wright, Haldane established the discipline of population genetics and played a central role in the ‘modern synthesis’, which reconciled the principles of Darwinian evolution and natural selection with the genetics of Mendel. Fisher had the insight to realise that continuous variation could be seen as the result of many individual genes of small effect, and was therefore compatible with Mendelian inheritance. Natural selection could change the frequency of alleles of these genes, leading to evolution. Haldane developed mathematical models of this process. He also analysed reallife examples of natural selection, including the famous example of industrial melanism in peppered moths in the North of England. Both figures fall within a broader historical context, in which the figure of Francis Galton casts a long shadow. Galton, half-cousin to Charles Darwin, was an old-fashioned polymath. He is perhaps best known for inventing the term ‘eugenics’ (as well ‘nature versus nurture’) and brought his considerable statistical expertise to bear in studies of heredity. On his death, he bequeathed funds to set up the Galton Laboratory and a Chair of Eugenics, a position first held by his protégé Karl Pearson. Another committed eugenicist, Pearson made AN INORDINATE FONDNESS FOR BEETLES J B S Haldane is almost as well known for his colourful turn of phrase as his landmark scientific studies. Among his most notable aphorisms concerned unconventional ideas in science: “Theories have four stages of acceptance. (1) This is worthless nonsense; (2) this is an interesting, but perverse, point of view; (3) this is true, but quite unimportant; (4) I always said so.” He also had a habit of conducting experiments on himself, ending up with crushed vertebrae and perforated eardrums, to which he responded: “The drum generally heals up; and if a hole remains in it, although one is somewhat deaf, one can blow tobacco smoke out of the ear in question, which is a social accomplishment.” It is disputed whether Haldane coined the immortal line, “An inordinate fondness for beetles” when asked what studies of evolution had told him about God. But it is often attributed to him and he frequently used the phrase and variants of it. enormous contributions to statistical methodology, and many of the tools used in science today have their roots in his work, from P-values to principal component analysis. He also pioneered mathematical approaches to the study of evolution, leading the ‘biometric’ school from which arose the statistical analyses underpinning the modern synthesis. Pearson was succeeded by R A Fisher, who in turn passed the baton on to Lionel Penrose. Penrose, a humane and enlightened researcher, was among the first to study the biological and genetic basis of mental retardation. He received a Lasker Award in 1960. A UCL contemporary of Karl Pearson’s was Charles Spearman (though the two did not get on), another who left a lasting impression on statistics. As well as his work on correlation, he is known for his work on general intelligence (or ‘g’). Figures like Haldane, Pearson and Penrose pioneered the application of genetics to human traits, including medical conditions. More generally, Haldane and Fisher were both important influences on the evolutionary theories developed by W D Hamilton. A knotty issue in evolution has always been how natural selection can lead to the appearance of altruistic behaviours that impose a cost on an individual but benefit others. Hamilton proposed that the key issue was relatedness, as helping kin would indirectly promote the propagation of genes. This line of thinking contributed significantly to the growth of ‘socio-biology’ as well as gene-centric ways of viewing evolution – popularised in Richard Dawkin’s landmark book The Selfish Gene. Hamilton was also an influence on John Maynard Smith. A student of Haldane, Maynard Smith converted from aeronautical engineer to evolutionary theorist. He made wide-ranging contributions, including influential early work on lifespan and ageing, the evolution of sex, and the application of game theory to evolution. His notion of the ‘evolutionarily stable strategy’ is a central pillar of modern thinking about animal behaviour. Below left: Karl Pearson. Below right: A demonstration by Professor Sir William Bayliss, with Professor Earnest Starling and Sir Henry Dale to the left. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 11 SECTION 2 THE CELLULAR WORLD Cells are the building blocks of life, and understanding the fundamental principles of cell biology will help to pinpoint key changes that lead to disease. Neurons obtained from embryonic stem cells. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 12 Developing vasculature in the retina. The structures of the cell, and much of its biochemistry, have been well described and fill many textbooks. Yet how they act together to guide the fate of the cell often remains mysterious. Visualising mitochondrial activity Mitochondria generate most of the energy on which cellular life depends. Given this critical role, it is not surprising that abnormalities in mitochondrial function have been implicated in numerous conditions, from diabetes to Parkinson’s and Alzheimer’s disease. As befits this central role in cell biology, mitochondria specialist Professor Michael Duchen collaborates extensively across UCL, investigating mitochondrial function in numerous disease states. A variety of imaging techniques reveal dynamic changes in the concentrations of key metabolites and calcium signals that regulate mitochondrial function. A variety of imaging techniques reveal dynamic changes in the concentrations of key metabolites and calcium signals that regulate mitochondrial function. Recent years have seen a growing realisation that mitochondrial dysfunction is central to Parkinson’s disease, where several genes influencing the risk of the disease affect mitochondria-related proteins6. In Alzheimer’s disease, Professor Duchen has generated intriguing findings on the possible role of -amyloid in the death of neurons (see page 14). In other collaborations, Professor Duchen has worked with cardiovascular researchers Professor Derek Yellon, Dr Derek Hausenloy and Dr Sean Davidson on the response of mitochondria to impaired oxygen supply (ischaemia and reperfusion). Much of this work focuses on the ‘mitochondrial permeability transition pore’. High levels of calcium in the mitochondrion trigger opening of this pore, leading to an outflow of ATP and ultimately death of the cell. Inhibiting pore opening limits the impact of reperfusion injury after ischaemia. It has been implicated in the mechanisms of preconditioning7 – restricting oxygen supply briefly to protect the heart from later ischaemia (see companion volume on Translation and Experimental Medicine). Mitochondria have also been implicated in kidney conditions. Interestingly, the imaging work of PhD student and nephrologist Andrew Hall has revealed that mitochondrial activity varies 6 Gandhi S et al. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33(5):627–38. 7 Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioninginduced protection. Circulation. 2004;109(14):1714–7. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 13 The fission yeast, Schizosaccharomyces pombe. Imaging of NADH in a cochlea explant culture. WHO’S BINDING WHO? DEATH OF A NEURON Precisely timed protein degradation is vital to cell division, but how does the critical protein-degrading complex know what to digest and when? Death of neurons in Alzheimer’s disease may result from loss of essential support from neighbouring cells. The anaphase-promoting complex (APC) plays a fundamental role in cell division. By attaching multiple ubiquitin tags, it marks key proteins such as cyclins for destruction at specific points in the cell cycle. Regulation of APC is thus crucial. As well as timing, regulation also has to address substrate specificity: how does APC know what to digest? Professor Hiro Yamano and colleagues have recently shed important light on these questions. To operate, APC relies on additional accessory proteins – Cdc20/Fizzy at early stages of cell division and Cdh1/Fizzyrelated at later stages. It was originally thought that these accessory proteins were required to activate APC, but their ability to bind APC substrates led to the idea that they were recruitment factors, delivering proteins to APC for ubiquitin tagging and then degradation. This view took a hit in 2006, when Professor Yamano and Professor Andrew Fry at the University of Leicester found that one APC substrate, Nek2A, could bind directly to APC even in the absence of Cdc20/Fizzy. This direct binding depended on a short sequence motif in the C-terminal tail of Nek2A. Crucially, this independent binding also enabled Professor Yamano to look separately at recruitment and ubiquitin tagging. Surprisingly, the results revealed that Cdc20/Fizzy was an APC activator after all. In the absence of Cdc20/Fizzy, Nek2A can bind to APC but it is not tagged with ubiquitin unless an N-terminal fragment of Cdc20/Fizzy is also present. Notably, though, this region of Cdc20/Fizzy is not the one required for it to bind and recruit other substrates. Hence there is a previously unsuspected interaction between Cdc20 and APC, crucial for ubiquitin addition, that requires specific sequences in the N-terminal region of Cdc20. As well as the insight into a process at the heart of cell division, the work may also open up a route to new therapeutic interventions. It may be possible to target this new point of interaction between Cdc20 and APC and specifically arrest the division of dividing cancer cells. Professor Yamano is now aiming to pinpoint the site on APC binding to the N-terminal region of Cdc20, with a view to screening for small-molecule inhibitors that could block the interaction. Hayes MJ et al. Early mitotic degradation of Nek2A depends on Cdc20independent interaction with the APC/C. Nat Cell Biol. 2006;8(6):607–14. Kimata Y, Baxter JE, Fry AM, Yamano H. A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol Cell. 2008;32(4):576–83. Alzheimer’s disease is characterised by the presence in the brain of protein tangles and plaques, the latter composed of fragments of -amyloid protein. Although its role in disease is controversial, -amyloid is toxic to neurons in culture. Yet, suggests the research of Professor Michael Duchen and colleagues, its impact on neurons may be an indirect consequence of its effects on glia, the cells that provide neurons with essential metabolic support. Professor Duchen’s research focuses primarily on mitochondria, damage to which can trigger cell death. But how might -amyloid affect the function of mitochondria? Over the past decade, the research of Professor Duchen and Dr Andrey Abramov has revealed a possible route – which, although complex, offers the enticing prospect of novel remedies. Professor Duchen’s principal tool has been co-culture systems of neurons and astrocytes (glial cells). If -amyloid is added to these cultures, neurons die. However, close examination revealed a curious phenomenon. In the first few hours after addition of -amyloid, it was astrocytes that showed a response rather than neurons. Perhaps then the primary problem was in astrocytes, which were prevented from providing essential support to neurons. Delving deeper, Professor Duchen identified a long slow loss of mitochondrial membrane potential in astrocytes as a key mediator of -amyloid’s effects. The picture emerging from several years’ work is that oxidative stress activates a DNA repair enzyme, PARP, which exhausts the supply of a key metabolite required for glycolysis. The failure of glycolysis depletes the substrates required by mitochondria, which fail – starving in the midst of plenty. Other factors also influence cell death through this pathway. For example, there is some evidence that the cholesterol content of the plasma membrane determines -amyloid’s toxicity. Indeed, Professor Duchen found that membrane cholesterol levels are significantly higher in astrocytes than neurons. Cholesterol enhances the ability of -amyloid to form pores in the plasma membrane, enhancing calcium influx that eventually cripples mitochondria. The identification of this pathway has revealed a new set of targets for intervention. Remarkably, in cultured cells, adding substrate for mitochondrial respiration protects astrocytes from -amyloid toxicity. Professor Duchen is now exploring the possibility of screening small-chemical inhibitors to target other points in the pathway as a route to new treatments for Alzheimer’s disease. Abramov AY, Canevari L, Duchen MR. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J Neurosci. 2003;23:5088–95. Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24(2):565–75. Abeti R, Abramov AY, Duchen MR. Beta-amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain. 2011;134(Pt 6):1658–72. Abramov AY, Ionov M, Pavlov E, Duchen MR. Membrane cholesterol content plays a key role in the neurotoxicity of -amyloid: implications for Alzheimer’s disease. Aging Cell. 2011;10(4):595–603. 14 BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 8 Hall AM, Unwin RJ, Parker N, Duchen MR. Multiphoton imaging reveals differences in mitochondrial function between nephron segments. J Am Soc Nephrol. 2009;20(6):1293–302. 9 Cantley J et al. Deletion of the von Hippel-Lindau gene in pancreatic beta cells impairs glucose homeostasis in mice. J Clin Invest. 2009;119(1):125–35. 10 El-Kadi AM et al. The legs at odd angles (Loa) mutation in cytoplasmic dynein ameliorates mitochondrial function in SOD1G93A mouse model for motor neuron disease. J Biol Chem. 2010;285(24):18627–39. 11 Campanella M et al. Regulation of mitochondrial structure and function by the F1Fo-ATPase inhibitor protein, IF1. Cell Metab. 2008;8(1):13–25. 12 Izawa D et al. Fission yeast Mes1p ensures the onset of meiosis II by blocking degradation of cyclin Cdc13p. Nature. 2005;434(7032):529–33. 13 Kimata Y et al. A mutual inhibition between APC/C and its substrate Mes1 required for meiotic progression in fission yeast. Dev Cell. 2008;14(3): 446–54. 14 Kimata Y, Kitamura K, Fenner N, Yamano H. Mes1 controls the meiosis I to meiosis II transition by distinctly regulating the anaphase-promoting complex/cyclosome coactivators Fzr1/ Mfr1 and Slp1 in fission yeast. Mol Biol Cell. 2011;22(9):1486–94. Fibroblast cells stained to show mitochondria (red: alive; green: dead or alive). markedly along a nephron, with the mitochondrial membrane potential being markedly higher in distal than proximal tubules8. Other studies have examined the role of mitochondria in betacell function and diabetes9, survival in intensive care, and neuromuscular conditions such as motor neuron disease10. A relatively new area of interest is the inhibitor protein IF1, which regulates activity of the mitochondrial ATP-generating machine, the F0F1 ATP synthase11. Although its main role is to generate ATP, powered by the flow of hydrogen ions, this enzyme complex can operate in both directions, breaking down ATP when it spins in reverse. IF1 appears to act as a brake on this reverse reaction, helping to ensure that ATP levels are not depleted. As yet, little is known about IF1, but its regulation of such a key aspect of mitochondrial function suggests it could play a major role in mitochondrial biology and disease processes. Cycle of life Control of the cell cycle and cancer is the principal interest of Professor Hiro Yamano, who joined the UCL Cancer Institute in 2010. He uses a popular tool in cell cycle research, the fission yeast Schizosaccharomyces pombe, which has a wellcharacterised cell cycle and is highly amenable to genetic dissection. Unusually, Professor Yamano complements work in fission yeast with biochemical studies in Xenopus oocyte extracts. The high degree of conservation of cell cycle mechanisms ensures that findings in one system can be related to those in the other. Professor Yamano’s main interest is APC/C (anaphasepromoting complex/ cyclosome), which attaches ubiquitin residues to key cell cycle proteins, targeting them for degradation. Cells, even cancer cells, arrest in mitosis if APC/C activity is blocked. Regulation of APC/C is thus of intense interest, and Professor Yamano has provided important insight into the mechanisms controlling APC/C activity and substrate specificity (see page 14). An additional interest is meiosis, the gamete-forming mode of cell division, which is more complex than mitosis as additional steps are needed to reduce chromosome number. This calls for additional layers of regulation of APC/C. In 2005, with Professor Masayuki Yamamoto in Tokyo, Professor Yamano showed that Mes1 protein was required to prevent the complete destruction of cyclins at the transition between the two main stages of meiosis, MI and MII12. Although cyclins do need to be degraded at this point, unlike mitosis, some residual cyclin needs to be spared for later stages to progress efficiently. In fact, Mes1 protein acts in a very interesting way. As well as being an inhibitor of APC/C, it is also a substrate. This creates a self-regulatory loop which ensures that APC/C is not entirely inactivated during the first phase of meiosis13. Most recently, Professor Yamano has uncovered a further layer of regulation of APC/C by Mes114. Safeguarding the oocyte Professor John Carroll’s cell of interest is the oocyte. Understanding how the oocyte is formed, and what happens after fertilisation, is not just of academic interest. Around one in seven couples experience difficulty in conceiving, often because of problems with eggs. A trend towards later conception is also focusing attention of the long-term fate of eggs in the ovary. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 15 used to drive the ion currents that maintain membrane potentials and hence the ability of nerves to generate action potentials. Differentiation of an embryonic stem cell. Professor Carroll has also found interesting ways in which meiosis differs from mitosis (see page 17). He is also keen to explore some of the long-term factors affecting the oocyte in the ovary. One key issue is DNA damage and repair – how does the oocyte maintain the integrity of its genome, so vital in cells that will give rise to entire new organisms, over such long periods? In addition, it is becoming clear that there is extensive cross-talk between the oocyte and its surrounding microenvironment in the ovary, and this has the potential to affect offspring many years after birth. Although the notion of ‘programming’ has tended to consider the impact of a mother’s physiology of a developing fetus, there is also evidence that maternal signals can also affect oocytes. Professor Carroll now splits his time between Monash University in Melbourne and UCL, where his colleagues Dr Hayden Homer and Dr Greg Fitzharris continue the work. Form and function Cells differ markedly in form, depending on their role in the body. Indeed, some possess entirely novel organelles – such as the Weibel–Palade bodies studied by Professor 16 Dan Cutler. These large cigar-shaped organelles, found in endothelial cells lining blood vessels, are packed full of von Willebrand factor, a polymeric protein important in blood clotting (see page 17). Weibel–Palade bodies also have a role in inflammation. This depends in particular on the presence in their membrane of a cell adhesion molecule, P-selectin. When the Weibel–Palade bodies fuse with the outer cell membrane, P-selectin is exposed to the bloodstream and ‘snags’ circulating leukocytes, causing them to begin a characteristic rolling along the vessel wall. Other cell adhesion molecules strengthen the binding and enable leukocytes to begin burrowing through the vessel wall into surrounding tissues. Professor Cutler has discovered that initial leukocyte recruitment by P-selectin also requires at least one other endothelial protein, CD63 – a well-known component of Weibel– Palade bodies of previously unknown function15. Loss of CD63 dramatically reduced leukocyte rolling and invasion of surrounding tissue. CD63 is thus a potentially exciting new target for anti-inflammatory drug development – being explored with the support of MRC Technology. Nerve cells are among the most specialised cells in the body. Structurally, they are highly modified, with long extensions that, in humans, can stretch up to a metre (or a stunning five metres in the giraffe). Hence the business end of a neuron, the synapse, can be a considerable distance from the control centre, the nucleus in the cell body. As Dr Josef Kittler has demonstrated, these specialisations are accompanied by distinct intracellular trafficking signalling systems, as well as a considerable degree of local autonomy. Local independence is important because synapses are highly dynamic structures that respond rapidly to incoming signals – more rapidly than is possible through signalling to the nucleus and changes in gene expression. One system allowing for rapid modulation of synapse behaviour is altered recycling of neurotransmitter receptors to and from the cell surface (see page 18). Dr Kittler is also interested in the trafficking of much larger structures, including organelles such as mitochondria. Although accounting for only around 2 per cent of body mass, the brain consumes 20 per cent of the body’s energy. This massive energy use is mainly BASIC LIFE SCIENCES UCL School of Life and Medical Sciences Most of a cell’s energy is generated by mitochondria. It turns out that entire mitochondria can be shipped to parts of the cell with high energy demands. Although it was known that mitochondrial recruitment was triggered by calcium influx, little was known about the mechanisms involved. Dr Kittler recently discovered that a protein with the artistic name Miro plays a critical role, tethering mitochondria to the molecular motors that transport material along the cell’s microtubule highways16. Crucially, binding of Miro to the molecular motors is inhibited by high calcium levels, causing mitochondria to detach once they have reached a point in the cell generating high calcium currents. As well as generating insight into basic mechanisms, Dr Kittler’s group is also exploring the potential role of these processes in disease. For example, neurotransmitter receptor trafficking has been found to be affected by huntingtin, the product of the mutated gene causing Huntington’s disease17. Impaired inhibition of neural function, because fewer receptors for inhibitory neurotransmitters are present at the synapse, could contribute to the motor and cognitive problems associated with Huntington’s disease. Dr Kittler is also collaborating with neurologists to explore 15 Doyle EL et al. CD63 is an essential cofactor to leukocyte recruitment by endothelial P-selectin. Blood. 2011;118(15):4265–73. 16 Macaskill AF et al. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61(4):541–55. 17 Twelvetrees AE et al. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65(1):53–65. Spindle fibres (red) in a maturing oocyte. Weibel–Palade bodies (blue). EGG TIMING BODY OF EVIDENCE An understanding of the molecular processes controlling oocyte development may provide insight into the origins of impaired fertility. Weibel–Palade bodies may not be the best known of organelles but they play a critical role in blood clotting. Unlike sperm, which are constantly generated throughout life, all oocytes are present in the ovary at birth. They are maintained in an immature state, part way through meiosis, until puberty, when the machinery of cell division is switched back on and oocytes mature in readiness for fertilisation. Working with mice oocytes, Professor John Carroll has identified several key ways in which these processes are controlled. Control of cell division, both mitosis and meiosis, is critically dependent on the so-called anaphase-promoting complex (APC). APC degrades cyclins, the key inhibitory proteins that stop a cell dividing. APC also breaks down an entirely different protein, securin, as part of a programme that enables chromosome to separate. In fact, Professor Carroll has discovered, these activities are closely connected, as securin competes with cyclins for APC. With excess securin, cyclins are not degraded effectively and cell cycle progression is stalled. APC has a further role, being required to hold oocytes in their early arrested state. While exploring factors influencing APC activity, Professor Carroll found that depletion of one particular protein, BubR1, unexpectedly led a proportion of embryos to slip off their early (prophase) arrest and re-enter meiosis. Follow-up work revealed that BubR1 acts through an APC co-factor, Cdh, which normally activates APC and maintains cell cycle arrest. However, while BubR1-deficient oocytes were able to reenter prophase, they never completed the first phase of meiosis, becoming stalled before the transition to anaphase. The diminished activity of APC leads to increased securin levels, which interfere with APC activity at the end of metaphase. Even when securin levels were reduced, however, BubR1deficient oocytes did not fully recover their ability to enter anaphase. It appears that loss of BubR1 has additional effects on chromosome attachment to the meiotic spindle, preventing cell division from proceeding even in the presence of functional APC. The results point to an early role for BubR1, mediated through Cdh1, to maintain prophase arrest, with securin an important target of active APC. Later, BubR1 has a separate role as part of the machinery that keeps APC in check until the cell is ready to enter anaphase. The findings reveal significant differences between early events in mitosis and meiosis, highlighting the importance of Cdh1 and securin in the latter. From a practical point of view, BubR1’s critical role in both prophase arrest and metaphase progression makes BubR deficiency a potentially significant factor in reduced fertility, because the reservoir of arrested oocytes is depleted or fewer oocytes become fertilisable eggs. The inner surface of blood vessels is lined with endothelial cells. Among their most notable features is the presence of strange cigarshaped organelles – known as Weibel–Palade bodies in honour of their discoverers, Ewald Weibel and George Palade (recipient of a Nobel Prize in 1974). Despite being important in both clotting and inflammation, Weibel–Palade bodies have been relatively neglected – an oversight Professor Dan Cutler has attempted to correct. One reason Weibel–Palade bodies are important is because they are the storage containers of von Willebrand factor, a crucial component of the blood clotting system. When blood vessels are damaged, von Willebrand factor is released as a long polymeric fibre to which platelets adhere. Abnormalities in von Willebrand factor function are actually the most common form of inherited blood coagulation disorder. Coordinated storage and release of von Willebrand factor is thus highly significant in its own right, as well as providing more general insight into cell trafficking and exocytosis. For von Willebrand factor and its storage compartment, form and function are highly correlated. Within the Weibel–Palade bodies, polymeric filaments are folded into organised tubular structures that shape their storage compartment into its characteristic shape. If this organisation is disrupted inside the cell, Weibel–Palade bodies become spheres containing multimers and shortened fibres that fail to recruit platelets efficiently. Once formed, Weibel–Palade bodies are trafficked through the cell and held at the periphery of the cell ready for secretion. This holding phase depends on Rab27a, a member of the Rab family of GTPases, and its effectors, including MyRIP and a member of the myosin family of proteins (myosin Va). These proteins anchor the bodies to actin fibres, and prevent release of immature von Willebrand factor polymers. Actin fibres also turn out to be critical to the exocytosis of Weibel– Palade bodies. Actin has long been known to play a role in secretion, but exact mechanisms have been difficult to unpick. By combining video and electron microscopy, Professor Cutler has been able to gain an unusually detailed view in time and space of individual exocytotic events. Filamentous actin initially anchors Weibel– Palade bodies and inhibits fusion with the plasma membrane, but following endothelial activation and just after fusion with the plasma membrane, a contractile actomyosin ring forms on the organelles. This ring appears to actively squeeze mature von Willebrand factor out of the cell. Michaux G et al. The physiological function of von Willebrand’s factor depends on its tubular storage in endothelial Weibel-Palade bodies. Dev Cell. 2006;10(2):223–32. Marangos P, Carroll J. Securin regulates entry into M-phase by modulating the stability of cyclin B. Nature Cell Biol. 2008;10(4):445–51. Nightingale TD, Pattni K, Hume AN, Seabra MC, Cutler DF. Rab27a and MyRIP regulate the amount and multimeric state of VWF released from endothelial cells. Blood. 2009;113(20):5010–8. Homer H, Gui L, Carroll J. A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science. 2009;326(5955):991–4. Nightingale TD et al. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J Cell Biol. 2011;194(4): 613–29. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 17 Presynaptic axons (red) and postsynaptic dendrites (green). Professor Philip Beales. COME TOGETHER FROM DEFENCE TO MIGRATION Regulating receptor numbers at the synapse is an important way of controlling neural activity. Two proteins known only for their roles in the complement system have turned out to have critical roles in embryogenesis. Synapses are highly dynamic structures. Their activity is modulated in numerous ways, even changing in response to the electrical signals they transmit. This ‘plasticity’ underpins many key aspects of brain function, including learning and memory. One way in which plasticity is achieved is by fine control of the numbers of neurotransmitter receptors present at the synapse, and Dr Josef Kittler and colleagues have uncovered some of the cellular mechanisms by which this achieved – and also how it can go awry in disease. Dr Kittler is particularly interested in the receptors for inhibitory neurotransmitters such as GABA (gamma-aminobutyric acid). These neurotransmitters act in opposition to excitatory neurotransmitters, and prevent excessive neural activation. The degree of inhibition mediated by GABA is known to depend on the numbers of GABA receptors present at the synapse. Hence, control of receptor numbers could provide a way to regulate the strength of inhibitory input to a neuron. Indeed, using high-definition imaging and other techniques, Dr Kittler has begun to identify the mechanisms by which GABA receptor numbers are controlled at the synapse. With Professor Stephen Moss, for example, he has shown that receptor numbers are regulated by recycling through endosomal pathways. These studies have revealed key regions of the receptor necessary for sorting, and that dictate whether receptors are tagged by ubiquitin for degradation or are trafficked back to the cell surface. More recently, he has teamed up with Dr Lewis Griffin in UCL’s Department of Computing, tracking individual receptors tagged with tiny but highly fluorescent markers (quantum dots). These studies have revealed that an excitatory stimulus leads to the break up of clusters of GABA receptors in the neuronal membrane. They have also revealed key biochemical changes linked to the break up of receptor clusters. The work on GABA receptor trafficking has significant medical potential. Loss of GABA receptors from synapses has been seen in several conditions, from epilepsy to neurodegenerative conditions. Indeed, in Huntington’s disease, Professor Kittler’s group has found that the abnormal protein underlying the condition, huntingtin, disrupts the intracellular delivery of GABA receptors to the synapse. Hence a better understanding of the molecular mechanisms controlling GABA receptor distribution promises to identify potential targets for a range of important medical conditions. Kittler JT et al. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit. Proc Natl Acad Sci USA. 2008;105(9):3616–21. Arancibia-Cárcamo IL et al. Ubiquitin-dependent lysosomal targeting of GABA(A) receptors regulates neuronal inhibition. Proc Natl Acad Sci USA. 2009;106(41):17552–7. Muir J et al. NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the 2 subunit. Proc Natl Acad Sci USA. 2010;107(38):16679–84. 18 BASIC LIFE SCIENCES UCL School of Life and Medical Sciences Because of the links between gene loss and symptoms, rare inherited diseases often provide insight into developmental processes. Recently, a highly unusual group of patients studied by Professor Philip Beales and colleagues has for the first time implicated proteins of the complement defence system in critical cellular events in early development. The work centred on 11 families affected by four extremely rare inherited conditions – Carnevale, Mingarelli, Malpuech and Michels syndromes, which have been seen in just 20 families worldwide. The overlapping constellation of symptoms in the four syndromes – characteristics facial abnormalities, cleft lip and palate, learning difficulties and other clinical features – has led to suggestions that they share underlying disease mechanisms. Regions of homozygosity shared by affected family members, and in different families, pointed to a section of chromosome 2 as the likely cause of the abnormalities. Sequencing of genes in this region revealed mutations in the COLEC11 gene in affected individuals. COLEC11 is not an obvious candidate for a developmental disease gene, as it codes for a component of the complement system, part of the body’s protection against infection. Its protein product is a member of the C-type lectin family, consisting of a collagen-like domain and a carbohydrate-binding domain. Elimination of COLEC11 function in zebrafish, however, confirmed its essential role in development. In its absence, zebrafish developed numerous defects, including striking abnormalities in craniofacial morphology. Although COLEC11 was normal in some families, homozygosity mapping in other families identified another potentially important region on chromosome 3. And within this region was another complement system gene, MASP1. Mutations in this gene were found in two families. In zebrafish, loss of MASP1 generated craniofacial abnormalities similar to those seen in fish lacking COLEC11. These striking effects, and associated pigment abnormalities, suggested that the genes were affecting the migration of neural crest cells, a finely controlled process required to sculpt the complex structures of the face. Indeed, studies in fish and cultured cells suggested that the proteins act as chemo-attractant guidance cues for migrating neural crest cells. The results suggest that the four syndromes are related, and should be grouped under the common label of M3C syndrome. Complement-related proteins have never been implicated in developmental conditions before, and it will be interesting to see if others are involved in developmental processes or inherited conditions. Rooryck C et al. Mutations in lectin complement pathway genes COLEC11 and MASP1 cause 3MC syndrome. Nat Genet. 2011;43(3):197–203. the potential involvement of such processes in epilepsy, which is characterised by excessive neural activity. A further strand of work is examining whether factors found to increase the risk of neuropsychiatric conditions, for example in genome-wide screens, affect trafficking in neurons. Early work on a protein implicated in schizophrenia (DISC1), for example, has revealed deficits in mitochondrial trafficking18. Cilia and ciliopathies In 1995, during his medical training, Professor Philip Beales encountered a patient with diabetes and an unusual mix of additional symptoms. Professor Beales suspected he knew what the problem was, and a later check of his textbooks confirmed his suspicions. The man had the rare genetic condition Bardet– Biedl syndrome. Since then Professor Beales has helped to identify several genetic causes of Bardet–Biedl syndrome and characterised their effects. Indeed, he has become a world authority on the large class of diseases which, like Bardet–Biedl syndrome, are associated with defective cilia. Cilia are perhaps best known for their ability to beat and create fluid currents across the surface of cells. But they also have an important non-motile role as cellular ‘antennae’, detecting and transmitting external signals. This function is particularly important during embryogenesis, when cells receive signals that control their developmental fate. Working with Dr Nicholas Katsanis in the USA and others, Professor Beales has done much to piece together the origins of Bardet–Biedl syndrome, which is characterised by a range of symptoms including progressive blindness, kidney problems, obesity, additional digits (polydactyly) and learning difficulties. By the late 1990s, several genes were known to be involved in the condition, but isolating them and working out what they did proved challenging. A significant breakthrough came in 2003, when Professor Beales and Dr Katsanis discovered that a new Bardet–Biedl syndrome gene, BBS8, had many of the hallmarks of a cilia protein19. The work thrust cilia firmly into the Bardet– Biedl syndrome spotlight. With Dr Katsanis and others, Professor Beales Elongated mitochondria in a cardiac cell line. went on to characterise other BBS genes affecting cilia structure and function. Furthermore, defective cilia also turned up in a range of other conditions, collectively referred to as ‘ciliopathies’. The realisation that cilia dysfunction could be a cause of congenital conditions, and characterisation of the protein components of cilia, provided a pool of possible candidate genes for ciliopathies. This led to the identification of another new gene, BBS520. It also helped Professor Beales identify mutations affecting IFT80, a protein involved in transport of material along the cilium, as a cause of juvenile asphyxiating thoracic dystrophy, where infants are born with an underdeveloped ribcage and other skeletal abnormalities21. Identification of faulty genes also enables their function to be studied in animal models, shedding new light on cilia function and their role in developmental processes. For example, they have turned out to be critical in establishing the characteristic asymmetry of human organ systems. Bardet–Biedl syndrome, for example, is often accompanied by a condition known as situs inversus, in which the normal asymmetry is reversed (a condition seen in Professor Beales’s original patient). Cell migration is also often affected, giving rise to characteristic craniofacial abnormalities, as seen in both Bardet–Biedl syndrome and a related condition, Hirchsprung’s disease22. In terms of cell biology, mutations have been found to affect at least two important cell signalling pathways – the hedgehog and Wnt 23 pathways. 18 Atkin TA, MacAskill AF, Brandon NJ, Kittler JT. Disrupted in Schizophrenia-1 regulates intracellular trafficking of mitochondria in neurons. Mol Psychiatry. 2011;16(2):122–4, 121. 19 Ansley SJ et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425(6958):628–33. 20 Li JB et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117(4):541–52. 21 Beales PL et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39(6):727–9. 22 Tobin JL et al. Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung’s disease in Bardet-Biedl syndrome. Proc Natl Acad Sci USA. 2008; 105(18):6714–9. 23 Gerdes JM et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39(11): 1350–60. A section through the retina. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 19 the adult, with cells of the right type ending up at the right place at the right time. Recently, however, there has been growing interest in analogous processes acting in adults. These exciting developments are highly relevant to repair and regeneration but also to cancer. The research of Professor Alison Lloyd centres on Schwann cells, which form the insulating myelin sheath around peripheral nerves. They have the curious property of being able to ‘dedifferentiate’ into a progenitor state after nerve damage, and then develop back into Schwann cells as nerves regrow (see page 21). Neural stem cells from the mouse hippocampus. Although Bardet–Biedl syndrome is a developmental condition, an understanding of its genetic basis opens up the prospect of interventions to ameliorate symptoms. For example, blindness is progressive, beginning in early adulthood, and is caused by gradual loss of photoreceptor cells (which are essentially highly specialised cilia). It may therefore be possible to intervene early in the process, to slow photoreceptor degeneration and preserve sight for longer. Size is important A central theme in cell biology, being addressed by Professor Buzz Baum, is how cells regulate their physical size and appearance. Here again cytoskeletal components play pivotal roles. One important but oddly neglected question is how cell size is regulated. Use of Drosophila cell cultures and RNA interference has revealed key signalling mechanisms involved in this process24. More recently, Professor Baum has worked with nanotechnologists to 20 explore the growth of single cells along narrow channels, limiting cell growth to one dimension. Cells grew to a characteristic ‘steady state’ length, governed by microtubule dynamics25. By contrast, other work on polarisation – specialisation of different ends of a cell – has implicated actin-based mechanisms. Columnar epithelial cells are ideal for studying such processes. They consist of sheets of identical cells whose basal ends show extensive protrusions. A combination of genetics and cell biology has revealed that this patterning depends on the creation of a gradient within the cell, which inhibits actin polymerisation except at the basal end of the cell26. Professor Baum has also explored how cellular interactions generate the spatial arrangement of bristles on the surface of Drosophila – a widely studied model of pattern formation. Working with computational biologists through the CoMPLEX initiative (see page 22), he has found that bristle patterning can be explained by simple but dynamic interactions between two signalling molecules on epithelial cells. Bristle formation is known to depend on signalling between Delta, present in membranes, and intracellular Notch in surrounding cells. As Notch then inhibits Delta expression, a pattern emerges in which Deltaexpressing cells are surrounded by a doughnut of Notch-expressing cells. However, these processes alone cannot explain regular bristle patterning. Professor Baum’s alternative model introduced cell dynamics, with cell protrusions making and breaking contacts between neighbouring cells, introducing ‘noise’ into the system. Live imaging confirmed dynamic interplay of cell protrusions27, while computational modelling revealed how noise could promote the appearance of regular structures28. Fateful decisions Developmental biology has long sought to elucidate the seemingly miraculous process by which a single cell, the fertilised egg, can generate all the cells of BASIC LIFE SCIENCES UCL School of Life and Medical Sciences Controlled differentiation may also be important in tissue repair. Professor Robin Ali, Dr Rachael Pearson and colleagues for example, have identified which particular photoreceptor precursor cells are best suited to repair of the degenerating retina (see page 21). 24 Sims D, Duchek P, Baum B. PDGF/VEGF signaling controls cell size in Drosophila. Genome Biol. 200912;10(2):R20. 25 Picone R et al. A polarised population of dynamic microtubules mediates homeostatic length control in animal cells. PLoS Biol. 2010;8(11):e1000542. 26 Georgiou M, Baum B. Polarity proteins and Rho GTPases cooperate to spatially organise epithelial actin-based protrusions. J Cell Sci. 2010;123(7):1089–98. 27 Cohen M, Georgiou M, Stevenson NL, Miodownik M, Baum B. Dynamic filopodia transmit intermittent DeltaNotch signaling to drive pattern refinement during lateral inhibition. Dev Cell. 2010;19(1):78–89. 28 Cohen M, Baum B, Miodownik M. The importance of structured noise in the generation of self-organizing tissue patterns through contact-mediated cell-cell signalling. J R Soc Interface. 2011;8(59):787–98. A transplanted cell making synaptic contact with a neuron. Interactions between Schwann cells (blue) and axons (green). AN EYE CELL FOR AN EYE THE ROUTE TO NERVE REPAIR Cell transplantation may be able to repair a degenerating retina. The striking regenerative powers of peripheral nerves depend on a complex cellular conversation. Progressive loss of retinal cells, a hallmark of several forms of vision impairment, has long been seen as amenable to cell transplantation therapy. The retina is relatively accessible and many inherited forms of degeneration typically affect just photoreceptor cells, leaving the other components of the visual system intact. Working with mice, Dr Rachael Pearson, Professor Robin Ali and colleagues have taken an important step towards demonstrating the feasibility of the cell transplantation strategy. Early attempts to replace degenerating rods, using stem cells, met with little success. Although cells survived, they typically integrated poorly into the retina and rarely differentiated into rods. Dr Pearson and colleagues therefore turned to more mature cells, already destined to become photoreceptors, called photoreceptor precursor cells. By labelling cells with green fluorescent protein, they were able to show that cells integrated into the retina of adult mice and took on the characteristic anatomy of rod cells. Even more impressively, transplanted cells also integrated into the retinas of models of inherited retinal degeneration, and in similar numbers (several hundred cells per eye). Most encouragingly, electrophysiological measurements and assessment of pupil responses implied that the new cells were functional and integrated into surviving neural circuitry. The key question, of course, is whether the therapy actually improves vision – and recent results suggest it can. Again working with a mouse model of retinal degeneration, the group transplanted far larger numbers of rod precursors – around 200,000 – at the optimal developmental stage. The integrated cells were seen to form characteristic synaptic connections in the retina, while brain imaging revealed that visual signals were being processed in visual areas of the brain. Most critically, the performance of animals on visually guided behavioural tests were markedly improved. The work therefore suggests that, with the right cell populations, cell transplantation therapy could ultimately be a viable option for sight loss due to retinal degeneration. Positive results have now been obtained with six different models of retinal degeneration, emphasising the potentially wide applicability of the approach. Dr Pearson and colleagues are now generating photoreceptor precursor cells from embryonic stem cells for use in transplants, and are exploring the possibility of generating cone cells as well as rods. Although damaged nerves in the central nervous system rarely regenerate, the peripheral nervous system has markedly better repair potential. This ability is dependent on Schwann cells, which form the insulating myelin sheath around peripheral neurons. But as Professor Alison Lloyd and colleagues have discovered, coordinated nerve regrowth is a cellular team effort. Schwann cells are highly unusual in their ability to switch repeatedly between differentiated and dedifferentiated states. This property is critical to peripheral nerve repair. If a nerve is damaged, the ‘downstream’ axon fragment is rapidly degraded. Its associated Schwann cells, by contrast, slip back into a progenitor state. Some also begin to migrate into the damaged area and promote regrowth of the severed axon. As the axon regrows, it follows the route marked by dedifferentiated Schwann cells back to its original target tissue. But Schwann cells are not the only cells at the site of injury. A whole army of fibroblasts also appear, as might be expected given their role in wound healing. Could they also be playing a role in nerve repair? Growing Schwann cells and fibroblasts in culture, Professor Lloyd and colleagues noticed an interesting shift in their behaviour. On their own, cultured Schwann cells normally repel one another, but with fibroblasts present they showed an uncharacteristic tendency to clump together. This behaviour was highly reminiscent of the ‘cell sorting’ seen in developmental processes, which often involves signalling molecules known as ephrins. Sure enough, one member of the ephrin family, ephrin B, was critical for fibroblast–Schwann cell signalling. This led to relocalisation of a cell adhesion molecule, N-cadherin, causing the Schwann cells to stick together. As luck would have it, Professor Lloyd had previously seen similar clumping behaviour in Schwann cells overexpressing the stem-cell factor Sox-2. Indeed, relocalisation of N-cadherin in Schwann cells in response to ephrin signalling was dependent on Sox-2. The findings suggest an elegant model in which ephrin signals from incoming fibroblasts prompt Schwann cells to adhere so they form tracts or ‘rails’ crossing the site of damage. The regenerating axon follows these rails, before reaching the dedifferentiated Schwann cells downstream of the site of damage. The results suggest ways in which to promote nerve repair but, potentially, the mechanism could be of wider significance. For example, cancers derived from Schwann cells, neurofibromas, can also spread by migrating along nerves, and it will be interesting to test whether this has any connection with the mechanisms uncovered in peripheral nerve repair. MacLaren RE et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–7. Pearson RA et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485(7396):99–103. Barber AC et al. Repair of the degenerate retina by photoreceptor transplantation. Proc Natl Acad Sci USA. 2013;110(1):354–9. Parrinello S et al. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143(1):145–55. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 21 CoMPLEX (Centre for Mathematics and Physics in the Life Sciences and Experimental Biology) aims to bridge the gap between biology and medicine and the physical sciences. COMPLEX: A MODEL FOR INTERDISCIPLINARY RESEARCH Physics, mathematics and computing have never been so important in life science research. As well as theoretical insight, the physical sciences generate invaluable experimental tools for capturing and analysing data, while mathematical and computational modelling are finding widespread application across biology. To promote such fruitful interactions, UCL’s CoMPLEX initiative aims to build links between biology and the physical sciences – defined broadly to include physics, chemistry, engineering, mathematics and computing – primarily by training a cohort of researchers who go on to work as integrated members of life science research groups. At the heart of CoMPLEX is a four-year PhD programme, launched in 1998, making it one of the UK’s first. Students spend an MRes year on intensive courses to build their biological knowledge, undertake three mini-projects, and finish with a summer-long research project. Throughout, there is a strong emphasis on modelling. The experience not only develops students’ skills but also enables them to make a more considered decision about their threeyear PhD project. 22 A critical aspect of this training is exposure to collaborations between life science and physical sciences researchers. In particular, these presentations are a ‘pitch’ to attract CoMPLEX students for PhD projects, during which students are jointly supervised by both life science and physical science researchers. The aim is to ensure that students are not just ‘hired hands’ brought in to do the tricky technical stuff but make a genuine intellectual contribution to a group’s work. Competition for places is fierce. More than 40 per cent have a first class degree and almost 25 per cent have already achieved a master’s with distinction. Similarly, CoMPLEX students are highly prized. Less than 20 per cent of suggested PhD projects are accepted, so supervisors have to offer appealing projects to attract students. Launched internally in 1998, CoMPLEX successfully applied for funding from the Engineering and Physical Sciences Research Council (EPSRC) to expand in 2003. Support was renewed in 2008 and additional funding has been obtained from the British Heart Foundation and others. Around 18 students are now enrolled each year. CoMPLEX is part of an interdisciplinary collaboration with the University of Oxford and Microsoft Research which has been awarded £6m EPSRC ‘landscape funding’ for postdoctoral research training in computational modelling. A further £1m which has been awarded by the BBSRC to a collaboration involving CoMPLEX, the Open University, Edinburgh and Birkbeck for an e-learning resource in systems biology. The subjects being tackled by CoMPLEX students are remarkably diverse, from the systems biology of the liver to the origins of life. CoMPLEX students also contributed to several projects featured in the publication, including Dr Josef Kittler’s work on neurotransmitter receptors (see page 18), Professor Buzz Baum’s modelling of cellular interactions (see page 20) and Professor Roberto Mayor’s analysis of neural crest cell migration (see page 26). BASIC LIFE SCIENCES UCL School of Life and Medical Sciences Fluorescence lifetime imaging of NADH in cultured cells. WORKING IT OUT TOGETHER Siân Culley is in the first year of her PhD, having specialised in the highly unusual combination of cell biology and particle physics in her degree: “I enjoyed those two things and luckily there were things I could do with it!” Her degree exposed her to laser technology and, after further experience during her MRes, this is the subject of her PhD. She is working on new methods of superresolution fluorescence microscopy, to improve resolution from around 200 nm – determined by the wavelength of light – to, hopefully, a few tens of nanometres or even lower. Despite the breadth of her earlier studies, the MRes was challenging: “It’s sometimes quite tricky. But because everyone comes from such a wide range of backgrounds, if you’re stuck on something they’ll be someone you can ask. There’s a very community-based feeling.” A desire to improve his mathematical skills was a key motivation for Nicolas Jaccard, who joined CoMPLEX after a degree in biotechnology engineering in Switzerland. Being new to UCL, he has appreciated the extensive contact with UCL researchers. “What really attracted me was the chance to meet all these UCL PIs and supervisors, because it can be very difficult if you are new. It’s a giant network you build in your first year.” Now in the second year of his PhD, he is exploring ways to culture stem cells in reactors – which he has himself modelled, designed and built. The emphasis of his work has shifted to image processing, driven by the need to find new ways to monitor cells in the specialised reactors. Having actively sought out UCL’s biochemical engineering department, he has also helped to bring them into the CoMPLEX fold. As well as scientific development, he is also appreciated the emphasis on generic skills training, such as scientific writing and presentation skills. “By the time you come to do your PhD you already know these things, so you can concentrate on your science. You’re much more efficient in your first few months.” Tom Blacker was one of the many CoMPLEX students arriving with little background in the life sciences, after a physics degree at Exeter. The MRes course was an ideal transition. “It’s a crash course in biology – you’re thrown in at the deep end. The nice thing is they get in lecturers from all around UCL. We’d been doing biology for a month and were getting lectured to by these real authorities.” For Tom, with an interest in the life sciences but unsure where to focus, CoMPLEX was the ideal course. “CoMPLEX offered this array of collaborations, and you get a chance to sample them all. You get a chance to dip in and see what you like. You can find your own niche in that interface between the physical and life sciences.” For his PhD, he is applying ultra-fast laser techniques to analyse metabolism in living tissues. He has also found the interdisciplinarity challenging. “It’s tough but exciting. You worry about becoming a jack of all trades because you want to be a master of both.” The dual supervisor approach has been essential, he suggests: “You are properly embedded in two labs. You’re really exposed to the way of working in both labs.” For Lewis Dartnell, CoMPLEX has been a springboard to academic success. After completing his PhD he obtained a postdoctoral position in UCL’s Institute of Origins and is now hoping to secure a fellowship position. In his MRes year, one of his mini-projects, on cancer gene networks, led to an academic paper. And the write up of his summer project, which suggested that the remarkable dynamic stripes of cuttlefish are used to create an optical illusion and confuse their shrimp prey, earned him second place in a Daily Telegraph science writing competition. His Biological Sciences degree from Oxford provided him with an excellent grounding but CoMPLEX was still, he suggests, “a baptism of fire: you’re expected to teach yourself an enormous amount of stuff off your own back. But it’s done in a very constructive way and you do get a lot of support.” Fascinated by the possibilities of life elsewhere in the universe, he has gone on to establish a niche in the emerging area of astrobiology. As well as exploring how ‘extremophiles’ from the most inhospitable regions of Antarctica respond to ionising radiation, he has also studied ways to detect the chemical ‘biosignatures’ that would indicate the past existence of life. “It’s a great time to be a young scientist in this field, surfing the wave.” He too was struck by the course’s supportive community spirit. “There isn’t an element of competition in projects – everyone helps each other as much as they can. It’s a great learning experience.” Victor Sojo arrived at CoMPLEX after a degree in chemistry and a master’s in computer science, both undertaken in Venezuela. His aim was to find a broadly based course where he could hone his mathematical skills. “It’s been challenging,” he admits, “but it’s what I came here for. I knew what I was letting myself in for!” Again, the support of fellow students has been a tremendous benefit. “They have no problem sharing everything they discover as they go along.” Still early in his MRes studies, Victor is not sure of his final specialisation, though he knows it will involve some aspect of evolution and the ‘big questions’ – where did life come from, why did sex evolve, how did human behaviours evolve? His wide range of skills should ultimately provide a good grounding. “When they hear what I’ve studied, some people say ‘that’s a crazy background’. Here, they say ‘that’s really cool, that’s just what we need’.” BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 23 SECTION 3 BUILDING TISSUES Developmental processes sculpt collections of cells into complex and beautiful forms. The mechanisms underlying these processes, and the genetic programmes that control them, are beginning to be identified in a range of model organisms. Cross-section through a teratoma. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 24 Mitochondria in heart muscle and endothelial cells. In some of the most famous experiments in developmental biology, during the 1920s Hans Spemann and Hilde Mangold showed that certain regions of an embryo could, when transplanted into a different embryo, direct the formation of well-organised new tissues. Spemann called these regions ‘organisers’, and the amphibian structure is known as Spemann’s organiser in his honour. Despite this insight, the exact nature of organisers were unclear until new molecular and genetic techniques enabled molecules and genes with organising powers to be identified. Pulling the nanostrings The organiser experiment illustrated the principle of ‘induction’, the power of certain cells to ‘induce’ new structures. In vertebrates, transfer of the organiser (‘Hansen’s node’) can generate a complete new nervous system. As well as important work on the mechanisms controlling axis development and The exact nature of organisers were unclear until new molecular and genetic techniques enabled molecules and genes with organising powers to be identified. cell migration during embryogenesis, Professor Claudio Stern has spent several decades identifying molecules involved in this remarkable process (see page 26). Although initially thought to be driven by a single signal, it is now becoming clear that the real situation is far more complex, involving many genes and at least three external signals. An analysis of the times at which genes are active has begun to suggest how they may act together. Professor Stern is now taking this a step further to see how changes in the activity of genes affects others in the network, using ‘NanoString’ technology to map changes in the expression of hundreds of key genes at high resolution. Working with computational biologists, he is developing integrated models of how the genetic regulatory network coordinates the complex dynamics of cell fate decisions underpinning neural identity. An interesting new area of work, on the genetic mechanisms influencing twinning, has emerged from past studies on axis formation. Lower organisms specify axes – front and back, left and right – very early in development, often in the egg itself. In vertebrates, however, axes appear much later. One consequence of this is that embryos can divide surprisingly late in development, giving rise to identical twins. Remarkably, a chick embryo made of 50 000 cells can be split into two and give rise to two entirely normal adults. To identify genetic influences on this process, Professor Stern will combine work on chick development with analyses of the armadillo which, uniquely, creates BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 25 Collective migration of cultured Xenopus neural crest cells (red). Xenopus frog embryo, four-cell stage. PRENATAL ATTRACTION BERT AND ERNI BUILD A NERVOUS SYSTEM An apparently paradoxical mix of attraction and repulsion can drive migrating cells towards the correct destination. A complex cascade of signalling directs the formation of the nervous system. Building an embryo depends on the highly coordinated migration of cells, notably from a structure known as the neural crest. Understanding how cells swarm en masse to the correct location has long intrigued researchers, and its similarities to the migration of cancer cells during metastasis makes it of considerable medical importance. Combining studies of frog and fish embryos with mathematical modelling, Professor Roberto Mayor and colleagues have found that collective migration of neural crest cells can be explained by comparatively simple reciprocal signalling between cells. Migrating cells in culture have long been known to show repulsive behaviour when they meet each other. This ‘contact inhibition of locomotion’ was first described more than half a century ago by Michael Abercrombie, who worked in the laboratory space now occupied by Professor Mayor’s group. Professor Mayor has shown that contact inhibition of locomotion also occurs in vivo, and enables cells to respond to chemoattractants. This chemotaxis seems to be dependent on the stabilisation of cell protrusions specifically in the direction of a chemical gradient. One issue with contact inhibition of locomotion is that, by itself, it would be predicted to lead to cell dispersal. Yet when cultured in vitro, neural crest organise themselves into packs and migrate en masse. Perhaps, Professor Mayor hypothesised, repulsive forces were being counterbalanced by attractive forces. In support of this idea, a simple computer model incorporating repulsion and attraction recreated cohesive directed locomotion along a channel bordered by ‘no-go’ areas. What might be responsible for the attractive forces? Looking for genes for secreted proteins expressed in migrating cells, Professor Mayor hit upon an excellent, albeit unexpected candidate: the complement protein C3, which is cleaved to create a peptide with known chemoattractant properties. Crucially, migrating (but not stationery) neural crest cells also express the receptor for C3. Furthermore, blocking interactions between the two abolished the ability of neural crest cells to move as a pack. Individual cells do have the capacity to migrate directionally. But the addition of an attractive interaction, and consequent group migration, seems to increase considerably the efficiency of directed locomotion. Moreover, the principles are strikingly similar to those governing swarming behaviour in many other organisms, from bacteria to starlings. The simple combination of repulsion and attraction may thus be an overarching principle for achieving group coordination of locomotion in biological systems across multiple scales. In 1924, Spemann and Mangold’s landmark experiments revealed that a small region of tissue transplanted into another embryo could induce the development of a complete new nervous system. Many years later, the view emerged that the transplanted ‘organiser’ only needed to inhibit a protein known as BMP, which stopped cells from developing along a default neural pathway. More than a decade’s work in Professor Claudio Stern’s laboratory has not only shown that this is an oversimplification but also identified a host of factors that form part of a complex regulatory network controlling early nervous system development. An early sign that BMP was not the complete picture came from work showing that a recipient embryo’s cells became sensitive to BMP inhibition only after five hours’ exposure to a graft. During this period, something must be happening to cells to render them responsive to BMP inhibition. To find out what, Professor Stern screened for genes that were active specifically during this early time window. Extensive follow-up of a dozen genes identified has begun to reveal the complex genetic circuitry controlling neural induction. An exciting early discovery was a gene called ERNI (early response to neural induction). As well as being switched on very early, its site of expression suggested it was being regulated by FGF8. Several other pre-BMP genes turned out to be activated by FGF8, implicating it as a critical early factor in neural induction. A second gene, Churchill, which codes for a zinc finger protein, plays a critical role in specifying which cells exposed to FGF8 – a widely used signalling molecules – will contribute to the nervous system and which will give rise to other tissues. Churchill may also be part of the system that controls responsiveness to BMP. Recent studies have begun to characterise the remaining genes identified in the screen, including a previously known gene (TrkC, encoding a nerve growth factor receptor) and two new genes, Asterix and Obelix. Notably, the times at which genes are switched on during normal neural induction revealed three waves of gene expression. Significantly, the analyses suggest that, as well as FGF8, at least two other signals must be driving the expression of key genes and defining which cells will give rise to the nervous system during development. Thus even this complex temporal programme of gene activity does not tell the full story of neural induction. Carmona-Fontaine C et al. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008;456(7224):957–61. Theveneau E et al. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell. 2010;19(1):39–53. Streit A et al. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406(6791):74–8. Sheng G, dos Reis M, Stern CD. Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell. 2003;115(5):603–13. Papanayotou C et al. A mechanism regulating the onset of Sox2 expression in the embryonic neural plate. PLoS Biol. 2008;6(1):e2. Pinho S et al. Distinct steps of neural induction revealed by Asterix, Obelix and TrkC, genes induced by different signals from the organizer. PLoS One. 2011;6(4):e19157. Carmona-Fontaine C et al. Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev Cell. 2011. 26 BASIC LIFE SCIENCES UCL School of Life and Medical Sciences four identical offspring from each fertilised egg. He will also investigate a human population from Nigeria with a high incidence of twins and conjoined twins, to identify possible genetic causes. A connected programme of work is focused on cultured chick embryonic stem cells. In part, this is to gain a better understanding of these cells, which appear to be surprisingly heterogeneous, showing marked variation in gene expression and significant differences from bona fide embryonic stem cells. In addition, they also provide a way to explore the developmental effects of programmes of gene expression. If key genes are identified in other studies, the cells will provide a ‘blank slate’ for assessing the developmental consequences of gene activity. Professor Stern has also explored the migration of neural crest cells, in collaboration with Professor Roberto Mayor. The striking collective migration of cells is reminiscent of swarming behaviour seen at all scales from bacteria to flocks of locusts and birds. Indeed, computational modelling suggests that relatively simple principles can generate this swarming behaviour. Professor Mayor has identified some of the molecules involved in coordinated migration of amphibian neural crest cells (see page 26). Curiously, this work implicated components of the complement system in development – as did Professor Philip Beales’ entirely independent work on rare human developmental conditions (see page 18). The processes that build a chick or a frog are more or less the same as those that build a human baby. These processes are of more than academic interest. The processes that build a chick or a frog are more or less the same as those that build a human baby. If the developmental programme does not play out correctly, a child may be born with significant physical or mental abnormalities, as well illustrated by Professor Beales’ work on ciliopathies. Similarly, problems with neural tube development can have serious consequences. More than a decade ago, Professor Andrew Copp identified problems with folate metabolism leading to non-closure of the neural tube as a possible cause of spina fida. His group has gone to provide further insight into the mechanisms of neural tube closure in experimental models. Fishing for clues Over the past decade, Professor Steve Wilson has put together one of Europe’s largest zebrafish labs. For developmental biology, zebrafish have many advantages. They are easy to grow, they can be manipulated genetically and, because their embryos are transparent, tissue development is easier to visualise. Furthermore, being vertebrates, their developmental processes resemble those seen in humans. Ovaries of the fruit fly, Drosophila melanogaster. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 27 Confocal image of four-day-old zebrafish visual system. Salamanders are well known for their unique ability to regenerate limbs and tails. This striking ability extends to other tissues, including jaws, eyes, intestine and even parts of the heart. Among several areas of research, Professor Wilson has examined various stages of eye development. These include some of the earliest signals regulating eye formation, which has revealed a key role for Wnt signalling in defining areas that will give rise to the adult eye29. Other work has focused at later stages and the development of the retina. Of particular interest are the events that lead to the sealing of the spherical eyeball (see page 29). Professor Wilson also has a growing interest in stem cells in the eye, and coordination of cell division and differentiation. In the flotte lotte mutant, for example, retinal progenitor cells continue to divide but fail to 28 differentiate into neurons. However, when transplanted next to functioning retinal neurons, they are induced to differentiate. Hence neurons seem to provide a signal that can overcome the defect in cell cycle progression. The work highlights the importance of stem cell niches and external signals in regulating progenitor cells30. To find out more about the genes involved in this regulation, and in other aspects of eye development, Professor Wilson’s group is currently undertaking a large-scale screen, mutating genes throughout the zebrafish genome. He is also collaborating with medically oriented groups keen to understand more about the function of disease genes. Hail the salamander Salamanders are well known for their unique ability to regenerate limbs and tails. This striking ability extends to other tissues, including jaws, eyes, intestine and even parts of the heart. Given how useful organ regeneration is, its existence within just one family is perhaps surprising. The standard evolutionary argument is that regeneration was the ancestral state but has been lost in most organisms, perhaps because of accompanying disadvantages such as increased risk of cancer. The question is of more than academic interest: salamanders could be a source of insight and inspiration for the burgeoning field of regenerative medicine. Professor Jeremy Brockes was drawn to this fascinating issue through one of its other unusual features. For nearly 200 years – since the BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 29 Cavodeassi F et al. Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/beta-catenin pathway. Neuron. 2005;47(1):43–56. 30 Cerveny KL et al. The zebrafish flotte lotte mutant reveals that the local retinal environment promotes the differentiation of proliferating precursors emerging from their stem cell niche. Development. 2010;137(13):2107–15. Limb regeneration in the newt. The zebrafish eye. A SALAMANDER SPECIALITY MIND THE GAP Salamanders’ remarkable ability to regrow their limbs may be something they alone have evolved, rather than being a general capacity lost by other vertebrates. Probing the mechanisms of fish eye development may shed light on the origins of human eye abnormalities. If a salamander loses a limb to a predator, it can grow an almost perfect replacement. As well as its intrinsic biological interest, the process has also been seen as potentially relevant to regenerative medicine. But, the work of Professor Jeremy Brockes and colleagues suggests, limb regeneration depends in part on components that are unique to salamanders. At least two features of salamander limb regeneration are notable. First, regrowth matches perfectly the amount of limb lost: if a limb is severed at the shoulder, an entire new arm develops; if it is cut at the wrist, just a hand is formed. Secondly, regeneration depends on a regrowing nerve. If the nerve is transected at the base of the limb, no regrowth occurs. In an elegant series of studies, Professor Brockes has been able to tie seemingly unrelated aspects of this mechanism together. Patterning of salamander limbs is known to respecified in a graded manner by retinoic acid. A search for genes whose activity was regulated by retinoic acid led to the identification of Prod1, a cell-surface protein, as a key factor in salamander limb patterning. Further, a screen for factors interacting with Prod1 identified its ligand – nAG (newt anterior gradient). Moreover, nAG had some very interesting properties. It is made by Schwann cells that encapsulate the regenerating nerve and at later stages in groups of cells in the specialised wound epidermis at the end of the stump. Most significantly, even in the absence of a redeveloping nerve, nAG on its own can drive the regeneration of an entire limb. Hence nAG is responsible for nerve dependency. The evolutionary significance of salamander limb regeneration has long been contentious. It is often assumed to be an ancestral trait that has been preserved in salamanders but lost in other vertebrate species with limbs. However, Professor Brockes’s studies with Dr Acely Garza-Garcia at the National Institute for Medical Research, Mill Hill, suggest that Prod1 is a salamander invention – no homologues exist in other species. These remarkable regenerative powers therefore may have evolved in salamanders. Although the results are significant for regenerative medicine, they do not support the idea that humans have a dormant ‘regenerative’ programme that could be reactivated to enable us to mimic the salamander’s skills. One of the advantages of research on zebrafish is the immediate relevance to human biology. Discoveries made in fish may give clues to mechanisms of disease in people and, conversely, the function of genes causing medical conditions can be explored in fish. Professor Steve Wilson’s work on eye development illustrates this two-way flow. An area of particular interest is the closure of the choroid fissure – a channel at the bottom of the eye that allows blood vessels and nerves to enter and exit the developing eye. Eventually, the fissure must be sealed to create the spherical eyeball, a process that requires carefully coordinated cell migrations. Occasionally, the fissure does not close completely, leading to the condition known as ocular coloboma, a rare congenital eye condition. Professor Wilson has been attempting to identify the genetic factors controlling the closure of the choroid fissure. Although it is known to depend on retinoic acid receptor signalling, the cellular targets and genetic programmes activated have proven hard to identify. Recently, in contrast to previous studies, his group has shown that there are actually two populations of cells affected by retinoic acid receptor signalling. Furthermore, markedly different sets of genes are activated in the two populations, suggesting two independent processes are at work to close the fissure. Potentially, these genes could be involved in ocular coloboma. In other work Professor Wilson has collaborated with clinical ophthalmologist Dr Nicky Ragge to investigate the genetic basis of branchio-oculo-facial syndrome (BOFS), a severe congenital condition affecting the face and eyes. Mutations affecting the TFAP2 transcription factor can cause this condition in humans, and blocking the function of the equivalent gene in fish leads to similar abnormalities, including coloboma. What has been less clear is why the severity of the defects varies so widely among BOFS patients. Professor Wilson’s team showed that, if the function of TFAP2 is partially reduced, this makes fish embryos much more susceptible to the effects of other genetic mutations affecting eye development. On their own, these mutations might have no obvious consequences but when they occur in embryos with compromised TFAP2 function, they lead to coloboma or even loss of the eyes altogether. The presence or absence of similar genetic variants in humans could explain why individuals have very different susceptibility to defects in TFAP2. da Silva SM, Gates PB, Brockes JP. The newt ortholog of CD59 is implicated in proximodistal identity during amphibian limb regeneration. Dev Cell. 2002;3(4):547–55. Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318(5851):772–7. Garza-Garcia A et al. Solution structure and phylogenetics of Prod1, a member of the three-finger protein superfamily implicated in salamander limb regeneration. PLoS One. 2009;4(9):e7123. Lupo G et al. Retinoic acid receptor signaling regulates choroid fissure closure through independent mechanisms in the ventral optic cup and periocular mesenchyme. Proc Natl Acad Sci U S A. 2011;108(21): 8698–703. Gestri G et al. Reduced TFAP2A function causes variable optic fissure closure and retinal defects and sensitizes eye development to mutations in other morphogenetic regulators. Hum Genet. 2009;126(6):791–803. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 29 Synapses on hippocampal neurons. Artificially coloured carbon nanotubes. WNTS BUILD A SYNAPSE CATCH THE TUBE Wnt signalling has a surprising role to play in strengthening synaptic connections. Carbon nanotubes are exciting new tools with many potential applications in biomedicine. The Wnt cell signalling pathway is one of the most widely studied in biology. It has been implicated in an enormous diversity of biological processes, during development and in adult organisms. In part, this flexibility arises from the variety of both Wnt signalling proteins – around 20 in humans – and Wnt receptors. Wnt proteins repeatedly turn up in key biological processes, and Professor Patricia C Salinas and colleagues have recently added another – control of synaptic strength. Wnt signalling is well known to be critical to development of the embryonic nervous system and to the formation of synaptic connections. The latest work suggests that Wnt proteins can also modulate the strength of connections after they have been established. In 2005 Professor Salinas showed that one particular Wnt protein, Wnt7b, promoted the formation of new dendrites in cultured hippocampal cells. Furthermore, this effect depended on a specific signalling pathway, through the scaffold protein known as Dishevelled (Dvl). Later work in knockout mice revealed that Wnt7a promoted fine modelling of complex synapses in the cerebellum, again by acting through the Dvl pathway. Recent work has expanded on these findings. In particular, Wnt7a appears to be playing a significant role in ‘synaptic plasticity’ – changes in the properties of synapses after they have transmitted a signal, an important factor in learning and memory. During the formation of new synapses in the mouse hippocampus, Wnt7a signalling was found to be dependent on the Wnt receptor, Frizzled-5 (Fz-5). Furthermore, neuronal activity led to increased numbers of Fz5 receptors at the synapse, enhancing Wnt signalling. Interestingly, Wnt7a appears to enhance connections at excitatory but not inhibitory synapses (at the latter, neurotransmitter release inhibits rather than activates neurons). These effects at excitatory synapses depended on calcium signals and the calcium/ calmodulin-dependent protein kinase II, which had previously been implicated in modulation of synaptic strength. The results suggest that Wnt7a, unlike other Wnt proteins, promotes the formation of just certain types of synapse. Potentially, abnormal Wnt function could therefore contribute to conditions in which the balance between excitatory and inhibitory signalling is disturbed, such as epilepsy. First rising to prominence in the 1990s, carbon nanotubes consist of sheets of carbon atoms rolled up into tubes just a nanometre or so in diameter. Among their many possible uses is as a delivery platform for therapeutic agents – an area where Professor Kostas Kostarelos and colleagues have generated highly promising results. Although pure carbon nanotubes are insoluble, they can be chemically modified to increase their solubility. They can also have biologically active molecules chemically attached to them – anything from anti-cancer drugs to DNA for gene therapy. Crucially, such ‘functionalised’ carbon nanotubes offer advantages over existing delivery technologies. In 2007, Professor Kostarelos and colleagues found that, although some nanotubes are taken up by standard endocytotic mechanisms, others penetrate the membrane directly, acting as a kind of ‘nano-syringe’. This would allow material to be delivered directly into the cytoplasm – one of the major challenges in cell engineering. This advantage is tempered somewhat by difficulties in targeting – receptor–ligand binding tends to promote endocytosis rather than direct entry. So Professor Kostarelos has looked for applications where biochemical targeting is not required, with a focus on ‘small interfering RNAs’ (siRNAs) to silence the expression of target genes. One exciting possibility is delivery of siRNA to localised areas of the brain. In rodent models of stroke, for example, the approach has been used to inhibit programmed cell death after oxygen starvation, thereby limiting tissue damage and promoting recovery. And in Parkinson’s disease, surgical techniques could be used to deliver siRNA directly to the dopamine-containing cells affected in the condition – a strategy being explored in collaboration with neurosurgeon Professor Marwan Hariz at the Institute of Neurology. It remains early days for nanotube-based therapeutics. In the long term their clinical use will hinge on safety as well as efficacy, so Professor Kostarelos is also studying the fate of carbon nanotubes within the cell and in body tissues. With extensive programmes in nanoscale delivery systems, he also aims to convince others of their enormous potential. One possible use is the reprogramming of cells into a pluripotent state, bringing carbon nanotube technologies into the burgeoning area of cellular engineering. Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nature Neurosci. 2005;8(1):34–42. Ahmad-Annuar A et al. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174(1):127–39. Sahores M, Gibb A, Salinas PC. Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis. Development. 2010;137(13):2215–25. Ciani L et al. Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca² +/Calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 2011;108(26):10732–7. 30 Kostarelos K et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nature Nanotechnol. 2007;2(2):108–13. Al-Jamal KT et al. Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc Natl Acad Sci USA. 2011;108(27):10952–7. Nunes A et al. In vivo degradation of functionalized carbon nanotubes after stereotactic administration in the brain cortex. Nanomedicine (Lond). 2012 [Epub ahead of print]. Singh R et al. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci USA. 2006;103(9):3357–62. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences Confocal microscope image of a section of the cochlea. pioneering studies of Tweedy John Todd in the 1820s – it has been known that limb regeneration is dependent on nerve regrowth. In recent years Professor Brockes has been able to tie together this dependency with the uncannily accurate regrowth of limbs, by which only the structures that have been lost are regenerated (see page 29). The paper describing the remarkable discovery that a single molecule, nAG, could substitute for the presence of a nerve, and drive the development of an entire new limb, was winner of the 2008 AAAS Newcomb Cleveland Prize, awarded to the ‘outstanding’ paper published in Science each year. What are the implications of these findings for our understanding of evolution and for regenerative medicine? The fact that the key mediator, Prod1, is a salamander-specific protein strongly suggests that salamanders have evolved some aspects of the mechanisms of limb regeneration, and these have not been lost by Neurons in the zebrafish brain. other organisms. Professor Brockes has continued to uncover some of the molecular mechanisms driving this process, including ‘upstream’ factors regulating Prod131 and Prod1’s downstream targets32. He also found that regulation of nAG expression by the nerve in limb development can explain why, under certain experimental conditions, limb regrowth can occur in the absence of innervation33. Regeneration thus appears to be a complex, highly regulated process. It is also not simply a reactivated embryonic developmental pathway. Similarly, the blastema, the mass of cells that develops at the site of injury and gives rise to the new limb, is a highly specialised structure, not simply a collection of reprogrammed stem cells. Nothing like it has been seen in mammals. into the systems-level specification of complex new tissue, in adults, will surely be of value to those working in regenerative medicine. A recurring feature in developmental biology is the reappearance of key proteins, or families of proteins, in a range of different developmental context. A classic example is the Wnt family of proteins. As well as having multiples roles in the initial wiring of the nervous system, Professor Patricia Salinas has uncovered a further neurobiological role for Wnt proteins – strengthening synaptic connections after nerve transmission, a key process in neural plasticity and hence learning and memory (see page 30). 31 Shaikh N, Gates PB, Brockes JP. The Meis homeoprotein regulates the axolotl Prod 1 promoter during limb regeneration. Gene. 2011;484 (1-2):69–74. 32 Blassberg RA et al. Functional convergence of signalling by GPIanchored and anchorless forms of a salamander protein implicated in limb regeneration. J Cell Sci. 2011;124 (Pt 1):47–56. 33 Kumar A et al. The aneurogenic limb identifies developmental cell interactions underlying vertebrate limb regeneration. Proc Natl Acad Sci USA. 2011;108(33):13588–93. Thus salamander limb regeneration is unlikely to translate directly to regeneration in people. Nevertheless, insights BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 31 SECTION 4 ALL SYSTEMS GO Reductionist approaches have dominated science for the past century. Yet biology is dominated by complex dynamic systems, and biological problems are increasingly being analysed at a systems level. Calcium signalling in a spinal motoneuron culture. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 32 Confocal microscope image of rat cerebellum (red: blood vessels; green: neurons). The biochemical advances of the mid-20th century revealed complex metabolic pathways such as the iconic Krebs cycle. The late 20th century saw a surge of interest in cell signalling, generating equally complex pathways and networks. A growing quest is to understand not just how a pathway operates in isolation but also how it fits into the greater systems-level understanding of the cell. A systems view of the cell A good place to apply integrative, systems-based approaches is in singlecelled organisms. The best-known single-celled eukaryotic models are the yeasts, and Professor Jürg Bähler is using one such organism – fission yeast, Schizosaccharomyces pombe – with the ambitious long-term aim of developing a complete systemslevel understanding of its behaviour. S. pombe was first extracted from East African beer at the end of the 19th century S. pombe was first extracted from East African beer at the end of the 19th century (‘pombe’ is Swahili for beer). (‘pombe’ is Swahili for beer). Its genome has been fully sequenced, and its 14 million base pairs contain around 5000 genes. Although sharing part of its name with the other main model yeast, Saccharomyces cerevisiae (brewer’s yeast), the two actually have little in common – they probably diverged around half a billion years ago – and in several ways S. pombe is a better model of mammalian systems. As well as a complete genome sequence, genetic tools are available to modify S. pombe genes. It grows easily and rapidly in the lab, and is thus well suited to large-scale studies of gene expression. Professor Bähler is particularly interested in its responses to external stress, such as nutrient limitation. It is already clear that control of gene expression in S. pombe is enormously complex. Although recent decades have been dominated by studies of protein transcription factors, they are only a small part of the story. Regulation is also happening at the RNA level. The amount of DNA transcribed far exceeds that coding for proteins, and it will be a major challenge to determine the role of noncoding RNAs (or perhaps, more accurately, non-protein coding RNA) in control of gene activity and cell behaviour. As well as environmental stress, Professor Bähler has a growing interest in ageing. Cellular pathways affecting ageing are beginning to be discovered (see page 37), and S. pombe is a valuable model in which to investigate them. These studies will take advantage of new work on 120 natural S. pombe isolates collected from 20 countries. Fission yeast strains show natural variation in lifespan, BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 33 Fission yeast, Schizosaccharomyces pombe. Levels of the gut hormone PYY affect food intake. THE HARD CELL FOOD ON THE BRAIN Fission yeast, Schizosaccharomyces pombe, is an ideal organism in which to investigate control of gene expression across the entire genome. The gut hormone PYY may be the key to weight control. Wilhelm BT et al. Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature. 2008;453(7199):1239-43. Weight control reflects a deceptively complex integration of multiple inputs. At its heart lies the body’s energy balance, which matches food intake with energy usage. Overlaying these homeostatic mechanisms are complex psychological influences that can affect food intake. In today’s ‘obesogenic’ environment, where high-calorie food is widely available and opportunities for exercise reduced, energy intake frequently exceeds energy use, ultimately leading to weight gain and obesity. A critical player in these processes, the work of Dr Rachel Batterham and colleagues suggests, may be the gut hormone peptide YY3–36 (PYY). Much is now known about the control of food intake and the critical role played by the hypothalamus. PYY, for example, is released after a meal and acts as a ‘satiety signal’ in the hypothalamus. Notably, infusion of PYY directly into the bloodstream reduces appetite and food intake. Nevertheless, it remains unclear how the wider neurobiological influences on food intake – those linked to emotional reactions (‘comfort eating’) or the pleasurable sensations associated with food, linked to activity in reward pathways – integrate with basic homeostatic processes. To address this question, Dr Batterham teamed up with Professor Steven Williams of King’s College London to assess brain activity and eating behaviour after infusion of PYY. As expected, food intake was markedly lower after people were infused with PYY. Crucially, though, while variation in food intake in the control group was associated primarily with the level of brain activity in the hypothalamus, in the PYY-treated group it was linked to activity in areas of the cortex and limbic system (emotional brain areas). Hence, in the absence of PYY, corresponding to an unfed state, food intake seems to reflect core homeostatic mechanisms. In the presence of PYY, however, it is far more dependent on emotional and cognitive processes, reflecting the pleasurable rather than functional aspects of eating. Dr Batterham has also generated PYY knockout mice to gain insight into PYY’s physiological actions. Notably, PYY was found to mediate the satiety-promoting effects of high-protein diets. PYY was also required for weight loss after gastric bypass surgery. In overfed obese mice, PYY levels were suppressed and when mice were returned to a healthy diet, PYY levels did not return to weight-appropriate levels. Suppression of PYY could therefore be one be one reason why weight loss is so hard to maintain. Thus PYY seems to play a key role in mediating the weight loss effects of gastric bypass surgery, currently the only effective treatment for patients with complex obesity. A fuller understanding of the role played by PYY could lead to much-needed interventions based on dietary modifications or pharmacological interventions. Lemieux C et al. A Pre-mRNA Degradation Pathway that Selectively Targets Intron-Containing Genes Requires the Nuclear Poly(A)-Binding Protein. Mol Cell. 2011;44(1):108–19. Batterham RL et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450(7166):106–9. Much has been learned about the control of gene expression from studies of individual genes. But to work out how a cell operates and responds to its environment, a more integrated approach is needed, to identify coordinated programmes of gene activity. New sequencing and other high-throughput technologies are now making this possible, and Professor Jürg Bähler has applied them with considerable success in the fission yeast Schizosaccharomyces pombe. S. pombe has many advantages as an experimental organism, including a well-characterised genome and an extensive toolbox for genetic manipulation. It ultimately offers the realistic possibility of a complete systems-level understanding of cellular function. Regulation of gene activity will of course be central to this understanding. In 2008, while at the Wellcome Trust Sanger Institute, Professor Bähler’s team took a major step towards understanding fission yeast’s genetic programmes in a landmark whole-genome analysis of gene expression, under a range of experimental conditions. One notable feature – also seen in other organisms, including humans – was the surprising amount of the genome transcribed into RNA. Only a few per cent of the genome is protein-coding, but well over 90 per cent is copied into RNA. The likelihood is that gene expression is controlled not just by proteins but also by legions of newly discovered non-coding RNAs – hinting at substantial degrees of complexity. Professor Bähler is continuing to explore genome-wide control of gene activity, in his own lab and in multiple international collaborations. As an example, splicing patterns depend on environmental stimuli and even, surprisingly, the rate at which genes are transcribed. In addition, work with colleagues in Canada has revealed that a new pathway of pre-mRNA degradation in the nucleus controls the expression of certain intron-containing genes. Other studies have identified histone deacetylases as critical repressors of gene expression, potentially of greater significance than histone methylation. And work with Professor Paul Nurse at Rockefeller University in the USA has revealed that fission yeast has the ability to modulate gene expression globally over a range of cell sizes. As a single cell, S. pombe is supposedly a ‘simple’ model organism. Even so, fully understanding its biology will undoubtedly be a daunting task. Hansen KR et al. H3K9me-independent gene silencing in fission yeast heterochromatin by Clr5 and histone deacetylases. PLoS Genet. 2011;7(1):e1001268. Zhurinsky J et al. A coordinated global control over cellular transcription. Curr Biol. 2010;20(22):2010–5. 34 Batterham RL et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4(3):223–33. Chandarana K et al. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes. 2011;60(3):810–8. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences Astrocytes in the hippocampus. and it will be interesting to search for gene variants affecting longevity and to identify their effects on gene expression and cell biochemistry. Systems-level behaviour can also be seen in the cells of higher organisms, an example being the endogenous clock mechanisms present in all cells. Professor David Whitmore has studied the circadian clock of zebrafish, which is entrained by light34 and depends on cryptochrome 1a35. Remarkably, the clock is active on the first day of fish development and requires no external stimulus to set in motion – fish kept in darkness and at constant temperature still show characteristic daily patterns in activity of key clock genes36. And not only can cells from very early embryos detect light, but being able to do so is a survival advantage37. Body talk Physiology is by its very nature an integrative discipline. At its most challenging, it requires integration not just of biochemical and cellular mechanisms but also of brain activity and conscious thought. Dame Professor Linda Partridge. Dr Alex Gourine is investigating an entirely subconscious process – the control of breathing by carbon dioxide. His team has identified the cells in the cerebellum responding to high carbon dioxide levels, as well as the signalling mechanisms used to transmit this information to other areas of the brain (see page 37). Dr Rachel Batterham, by contrast, is tackling the deceptively complex issue of appetite control and eating, particularly the effects of a signalling molecule known as peptide YY. Ultimately, this may provide a route to new weight control measures (see page 34). Perhaps the most challenging of all is a systems-level understanding of brain function. One approach offering considerable promise is computational, exemplified by the work of Professor Peter Dayan and colleagues in the Gatsby Computational Neuroscience Unit (see page 36). The Unit’s work is based on the use of computational and mathematical modelling to understand brain function, particularly in areas such as plasticity, neural dynamics and population coding, applied in areas such as perception, vision and decision-making. Extensive collaborations with practical Heightened platelet activation could be a reason why some patients are at increased risk of a heart attack after an emotional experience. neuroscience underpin a two-way dialogue between theory and experimental studies (see companion volume on Neuroscience and Mental Health). Humans are inherently social animals, so arguably a fully integrative view of human biology also needs to reflect the impact of social interactions. Until recently, little attention was given to the physiological impact of social and psychological factors. That picture is changing, with the recognition that there is considerably interplay between psychology and brain function, endocrinology, and the immune system. Pioneering work bridging these diverse domains has been carried out by Professor Andrew Steptoe and colleagues. Much of this work is population-based, but a central theme of his work is to understand the mechanisms underpinning population-level effects. For example, heightened platelet activation could be a reason why some patients are at increased risk of a heart attack after an emotional experience38. Ageing: from molecules to minds Superficially, ageing might be equated simply with years lived. But biological age is not the same as chronological age – a 60 year old may have the body of a 30 year old or vice versa. In fact, ageing is a complex and poorly understood process, with characteristic changes at molecular, cellular and tissue levels. Notably, many conditions are associated with increasing age. If the mechanisms underpinning ageing could be identified, it might be possible to intervene and slow not just the ageing process but also 34 Whitmore D, Foulkes NS, SassoneCorsi P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature. 2000;404(6773):87–91. 35 Tamai TK, Young LC, Whitmore D. Light signaling to the zebrafish circadian clock by Cryptochrome 1a. Proc Natl Acad Sci USA. 2007;104(37):14712–7. 36 Dekens MP, Whitmore D. Autonomous onset of the circadian clock in the zebrafish embryo. EMBO J. 2008;27(20):2757–65. 37 Tamai TK, Vardhanabhuti V, Foulkes NS, Whitmore D. Early embryonic light detection improves survival. Curr Biol. 2004;14(3):R104–5. 38 Strike PC et al. Pathophysiological processes underlying emotional triggering of acute cardiac events. Proc Natl Acad Sci USA. 2006;103(11):4322–7. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 35 Dialling down TOR activity – already possible with rapamycin – could therefore be beneficial across a wide spectrum of ageing-related diseases. the development of these conditions. Serotonin and dopamine may act in opposition. ACTION STATIONS Computational models may be able to integrate the contrasting effects of dopamine and serotonin. Humans, like all animals, are constantly faced with choices. How do we know what the right course of action is at any given moment? Despite the enormous complexity inherent in such a question, Professor Peter Dayan and colleagues are developing computational models to provide a conceptual framework for decision making, looking in particular at two critical modulators of behaviour, dopamine and serotonin. Superficially, decision making is straightforward: any organism should choose options that maximise rewards and minimise punishments, over the long term. To make these judgements, an animal needs to have absorbed learning from past experience. However, this simple notion suffers significant problems. In any typical environment, there is so much information to process and so many sequences of possible choices that it is computationally challenging to arrive at optimal decisions. Further, important aspects of choice are pre-programmed by evolution – having to learn by experience that tigers are dangerous would be a disastrous strategy. In the face of these challenges, human and animal decision making employs a more sophisticated approach, combining multiple mechanisms, each of which works well in a limited set of circumstances. The UCL team’s approach is to build computational models to characterise these mechanisms and their complex interactions. Dopamine and serotonin lie at the heart of two such systems. One long-standing but still controversial idea is that dopamine and serotonin oppose each other’s influences, with dopamine responsible for reward and serotonin for punishment. The newer computational models point to richer forms of opponency, integrating the ‘vigour’ with which goals are pursued. On this axis, dopamine is responsible for invigoration and serotonin for inhibition. The interactions between these opponencies leads to a complex set of situations in which the systems may reinforce one another or come into conflict, scenarios that can be modelled in quantitative computational models and tested experimentally. While a model based on these two modulators can scarcely explain all the complexity of decision making, it does provide a framework for exploring the impact of these two critical molecules in human behaviour. Ultimately, it may also provide input into understanding some of the suboptimal decisionmaking seen in a range of debilitating neurological and psychiatric conditions such as Parkinson’s disease, depression and anxiety, which are characterised by abnormalities in these critical brain chemicals. Guitart-Masip M, Beierholm UR, Dolan R, Duzel E, Dayan P. Vigor in the face of fluctuating rates of reward: an experimental examination. J Cogn Neurosci. 2011;23(12):3933–8. Boureau Y-L, Dayan P. Opponency revisited: Competition and cooperation between dopamine and serotonin. Neuropsychopharmacology. 2011 doi:10.1038/npp.2010.151. 36 Hopes that this might be possible have been boosted by numerous studies showing that lifespan can be reliably extended in numerous model organisms, from yeast to mice, by genetic manipulation or by controlling food intake – caloric or dietary restriction. Dietary restriction is too extreme to be a practical option for people. But if the biochemical pathways by which it acted could be identified, it might be possible to mimic its effects. An early candidate was the sirtuin pathway, of particular interest as it was affected by the plant chemical resveratrol, found in the skin of red grapes and (in minute quantities) in red wine. Despite much early excitement, the sirtuin story may be on the wane (see page 37). Meanwhile, an alternative set of pathways being studied by Professor Linda Partridge, Dr David Gems and their colleagues is looking more promising. Dietary restriction triggers an adaptive response that enables cells to survive longer in resourcepoor conditions. Central to this response is a protein known as TOR (target of rapamycin). This and a second pathway known to affect ageing, the insulin-like growth factor-1 pathway, converge on an important regulatory enzyme, ribosomal S6 protein kinase 1 (S6K1). Recent exciting work, carried out with Professor Dominic Withers, now at Imperial College, revealed that S6K1 could be a significant player in mammalian ageing39. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences Female knockout mice lacking S6K1 lived longer than controls and showed significantly fewer signs of age-related decline. TOR, S6K1 or downstream factors are therefore potential targets for pharmacological intervention. Indeed, there is already evidence from experimental models that rapamycin has lifespanenhancing properties40. Such work puts cell metabolism at the heart of ageing. Conventionally, ageing has been ascribed to ‘wear and tear’, with a central role played by charged reactive oxygen species generated by respiration. Yet this appealing hypothesis may not be the full picture – preventing oxidative damage, for example, has little impact on lifespan41. Indeed, a radical alternative has been proposed that places TOR at the heart of ageing. Problems may arise because baseline TOR activity is too high. This drives a set of cellular changes that, over the long term, have harmful consequences in a wide variety of cell types. According to this view, ageing is not about damage and loss of function, but of overactivity. Dialling down TOR activity – already possible with rapamycin – could therefore be beneficial across a wide spectrum of ageing-related diseases. 39 Selman C et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949): 140–4. 40 Bjedov I et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11(1):35–46. 41 Doonan R et al. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008;22(23):3236–41. Astrocytes, carbon dioxide sensors in the brain. The nematode worm Caenorhabditis elegans. EVERY BREATH YOU TAKE AWKWARD QUESTIONS IN AGEING RESEARCH Surprisingly, astrocytes have turned out to be the critical carbon dioxide-detecting cells in the brain. When it comes to life extension, some findings look more robust than others. Breathing both supplies the body with oxygen and disposes of waste carbon dioxide (CO2). Monitoring of bloodstream oxygen and CO2 levels are both important homeostatic mechanisms, carried out by carotid bodies and the brainstem, respectively. Although the brainstem has been known for many years to play a critical role in respiratory CO2 sensing, only recently have the critical cells been identified, thanks to the work of Dr Alexander Gourine and colleagues. In 2005, with Professor Michael Spyer, Dr Gourine identified the purine nucleotide ATP as the critical signalling molecule linking CO2 levels to breathing rate. Best known as a cellular energy source, ATP also has an important role in intercellular signalling, binding to a specific class of receptors. In rats, blocking these receptors in chemosensitive areas of the brainstem abolished the stimulatory effects of increased CO2 levels on breathing rate. More recently, Dr Gourine and colleagues in Bristol and Stanford honed in a particular class of cells in the brainstem, astrocytes, as possible brain chemosensors. Astrocytes are highly numerous in the brain – they actually outnumber neurons – but have generally been seen simply as providing physical and metabolic support for neurons. Recently, however, there has been a growing awareness that they also play more functional roles. Indeed, using molecular imaging, Dr Gourine and colleagues identified significant calcium fluxes in astrocytes in response to small changes in pH, leading to the release of ATP, activation of nearby neurons and an increase in respiratory activity. Furthermore, using optogenetic techniques – genetically introducing ion channels that open in response to light of a specific wavelength – they were able to show that cell-specific light-induced calcium activation of astrocytes was sufficient to trigger changes in breathing, mimicking the effects of CO2. Astrocytes are well positioned in the brainstem to carry out their chemosensing role, being intimately connected both to incoming blood vessels and the neurons responsible for controlling breathing activity. Interestingly, they appear to be a specific type of astrocyte as those present elsewhere in the brain do not respond to pH changes. These findings add weight to the idea that astrocytes are more important to information processing in the brain than once suspected – an idea becoming widely accepted but currently backed up by little convincing experimental evidence. Genetic studies in model organisms – such as yeast, fruit flies, nematode worms and mice – have identified genes that affect lifespan. As Professor David Gems, Professor Linda Partridge and their colleagues have shown, however, great care is needed before it can be said with certainty that a gene affects ageing. Ageing is a complex and poorly understood biological process. Several factors have been reliably shown to lengthen lifespan (see main text) and in several organisms genetic changes have been found that cause organisms to live considerably longer than usual. In such studies, it is important to be sure that an observed phenotype is actually the result of a specific genetic change. As is often pointed out, correlation does not imply causation. An early example of this came from work on flies, in particular a mutant fly known as Indy (short for I’m not dead yet), which lives twice as long as normal flies. The UCL group planned to use Indy flies as positive controls. But to their surprise, they found that, when crossed into other genetic backgrounds, Indy mutations had no impact on lifespan. In one case, increased longevity was seen – but the new strain did not include the Indy mutation. Longevity in the original Indy strain was also abolished when antibiotics were used to kill Wolbachia, a bacterium often found in insect cells. More recently, and potentially more seriously, similar results have been obtained with sirtuin mutations. Sirtuins have generated considerable excitement since the discovery that overexpression could extend lifespan in a range of experimental organisms. Despite this excitement, sirtuins have also attracted controversy, with sometimes conflicting findings on their role in ageing. Professor Gems and Professor Partridge’s research has dealt them a further blow. Outcrossing experiments in the worm, for example, eliminated increases in longevity even though sirtuin overexpression was maintained. Instead, longevity was associated with a different genetic change associated with sensory neuron function. In flies, sirtuin overexpression did remain linked to longer life compared to unadulterated flies, but not when compared with genetically modified controls without sirtuin overexpression. Laboratory strains are artificial, and it is possible that adaptation to laboratory life may affect lifespan. Rather than truly extending life, it is possible that some supposedly longevity-enhancing genetic changes are simply counteracting this lost survival ability. Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436(7047):108–11. Toivonen JM et al. No influence of Indy on lifespan in Drosophila after correction for genetic and cytoplasmic background effects. PLoS Genet. 2007;3(6):e95. Burnett C et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477(7365):482–5. Gourine AV et al. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329(5991):571–5. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 37 An evolutionary perspective on human behaviour integrates genes, environment and culture. GENES, CULTURE AND HUMAN BEHAVIOUR Charles Darwin recognised that humans, like all other organisms, were subject to the forces of natural selection. Today, the idea is anathema to some on principle. Even for those happy to accept biological explanations for the evolution of modern humans, the enormously greater role of culture may seem to have lessened the significance, and explanatory power, of biological evolutionary forces. Yet there is a strand of anthropology, typified by the work of Professor Ruth Mace, who leads a Human Evolutionary Ecology Group in the Department of Anthropology, which views human activities through an evolutionary lens. Notably, the work often generates insights that would not have been apparent through other approaches. Although an evolutionary perspective naturally brings in the question of genes, it also places humans firmly within a geographical and cultural context, and is highly empirical, thus avoiding some of the pitfalls of simplistic evolutionary psychology. To minimise the impact of complex artificial environments, research often focuses on traditional societies (though it is increasingly hard to find any that are genuinely untouched by modern life), but also addresses life in developed societies. 38 Naturally, reproduction is a key theme. In industrial societies, it is also an evolutionary conundrum, as the general association between wealth and reproductive success (numbers of offspring) is broken. Is low fertility adaptive? How economics affects reproductive decision making is a complex area, but work with the ‘Children of the 90s’ cohort in Bristol has provided evidence of a ‘quality–quantity tradeoff’1. Smaller families may now be favoured so that individual offspring are not disadvantaged by sibling competition, lower parental investment, or loss of economic benefits owing to child-bearing. The evolutionary perspective also sheds light on a curious and unexpected consequence of a scheme to improve water supply in rural Ethiopia. Women spent less time and energy obtaining water, but these benefits were associated with a sudden surge in fertility. Evolutionary theory suggests that any energy saved on ‘survival’ activities will be routed into reproduction, and that is exactly what appeared to happen2. Follow up studies are examining the factors influencing take up of contraception in such rural communities. Another theme is the potential evolutionary significance of the menopause. Traditional theories suggest that it is simply a consequence of ageing and greater longevity. An alternative idea is that it provides selective benefits, as grandmothers can provide input into their daughters’ child-rearing. Indeed, empirical studies have shown benefits associated with input from grandmothers. One of the most controversial areas of evolutionary theory is that of group selection – the idea that natural selection can operate on groups rather than just individuals. The work of W D Hamilton (see page 10) suggested how genetic relatedness – kinship – could explain socially beneficial behaviour. Selfless behaviour that benefits a relative can ensure better propagation of genes in the long run. But humans are also notable for the degree of cooperation between unrelated individuals. Belonging to a cooperating group may directly benefit an individual. A more provocative suggestion is that selective pressures are operating on the group itself. A group may prosper even if individuals within it do not. Although still a minority view – group benefits can usually be assigned to individuals – it is possible to envisage a process of ‘cultural evolution’ that can operate at a group level. Successful cultural traits can adapt, compete and provide a selective advantage, promoting their wider dissemination. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences Cultural group selection has been proposed as a model to explain cooperation, which is frequently tested in economic games across different cultures. However, the idea that cooperation is a stable feature of societies is not supported by Professor Mace’s recent work showing that even within one cultural group, the Pahari Korwa of central India, cooperation varied markedly between subpopulations, reflecting local demographic and ecological factors. On the other hand, a study examining striking patterns in language distribution offers more support for cultural group selection. The size of language areas shows a marked gradient, with areas growing in size further from the equator. After testing a wide range of environmental and other possible explanations, only political complexity emerged as a likely contributory factor. Thus languages may spread when a society becomes large and complex enough to assimilate neighbouring regions. In other words, one culture outcompetes another. This focus on culture also leads to the interesting notion of ‘cultural phylogenetics’ – family trees of cultural traits modelled on those used to represent evolutionary relationships among living organisms. This approach has been used to deduce the ancestral matrimonial relationships in Austronesian societies, a shift from matrilineal kinship after the adoption of animal farming 3 and, more dramatically, the rise and fall of civilisations (see page 44). With Dr Andrea Migliano recently joining the group, future work will have a greater focus on genetics. In notable earlier work, Dr Migliano related the short stature of African pygmies to their dangerous environment. She suggested that evolution had favoured rapid development and early sexual maturity, as life expectancy is low4. Short stature was not directly selected for, but a byproduct of selection for early reproductive maturity. Milk and modern culture Professor Mark Thomas’s work on lactase persistence (see page 44) also illustrates the interplay between culture and human evolution. A notable point about lactase persistence is that the mutation only became advantageous under the appropriate cultural conditions – after milkproducing animals had been domesticated. human behaviour could only take root when populations grew to a critical density. This contingency underpins a theory developed by Professor Thomas to explain the emergence of sophisticated ‘modern’ human behaviour – the first use of symbolic representations, art, toolmaking and music. This remarkable blossoming, which marks humans out from other animals, began in the late Stone Age, around 45,000 years ago. Curiously, though, similar traits appeared much earlier, around 90,000 years ago, in sub-Saharan Africa, but petered out. The reason, suggests Professor Thomas, relates to population density. Modern This conclusion was supported by modelling of skill propagation. Skills can be passed on but rarely with complete fidelity, creating variation in skill levels in the next generation. Occasionally, though, an individual will develop superior skills. The total skills level in the population can therefore increase, if the population is large enough for the imperfect transmission of skills to be outweighed by the presence of sufficient skilled individuals from whom a learner can choose to copy. Using realistic assumptions based on ethnographic data, Professor Thomas was able to simulate these processes in the stone age. Essentially, simulations suggested that population size was the critical factor in achieving skills accumulation in a population5. The ‘tipping point’ population densities appeared in Eurasia around 45,000 years ago, but they also were also achieved in sub-Saharan Africa 45,000 earlier. Other parts of the world took longer to reach threshold population densities, which could explain why modern behaviour took root later even though modern humans were present. One puzzle is why modern behaviour did not survive in subSaharan Africa. Detailed demographical change is uncertain, and there is at least some evidence of worsening climate, which would have led to swings in population sizes and minor bottlenecks snuffing out incipient complex culture. The model has distinct advantages over biological explanations – that cognitive changes directly drove the new behaviours. It would be hard to explain the emergence of modern behaviour twice, while the speed at which modern culture emerged and spread argues against direct genetic and biological adaptation. A more likely scenario is that anatomically modern humans, thought to have evolved around 195,000 years ago, may have had the cognitive capacity for modern behaviour but it could only become embedded and transmitted once populations grew above a threshold size. The rest, as they say, is history. High population densities may have had further important impacts. Once people began to live closer together, with domesticated animals, new niches and opportunities were created for pathogens. Professor Thomas and Dr Ian Barnes have discovered that human evolution driven by pathogens may also have been shaped by demography. By examining the prevalence of a gene variant associated with protection against tuberculosis, they found a correlation between the length of time an urban area had existed and the prevalence of the protective allele6. 1 Lawson DW, Mace R. Optimizing Modern Family Size: Trade-offs between Fertility and the Economic Costs of Reproduction. Hum Nat. 2010;21(1): 39–61. 2 Gibson MA, Mace R. An energy-saving development initiative increases birth rate and childhood malnutrition in rural Ethiopia. PLoS Med. 2006;3(4):e87. 3 Holden CJ, Mace R. Spread of cattle led to the loss of matriliny in Africa: a coevolutionary analysis. Proc R Soc Lond Ser B: Biol Sci. 2003; 270(1532):24–33 4 Migliano AB, Vinicius L, Lahr MM. Life history trade-offs explain the evolution of human pygmies. Proc Natl Acad Sci USA. 2007;104(51):20216–9. 5 Powell A, Shennan S, Thomas MG. Late Pleistocene demography and the appearance of modern human behavior. Science. 2009;324(5932):1298–301. 6 Barnes I, Duda A, Pybus OG, Thomas MG. Ancient urbanization predicts genetic resistance to tuberculosis. Evolution. 2011;65(3):842–8. Neolithic mother figure idol. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 39 SECTION 5 ORIGINS: GENES AND EVOLUTION Genes provide insight current-day biology, but also act as a time machine for looking back at the evolution of life, including humans. Charles Darwin’s notebook entry on his views of family relations among species. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 40 Gene sequences now provide a way to identify family relationships. It is fitting that UCL occupies the site of Charles Darwin’s former house, on London’s Gower Street. Some of the most significant developments in 20th century evolutionary thinking occurred in UCL (see page 10), and today UCL researchers continue to make important contributions to evolution research. The most obvious difference between Darwin’s and modern research in evolution is the central position of DNA. Differences in DNA sequence generate the phenotypic variation on which natural selection acts. As a result, genomic DNA sequences provide a record of past evolutionary history. Darwin’s profound insight that all organisms were related, with new species being generated from existing ones, connected all livings things in a phylogenetic tree of life. Although shared physical characteristics can be used to position organisms on a phylogenetic tree, DNA provides a more fundamental way to identify The explosive growth in DNA sequence data has created extraordinary new opportunities for research in multiple fields, from ecology to oncology. Yet analysing this torrent of data is a formidable challenge. family relationships. Indeed, morphology can be positively misleading, as Professor Max Telford’s research has illustrated. Simple-looking worms may look like genuine flatworms (platyhelminthes) but actually sit on an entirely different branch of the tree of life (see page 43). The explosive growth in DNA sequence data has created extraordinary new opportunities for research in multiple fields, from ecology to oncology. Yet analysing this torrent of data is a formidable challenge. Professor Ziheng Yang has developed widely used computational methods and statistical applications, and has collaborated with groups in UCL and globally to analyse genetic data. One area of interest is the timing of key evolutionary events. Over deep time, timescales have primarily been obtained from geophysical analysis of fossil finds. More recently, DNA sequence comparisons have provided an alternative way to look back in time. The general principle is that two related gene sequences will independently accrue changes over time after they diverge from a shared ancestral sequence. If DNA changes are assumed to arise at a constant rate, then the time of divergence can be calculated. This ‘molecular clock’ approach has been widely used, but has significant drawbacks – not least the fact that the clock has probably not ticked at a constant rate. Professor Yang has developed statistical techniques that incorporate uncertainty into clock pacing, and has been involved in several projects reconciling fossil and DNA evidence. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 41 Skulls in UCL’s Grant Museum of Zoology. Fossil and genetic dating often show large discrepancies. For example, the fossil record suggests that multicellular animals burst onto the evolutionary scene between 500 and 590 million years ago, but genetic analyses put their origins – based on molecular clock assumptions – far earlier, at 700 million years or earlier. A Bayesian model, which allows the molecular clock to vary over time, brought this figure down to 580 million years – more in line with fossil dates42. A similar approach has been used to narrow the discrepancy in the origins of ray-finned fishes – which account for more than half of all vertebrate species43. With colleagues in Nottingham, Cambridge and the USA, Professor Yang has also attempted to integrate fossil and molecular evidence to date the key events in primate evolutionary history. This work suggested that the first anthropoids (monkeys and apes) appeared around 47.2 million years ago44. Other analyses have suggested that for most of hominoid evolution, population sizes were 5–10 times larger than for modern humans, confirming that humans have diverged from a remarkably small starting population45. 42 Notably, these studies have suggested that molecular data cannot by themselves generate accurate speciation dates. Despite its inherent limitations, the fossil record provides essential information about absolute times that needs to be incorporated into models for molecular data. Better dating is going to depend on additional fossils, and better analysis of them, rather than on more sequence data46. DNA sequence analysis can also be used to identify sites that have undergone ‘positive selection’ – changes resulting from Darwinian natural selection. The challenge here is to distinguish changes due to positive selection from those that have simply spread by chance. The general approach is to compare DNA changes that alter amino acid sequences with those that do not. If proteinaltering changes occur faster than those than those that preserve protein sequence, they are likely to have been actively selected for. Using this approach, Professor Yang and colleagues at Cornell University were able to infer that female reproductive proteins, like male reproductive proteins, have been evolving Professor Mark Thomas. rapidly, driven by positive selection, possibly due to sexual selection or sexual conflicts47. Computational tools for these analyses are widely used, particularly by virologists looking for positively selected amino acid residues in rapidly evolving viral genomes. This approach was used to map signs of positive selection in the HIV-1 envelope protein gene over the past 20 years in Japan48. adopt the farming lifestyle? DNA evidence from ancient remains strongly suggests that Europe’s farmers were entirely distinct from earlier hunter-gatherers. It was the people, not just the technology, that swept across Europe49. Human evolution 42 Aris-Brosou S, Yang Z. Bayesian models of episodic evolution support a late precambrian explosive diversification of the Metazoa. Mol Biol Evol. 2003;20(12):1947–54. Genetics also provides an opportunity to explore recent events in human history, complementing traditional archaeological studies. Professor Mark Thomas’s unusual interdisciplinary perspective combines practical work on ancient DNA with computational modelling and an interest in demography and cultural as well as biological evolution. DNA evidence has helped to answer one of the most contentious issues in early European history. Around 8000 years ago, Europe was transformed from a land of hunter-gatherers to agriculture-based societies. But were the huntergatherers displaced by a wave of farmers from the Middle East, or did they BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 43 Hurley IA et al. A new time-scale for ray-finned fish evolution. Proc Biol Sci. 2007;274(1609):489–98. 44 Wilkinson RD et al. Dating primate divergences through an integrated analysis of palaeontological and molecular data. Syst Biol. 2011;60(1): 16–31. 45 Burgess R, Yang Z. Estimation of hominoid ancestral population sizes under bayesian coalescent models incorporating mutation rate variation and sequencing errors. Mol Biol Evol. 2008;25(9):1979–94. 46 Warnock RC, Yang Z, Donoghue PC. Exploring uncertainty in the calibration of the molecular clock. Biol Lett. 2011 [Epub ahead of print] 47 Swanson WJ, Yang Z, Wolfner MF, Aquadro CF. Positive Darwinian selection drives the evolution of several female reproductive proteins in mammals. Proc Natl Acad Sci USA. 2001;98(5):2509–14. 48 Yoshida I et al. Change of positive selection pressure on HIV-1 envelope gene inferred by early and recent samples. PLoS One. 2011;6(4):e18630. 49 Bramanti B et al. Genetic discontinuity between local hunter-gatherers and central Europe’s first farmers. Science. 2009;326(5949):137–40. An acoelomorph, a distant human relation. Professor Ziheng Yang. SHAKING THE TREE OF LIFE BUILDING A TREE OF LIFE A nondescript worm from a Swedish fjord has an unexpectedly important position in the tree of life. Powerful statistical methods can be used to identify species relationships based on DNA data. Initial attempts to draw up a tree of life were based on shared physical features but they now typically reflect DNA sequence comparisons. Using such techniques, Professor Max Telford has had the rare distinction of identifying an entirely new phylum – one of only around 35 in the animal world – based on the analysis of a worm-like creature living deep in the mud of a Swedish fjord. The most interesting phylogenetic questions typically centre on the major branch points of the tree of life. In the animal world, a key point was when the deuterostomes split from the protostomes (the two groups differ in the order in which the mouth and anus form during embryonic development). The protostome lineage gave rise to arthropods, nematodes, annelids and molluscs, while deuterostomes include vertebrates and echinoderms. Professor Telford’s initial interests lay with the flatworms (platyhelminthes), protostomes thought to resemble the earliest bilaterally symmetrical organisms. In the late 1990s, to much surprise, a group analysed the DNA of one obscure member of this family, Xenoturbella, and concluded that it was not a flatworm after all but a member of the mollusc family. Since Xenoturbella has little in common with molluscs, Professor Telford was sceptical. Indeed, bivalve molluscs are Xenoturbella’s favourite food, suggesting that the DNA results reflected contamination. By carefully excising the gut before extracting DNA, his team was able to generate a DNA profile that excluded the worm’s lunch and convincingly removed the link to molluscs. However, one controversy followed another. Xenoturbella might not have been a mollusc but it was not a flatworm either. In fact, it was almost unique, forming a lonely sister taxon to the three other deuterostome phyla (chordates, hemichordates and echinoderms). Xenoturbella was actually a relative of humans, albeit a distant one – their common ancestor lived around 600 million years ago. In 2011, Professor Telford and collaborators sprung a further surprise. Another group of morphologically simple flatworms, acoelomorphs, had been believed to represent an early branch of animal evolution – a link between the earliest radially symmetrical animals (such as jellyfish) and more complex bilaterally symmetrical animals. Unfortunately, additional DNA sequence information suggested the acoelomorphs were, like Xenoturbella, deuterostomes, providing Xenoturbella with company in its new phylum. The results raise the intriguing possibility that both Xenoturbella and acoelomorphs are not ‘primitive’ but have ‘evolved simplicity’, losing many of the more elaborate features seen in other deuterostomes. Additional insight should come from more detailed genome sequencing and analysis of representative species, currently being carried out by Professor Telford and colleagues in Oxford, Spain, Japan and Germany. In his notebook, Charles Darwin sketched the world’s first phylogenetic tree, indicating how he thought new species developed from existing ones. Although family relationships have typically been based on morphological similarities, DNA-based methods are increasingly widely used. A method developed by Professor Ziheng Yang with Professor Bruce Rannala at the University of California Davis delimits species on the basis of genetic or genomic data. Although adopted by others, the method has raised questions about the place of wholly genetic-based approaches in taxonomy. The species concept is one of biology’s most controversial, with some 30 different definitions proposed to date. Traditionally, taxonomists define species on the basis of morphological or behavioural features. However, this approach has several drawbacks. In particular, taxonomic practice varies across fields, introducing subjectivity into species designations. The evolution of gene sequences from ancestral forms in related organisms provides an alternative way to assess family relationships. Professor Yang’s method uses Bayesian statistical methods to accommodate the uncertainty associated with low sequence divergence and potential ambiguities in family tree reconstruction. It also allows existing phylogenetic information – based on morphological characters or molecular data – to be incorporated. A prototype family tree for the population is specified as a starting point and the model tests which variation of it is most compatible with the genetic data. Applied to test gene sequence datasets, the method successfully positioned four well-recognised rotifer species and correctly concluded that samples from six ethnically diverse human populations all belonged to the same species. Its third task was to categorise a group of five North American fence lizards of disputed evolutionary ancestry. Until recently, four of the species were grouped as a single, morphologically diverse species, but an analysis of 29 nuclear genes from 17 individuals strongly supported a phylogenetic tree with five distinct species, confirming an earlier analysis of mitochondrial sequences. Compared with traditional taxonomic practices, the new method has several advantages. It has a strong foundation in evolutionary history and population genetic theory. It is far more objective, so results are more comparable across species groups, and can be applied across the entire tree of life. A US team has already used the method to clarify species relationships among African forest geckos. However, it has also stimulated an energetic debate about the use of genetic data alone to delimit species. Without wishing to be drawn on the issue, Professor Yang points out that, at the very least, the avalanche of sequence data provides a way for taxonomists to test their concepts and hypotheses. Bourlat SJ et al. Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature. 2006;444(7115):85–8. Philippe H et al. Acoelomorph flatworms are deuterostomes related to Xenoturbella. Nature. 2011;470(7333):255–8. Yang Z, Rannala B. Bayesian species delimitation using multilocus sequence data. Proc Natl Acad Sci USA. 2010;107(20):9264–9. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 43 The lactase persistence allele probably arose in east Europe. Children from Fenualoa in the Reef Islands. THE WHITE STUFF THE RISE AND FALL Lactase persistence illustrates the powerful interplay between genes and culture. Complex societies develop in small steps, but can slide back several stages at once. Like all mammals, human babies are brought up on a diet of mother’s milk. Unlike all other mammals, however, many humans retain the capacity to digest its principal sugar, lactose, into adulthood. This trait, lactase persistence, shows a global association with pastoralism and dairy farming, and Professor Mark Thomas and colleagues have combined genetic, archaeological and anthropological evidence to explain the links between genetic and cultural changes of profound historical importance. The ability to digest lactose in adulthood is a classic Mendelian trait. In the default state, lactase production is switched off during childhood. Lactase persistence results from a single nucleotide change in the promoter of the lactase gene. In areas such as northwest Europe, the prevalence of lactase persistence is very high (approaching 100 per cent in Ireland), yet in other parts of the world – notably the Far East, where few people drink milk – it is almost zero. The genetic evidence strongly suggests that lactase persistence in Eurasian populations arose once and then spread rapidly. The vast majority of people share the same mutation and DNA sequences show all the hallmarks of positive selection. Interestingly, though, lactase persistence seems to have risen independently on several occasions – at least three completely different mutations occur in African and Middle Eastern population with pastoral lifestyles. A central question is which came first – milk consumption or the genetic change? Studies of ancient human remains from multiple sites across Europe, mostly dating from 5000–6000 years ago, provided a likely answer. No evidence was found for the Eurasian lactase persistence allele. Since evidence of milk fats has been found on pots from the period, the mutation appears to have arisen after dairying had been established. A computer model, integrating genetic and archaeological evidence, has painted a more detailed picture of the likely sequence of events. The lactase persistence allele was probably first selected for around 7500 years ago in a region of central/ east Europe, before spreading in a wave across Europe (and elsewhere). This corresponds with the regional rise of the ‘Linearbandkeramik’ culture and cattle-based dairy farming. Notably, once both the culture (dairy farming) and the genetics (lactase persistence) were in place, the mutual advantages set the stage for rapid expansion. Initially, milk was probably used for products such as cheese and yoghurt, which contain less lactose. Once unadulterated milk became a suitable food source – nutritious, available throughout the year, and uncontaminated – humans would have been much better equipped to colonise the challenging north European environment. Can past events be reconstructed on the basis of present-day evidence? Professor Ruth Mace, Dr Thomas Currie and colleagues have applied rigorous quantitative tools used in phylogenetic analyses to shed light on the rise – and fall – of cultures in SouthEast Asia and the Pacific. Biological evolution has been marked by the appearance of ever-greater levels of organisation. Similarly, the ways in which human societies are organised vary in complexity, with complex political systems developing from more simple ones. Typical levels of organisation range from ‘tribes’ to ‘chiefdoms’ to ‘states’. Exactly how this happens has been controversial, however. At its simplest, cultures could develop sequentially through a series of organisational arrangements, like steps on a ladder. But could they also skip stages – jumping two rungs on the ladder? Or do societies arrive at the same organisational endpoint via different pathways? And although most work has looked at evolution of greater complexity, can societies also descend the ladder, becoming more simple? Interestingly, these transitions can be studied using the tools developed to analyse biological evolutionary relationships. The reason lies in the evolution of language, which shows striking similarities with biological evolution, particularly the concept of ‘descent with modification’. As a result, relationships between languages, like species, can be represented in phylogenetic trees. Political organisation can be overlaid on such ethnolinguistic phylogenetic trees, revealing how political organisation has changed over time. Using this approach, Professor Mace and Dr Currie evaluated six possible models for evolution of political complexity, ranging from a unidirectional ladder to a ‘free for all’ where all transitions are possible. The best fit was with a model allowing only singlestep upward evolution – high-complexity societies cannot form spontaneously from low-complexity beginnings. Good support was also found for a model in which societies can also slide down the complexity scale, sometimes dropping several steps at once. The results are consistent with the idea that states have to evolve through a series of intermediary steps, generally by the fusion of smaller organisational units. Over historical timescales, such changes have been witnessed in Madagascar in the late 1700s and Hawaii in the early 1800s. They also confirm that societies can revert to more simple organisations. More generally, the work illustrates how quantitative, hypothesis-based research can complement traditional disciplines such as archaeology in elucidating human history. Ingram CJ et al. A novel polymorphism associated with lactose tolerance in Africa: multiple causes for lactase persistence? Hum Genet. 2007;120(6): 779–88. Currie TE, Greenhill SJ, Gray RD, Hasegawa T, Mace R. Rise and fall of political complexity in island South-East Asia and the Pacific. Nature. 2010;467(7317):801–4. Burger J et al. Absence of the lactase-persistence-associated allele in early Neolithic Europeans. Proc Natl Acad Sci USA. 2007;104(10):3736–41. Itan Y et al. The origins of lactase persistence in Europe. PLoS Comput Biol. 2009;5(8):e1000491. 44 BASIC LIFE SCIENCES UCL School of Life and Medical Sciences advantage, it is likely to become embedded and passed on. Culture can therefore be considered from an evolutionary perspective. Professor Thomas’s research frequently reflects the interplay between genes and culture (see page 44), while in the Department of Anthropology, Professor Ruth Mace is leading a group that has adopted an evolutionary perspective on human culture and behaviour, embracing subjects as varied in scale as individual decisions on contraception and the rise and fall of entire political systems (see page 44). A model developed by Professor Thomas, incorporating this ‘Anglo-Saxon apartheid-like social structure’, was able to explain modern distributions of Y chromosomes. More controversially, Professor Thomas’s work has also uncovered unexpected features of British genetic history. Traditionally, the fifth-century Anglo-Saxon conquest of Britain was viewed as a take-over by an influx of people from Continental Europe. Archaeologists later began to favour an alternative model in which only a small elite invaded but had a strong cultural influence. However, analysis of Y chromosome sequences indicated that large numbers of AngloSaxon men made Britain their home. The discrepancy between archaeological and genetic estimates of the scale of the Anglo-Saxon migration could be resolved if smaller numbers of immigrants settled across Britain but used their superior wealth and power to outbreed indigenous Britons. A model developed by Professor Thomas, incorporating this ‘Anglo-Saxon apartheid-like social structure’, was able to explain modern distributions of Y chromosomes50. Archaeologists have been reluctant to accept this idea, though more detailed analyses lend support to this picture. Professor Thomas’s ability to analyse and extract meaning from genetic data has led him into a range of unusual collaborations. Having been the first to read woolly mammoth DNA sequences, he has also been involved in a project identifying fallow deer as the closest living relative of the extinct Irish elk51 – notable for having the largest antlers ever seen, some 10 feet across. A collaboration with Swedish and Spanish scientists suggested that genetic diversity in the endangered Iberian lynx has been low for thousands of years, so may not necessarily impede its future survival52. And, bizarrely, all living polar bears can trace their maternal ancestry to brown bears that lived in the British Isles during the last Ice Age53. Big, and clever Sometimes, historical studies have contemporary relevance. In work led by Professor Marta Korbonits at Barts and The London School of Medicine and Dentistry, Professor Thomas helped to show that the mutation affecting an 18thcentury ‘Irish giant’ is still around today54. Analysis of DNA extracted from the teeth of the Irish giant, Charles Byrne, whose remains are on display in the Hunterian Museum of the Royal College of Surgeons, revealed a mutation in a gene known to lead to tumours of the pituitary gland. He and Professor David Balding calculated that mutation probably arose around 1500 years ago, and perhaps 200–300 people currently carry it. Those potentially at risk can now be tested for carrier status and patients can be identified early and treated. Although some animals show signs of culture, in none is it as highly developed as in humans. Like genetically encoded information, culture is also passed on from generation to generation and is subject to selective pressures – if a cultural tradition offers a selective Genes and health The human genome has been shaped by our evolutionary history. Whether it is still being shaped by natural selection is a moot point, but this legacy does have significant implications for our health. Although humans are genetically very similar, the variation that does exist has significant implications for our health. Extensive searches are underway to identify genetic loci affecting health, with many UCL researchers involved in multicentre genome-wide studies across multiple diseases (see, for example, Professor Nick Wood’s work in neurodegenerative diseases, featured in the companion volume on Neuroscience and Mental Health and Professor Steve Humphries and Professor Aroon Hingorani’s work in the volume on Translation and Experimental Medicine). Statistical analysis is also fundamental to association studies, calling on the expertise of statistical geneticists such as Professor David Balding. As well as contributing to several medical genomewide association studies, Professor Balding has also worked in agricultural and dog genetics, and has made a number of notable contributions to forensic use of genetics. Although individually less common, collectively single-gene disorders and developmental syndromes have major health consequences, and their impact on individuals can be profound. Researchers such as Professor Philip Beales (see page 18) and Professor Peter Scambler have provided important insight into developmental disorders. And researchers in the Institute of Ophthalmology, including Professor Shomi Bhattacharya, Professor David Hunt and Professor Anthony Moore, have identified numerous genes underlying a host of eye diseases. 50 Thomas MG, Stumpf MP, Härke H. Evidence for an apartheid-like social structure in early Anglo-Saxon England. Proc Biol Sci. 2006;273(1601):2651–7. 51 Lister AM et al. The phylogenetic position of the ‘giant deer’ Megaloceros giganteus. Nature. 2005;438(7069): 850–3. 52 Rodríguez R et al. 50,000 years of genetic uniformity in the critically endangered Iberian lynx. Mol Ecol. 2011;20(18):3785–95. 53 Edwards CJ et al. Ancient hybridization and an Irish origin for the modern polar bear matriline. Curr Biol. 2011;21(15):1251–8. 54 Chahal HS et al. AIP mutation in pituitary adenomas in the 18th century and today. N Engl J Med. 2011;364(1):43–50. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 45 BASIC LIFE SCIENCE RESEARCH AT UCL Component institutes Most of the basic life science research at UCL is carried out by groups in the Faculty of Life Sciences, which comprises: • UCL Division of Biosciences • Gatsby Computational Neuroscience Unit • MRC Laboratory for Molecular Cell Biology The Basic Life Sciences Domain encompasses researchers across the whole of the UCL School of Life and Medical Sciences and their work with colleagues outside the school. Domain Chairs: Professor Michael Duchen, Dr Paola Oliveri www.ucl.ac.uk/slms/domains/basic-life-science • UCL School of Pharmacy Dean: Professor Mary Collins www.ucl.ac.uk/lifesciences-faculty/homepage UCL in London Partners Researchers in the UCL School of Life and Medical Sciences occupy a range of buildings on UCL’s central Bloomsbury Campus, at the nearby Royal Free Hospital and Whittington Hospital/Archway Campus sites, and other central London locations. UCL School of Life and Medical Sciences works closely with a range of local, national and international partners. Of particular significance are its close links to local NHS bodies, collectively forming UCL Partners, one of just five UK Academic Health Science Centres. These links underpin UCL’s NIHR Biomedical Research Centres at UCLH, the UCL Institute of Child Health (with Great Ormond Street Hospital) and the UCL Institute of Ophthalmology (with Moorfields Eye Hospital). 1 UCL Main Campus 2 UCL Hospital 3 Great Ormond Street Hospital and UCL Institute of Child Health 4 Moorfields Eye Hospital and UCL Institute of Ophthalmology 5 Royal Free Hospital and UCL School of Medicine 6 Whittington Hospital and Archway Campus With the MRC, Wellcome Trust and Cancer Research UK, UCL is also a founding partner of the Francis Crick Institute, led by Professor Sir Paul Nurse, which is due to open in 2015. UCL also establishes wider partnerships in the UK, for example with Imperial College to set up the London Centre for Nanotechnology, and with Imperial, King’s College London, the MRC and GlaxoSmithKline on the ‘Imanova’ clinical imaging initiative. 6 5 2 1 4 3 46 The School has also developed ties with nearby academic centres, including the London School of Hygiene and Tropical Medicine and Birkbeck College. As well as many joint research initiatives, the institutions also liaise at a strategic level. London As well as numerous international research collaborations, UCL has developed a strategic alliance with Yale University, the Yale–UCL Collaborative, to promote cross-fertilisation and joint ventures across education, research and application. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences Support: Resource centres and platforms Research income The scale of UCL’s research enables it to provide a range of technical infrastructure platforms to support research. These include outstanding facilities and technical expertise in molecular and cellular imaging, as well as pre-clinical and clinical imaging, and several sites specialising in high-throughput sequencing and genome analysis. ‘Live’ grants as at 1 September 2011 Other core platform technologies cover small-chemical libraries, proteomics, biological services and transgenics, and informatics. UCL researchers are also involved in numerous biobanking initiatives and cohort studies, providing access to extensive collections of materials and data. UCL also provides capital infrastructure funding to enable labs to develop their equipment base. For clinical research, a Research Support Centre provides access to essential support for work on people and patients, including liaison with the UCLH/UCL NIHR Biomedical Research Centre, UCL Clinical Trials Unit and UCLH/UCL Clinical Research Facility. The Translational Research Office works to promote the translation of research into therapies, techniques and products with therapeutic value. www.ucl.ac.uk/platforms/ www.ucl.ac.uk/slms/research_support_centre NIHR and other UK Government £177.1m MRC £194.6m Other UK Research Councils UK charities £83.3m £500.4m £53.6m Commercial (UK and international) EU£78.4m £62.6m Other international, inc. NIH Other£14.7m UCL Research Strategy The UCL Research Strategy calls for a transformation of the understanding of the role of our comprehensive research-intensive university in the 21st century. Total£1164.7m Figures refer to research within the UCL School of Life and Medical Sciences. NIHR: National Institute for Health Research; MRC: Medical Research Council; NIH: National Institutes of Health. In addition to highlighting the need to nurture and celebrate individual curiosity-driven research, the strategy sets out for UCL an innovative cross-disciplinary research agenda – designed to deliver immediate, medium- and long-term benefits to humanity. UCL will marshal the breadth of its expert perspectives, in order to address issues in their full complexity and contribute to the resolution of the world’s major problems. Its key aims are to: • continue to foster leadership grounded in excellence in discipline-based research • expand the distinctive cross-disciplinarity of our research, collaboration and partnerships • increase the impact of our global university’s research, locally, regionally, nationally and internationally. BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 47 Sponsors of research We are grateful to all the individuals and organisations who support research in the UCL School of Life and Medical Sciences. Abbott France, Abbott Laboratories, Ablynx NV, Academy of Medical Sciences, Action Medical Research, Action on Hearing Loss, Adam Dealy Foundation, Against Breast Cancer, Age UK (Formerly Research Into Ageing), Agennix AG, Aims 2 Cure, Alcohol Education and Research Council, Alder Hey Children’s NHS Foundation Trust, Alexion Pharmaceuticals, Allergan Inc., Alpha-1 Foundation, Alzheimer’s Society, Alzheimer’s Research UK, Amyotrophic Lateral Sclerosis Association, Anatomical Society of Great Britain & Ireland, Anna Freud Centre, Anthony Nolan Bone Marrow Trust, Apatech Ltd, Apitope Technology (Bristol) Ltd, Aqix Ltd, Argonne National Laboratory, Ark Therapeutics Ltd, Arthritis Research UK, Arts and Humanities Research Council, Assisted Conception Unit, Association for International Cancer Research, Association Francaise Contre les Myopathies, Association Monegasque Contre Les Myopathies, Association of Coloproctology of Great Britain and Ireland, Asthma UK, Astra Zeneca (UK) Ltd, Ataxia UK, Autonomic Disorders Association – Sara Matheson Trust, AVI BioPharma Inc., AXA Research Fund, Bachmann-Strauss Dystonia and Parkinson Foundation, Baily Thomas Charitable Trust, Baily Thomas Charitable Trust, Barts and The London Charity, Batten Disease Family Association, Baxter Healthcare Corp., Bayer – AG, Bayer SAS, Big Lottery Fund, Bill & Melinda Gates Foundation, Biochemical Society, Biocompatibles Ltd, Biogen, Biogen Idec Inc., Biomarin Pharmaceutical Inc., Biorex R&D, Biotechnology and Biological Sciences Research Council, Birkbeck College, Biss Davies Charitable Trust, Boehringer Ingleheim, Bone Cancer Research Trust, Brain Research Trust, Breast Cancer Campaign, Bristol Myers Squibb, British Academy, British Council for Prevention of Blindness, British Heart Foundation, British HIV Association, British Lung Foundation, British Medical Association, British Neurological Research Trust, British Orthodontic Society, British Pharmacological Society, British Psychological Society, British Retinitis Pigmentosa Society, British Skin Foundation, British Society for Haematology, British Tinnitus Association, British Urological Foundation, BUPA Foundation Medical Research Charity, Burdett Trust for Nursing, Burroughs Wellcome Fund, Cambridge University Hospital NHS Foundation Trust, Camden and Islington Health Authority, Canadian Institutes of Health Research, Cancer Fund, Cancer Research Institute USA, Cancer Research UK, Carbon Trust Ltd, Carl Zeiss Surgical GMBH, Celera Corp., Cell Medica Ltd, Centocor Inc., Central and East London CLRN, Central Research Fund, Cephalon Inc., Charles Wolfson Charitable Trust, Chemel AB, Child Growth Foundation, Child Health Research Appeal Trust, Children Living with Inherited Metabolic Diseases (CLIMB), Children With Cancer UK, Children’s Brain Diseases, Children’s Cancer and Leukaemia Group, Children’s Liver Disease Foundation, Children’s Research Fund, Children’s Trust, Chordoma Foundation, Chronic Fatigue Syndrome Research Foundation, Chronic Granulomatous Disease Trust, Chugai Pharma Europe Ltd, Cincinnati Children’s Hospital Medical Center, Circulation Foundation, CLEFT – Bridging The Gap, Clement Wheeler Bennett Trust, CMT UK, Cobra Bio-Manufacturing PLC, Cochlear Research and Development Ltd, Coda Therapeutics Inc., Cogent (Holdings) Ltd, Colgate-Palmolive Europe, College of Optometrists, Colt Foundation, Creating Resources for Empowerment and Action Inc., Cure Parkinson’s Trust, Cure PSP – Society for Progressive Supranuclear Palsy, Cyberonics Inc., Cystic Fibrosis Research Trust, Cystinosis Foundation Ireland, Cystinosis Research Network Inc., David and Elaine Potter Charitable Foundation, Davis Schottlander & Davis Ltd, Deafness Research (Formerly Defeating Deafness), Defense Advanced Research Projects Agency, Department for Children, Schools and Families, Department for Education and Skills, Department for International Development, Department of Health, Department of Health and Human Services, Department of Trade and Industry, Dermatitis and Allied Diseases Research Trust, Deutsche Forschungsgemeinschaft, Diabetes Research and Wellness Foundation, Diabetes UK, Diagenode SA, Doctors Laboratory, Dowager Countess Eleanor Peel Trust, Duchenne Parent Project, Dystonia Medical Research Foundation, Dystrophic Epidermolysis Bullosa Research Association, East Midlands Specialised Commissioning Group, Economic and Social Research Council, Edinburgh University, Edmond J Safra Philanthropic Foundation, Effort – Eastman Foundation, Efic, Eisai (London) Research Laboratories Ltd, El.En. S.p.A, Elan Pharmaceuticals Ltd, Eli Lilly and Co. Ltd, Emergency Nutrition Network, Engineering and Physical Sciences Research Council, Epic Database Research Company Ltd, Epilepsy Action, 48 Epilepsy Research UK, Eular – European League Against Rheumatism, Eurocoating S.P.A, European and Developing Countries Clinical Trials, European Association for the Study of Liver, European Commission, European Huntington’s Disease Network, European Organisation For Research and Treatment of Cancer, European Orthodontic Society, European Parliament, European Respiratory Society, European Society for Immunodeficiencies, Eve Appeal, Experimental Psychology Society, F Hoffmann La Roche Ltd, Fidelity Foundation, Fight For Sight, Fondation de France, Food Standards Agency, Foundation for Fighting Blindness, Foundation for Liver Research, Foundation for the Study of Infant Deaths, Foundation Leducq, Frances and Augustus Newman Foundation, Frost Charitable Trust, Fundacao Bial, Gatsby Charitable Foundation, Gen-Probe Life Sciences Ltd, Genentech Inc., General Charitable Trust of ICH, General Medical Council, Genethon, Genex Biosystems Ltd, Genzyme Corp., Gilead Sciences Inc., GlaxoSmithKline, Glaxosmithkline (China) R&D Co. Ltd, Global Alliance for TB Drug Development, Government Communications Planning Directorate, Great Britain Sasakawa Foundation, Great Ormond Street Hospital Charity, Great Ormond Street Hospital Special Trustees, Grifols UK Ltd, Grovelands Priory Hospital, Grunenthal GMBH, Guarantors of Brain, Guide Dogs for the Blind Association, Gynaecological Cancer Research Fund, H J Heinz Co. Ltd, Harbour Foundation, Health and Safety Executive, Health Foundation, Health Protection Agency, Healthcare Commission, Healthcare Quality Improvement Partnership, Heart Research UK, Helpage International – Africa Regional Development, Henry Smith Charity, Hestia Foundation, High Q Foundation, Histiocytosis Research Trust, Hospital For Sick Children, Human Early Learning Partnership, Human Frontier Science Program, Human Genome Sciences Inc., Huntington’s Disease Association, Ichthyosis Support Group, Illumina Cambridge Ltd, Imperial College Consultants Ltd, Imperial College of Science, Technology and Medicine, Inhibox Ltd, Institut de Recherche Servier, Institut Straumann AG, Instrumentarium Science Foundation, Intensive Care Society, International Association for the Study of Pain, International Balzan Foundation, International Child Development Programme, International Glaucoma Association, International Primary Care Respiratory Group, International Serious Adverse Events Consortium, International Spinal Research Trust, Ipsen Fund, Ipsen Ltd, Iqur Ltd, ISTA Pharmaceuticals, ITI Foundation, Jabbs Foundation, James S McDonnell Foundation, James Tudor Foundation, Janssen Pharmaceutica NV, Janssen-Cilag Ltd, Japan Society for the Promotion of Science, Jean Corsan Foundation, Jerini Ophthalmic Inc., John Templeton Foundation, John Wyeth & Brother Ltd, Johns Hopkins University, Johnson & Johnson Consumer Services EAME Ltd, Juvenile Diabetes Foundation, Katherine Dormandy Trust, Kay Kendall Leukaemia Fund, Kidney Research UK, Kids Company, Kids Kidney Research, King’s Fund, King’s College London, Legal and General Assurance Society Ltd, Leonard Cheshire Disability, Leukaemia and Lymphoma Research, Leverhulme Trust, Lincy Foundation, Linkoping University, Linnean Society of London, Lister Institute of Preventive Medicine, Liver Group, London Borough of Camden, London Deanery, London School of Hygiene and Tropical Medicine, Lowe Syndrome Trust, Lowy Medical Research Institute, Ludwig Institute for Cancer Research, Lund University, Lupus UK, Lymphoma Research Trust, Macmillan Cancer Relief (UK Office), Macular Disease Society, Marc Fisher Trust, Marie Curie Cancer Care, Mars Symbioscience, Mary Kinross Charitable Trust, Mason Medical Research Foundation, Matt’s Trust Fund for Cancer, Maurice Hatter Foundation, Max Planck Institute for Molecular Genetics, Max Planck Institute of Biology and Ageing, Medac GmBH, Medical Research Council, Medical Research Council of Canada, Medical Research Foundation, Melford Charitable Trust, Mend Central Ltd, Meningitis Research Foundation, Meningitis Trust, Merck Ltd, Merck Serono, Mermaid, Michael and Morven Heller Charitable Foundation, Michael J Fox Foundation for Parkinson’s Research, Middlesex Hospital Special Trustees, MIND, Mologic Ltd, Monument Trust, Moorehead Trust, Moorfields Eye Hospital (LORS), Moorfields Eye Hospital Development Fund, Moorfields Eye Hospital Special Trustees, Moorfields Hospital NHS Foundation Trust, Motor Neurone Disease Association, Moulton Charitable Trust, Mr and Mrs Fitzpatrick, MRCP(UK), MSS Research Foundation, Multiple Sclerosis International Federation, Multiple Sclerosis Society of Great Britain and Ireland, Mundipharma Research Ltd, Muscular Dystrophy Association, Muscular Dystrophy Campaign, Myasthenia Gravis Association, Myeloma UK, National Association for Colitis and Crohn’s Disease, National Brain Appeal, National Cancer Institute, National Centre for Social Research, National Centre for the BASIC LIFE SCIENCES UCL School of Life and Medical Sciences Replacement, Refinement and Reduction of Animals in Research, National Contest for Life, National Eye Institute, National Geographic, National Health and Medical Research Council, National Institute for Health and Clinical Excellence, National Institute for Health Research, National Institute of Academic Anaesthesia, National Institute of Mental Health, National Institutes of Health, National Kidney Research Fund, National Multiple Sclerosis Society, National Osteoporosis Society, National Screening Committee, Natural Environment Research Council, NCL Stiftung, Netherlands Organisation for Scientific Research, Neuroblastoma Society, New England Research Institutes Inc., Newlife Foundation For Disabled Children, NHS Blood and Transplant, NHS Executive, NHS Patient Safety Research Programme, Nicholls Foundation, Nicox SA, NIHR School of Primary Care Research, Nippon Telegraph and Telephone Corporation, No Surrender Charitable Trust, Nobel Biocare AB, North Essex Mental Health Partnership NHS Trust, Northern California Institute for Research and Education, Novartis Pharma AG, Novartis Pharmaceuticals Corp., Novartis Pharmaceuticals UK Ltd, Novo Nordisk Pharmaceuticals Ltd, Nuffield Foundation, Ocean Park Conservation Foundation, Ocera Therapeutics Inc., Octapharma, Office for National Statistics, Options Consultancy Services Ltd, Organisation for the Understanding of Cluster Headache, Organon Laboratories Ltd, Orphan Europe (UK) Ltd, Ovarian Cancer Action, Overweight and Heart Diseases Research Trust, Oxalosis and Hyperoxaluria Foundation, Oxford Optronix Ltd, Oxigene Inc., Ozics OY, Paediatric Rheumatology Discretionary Fund, Palaeontological Association, Pancreatic Cancer UK, Parkinson’s Disease Society, Path Vaccine Solutions, Pathogen Solutions UK Ltd, Pathological Society of Great Britain and Ireland, Paul Hamlyn Foundation, PCI Biotech, Pelican Cancer Foundation, Peptide Protein Research Ltd, Pervasis Therapeutics Inc., Peter Samuel Fund, Petplan Charitable Trust, Pfizer Ltd, Philips Medical Systems NL BV, Philips Oral Healthcare Inc., Physiological Society, Planer Plc, Polycystic Kidney Disease Charity, Primary Immunodeficiency Association, Procter and Gamble Technical Centre Ltd, Progressive Supranuclear Palsy (PSP Europe) Association, Prostate Action, Prostate Cancer Research Centre, PTC Therapeutics Inc., Qatar National Research Fund, Race Equality Foundation, Rank Bequest, Raymond and Beverly Sackler Foundation, Raynaud’s and Scleroderma Association, Repregen Ltd, Research in Motion Ltd (Canada), Research into Childhood Cancer, Rheumatology Discretionary Fund, Rho Inc., RMS Innovations UK Ltd, Roche Bioscience, Roche Products Ltd, Rockefeller Foundation, Roddick Foundation, Ronald McDonald House Charities UK, Rosetrees Trust, Roslin Cells Ltd, Royal Academy of Engineering, Royal Centre for Defence Medicine, Royal College of Anaesthetists, Royal College of General Practitioners, Royal College of Ophthalmologists, Royal College of Paediatrics, Royal College of Physicians, Royal College of Radiologists, Royal College of Surgeons of England, Royal Free Cancer Research Trust, Royal Free Hampstead NHS Trust, Royal Free Hospital Special Trustees, Royal National Institute for the Blind, Royal Society, Samantha Dickson, Sanofi Pasteur, Sanofi-Aventis, Santhera Pharmaceuticals Ltd, Sarah Cannon Research UK Ltd, Sarcoma Alliance for Research Through Collaboration, Save The Children, Science and Technology Facilities Council, Scope International AG, Selcia Ltd, Sheffield Teaching Hospitals NHS Foundation Trust, Shire Human Genetic Therapies AB, Siemens plc, Sir Halley Stewart Trust, Sir Jules Thorn Charitable Trust, Skeletal Cancer Action Trust Plc, SMA Trust, Smith & Nephew Plc, Society for Endocrinology, Society for Pediatric Radiology, Sport Aiding Medical Research For Kids (SPARKS), St George’s Hospital Medical School, St Peter’s Research Trust, Stanford University, Stanley Medical Research Institute, Stanley Thomas Johnson Foundation, Stanmore Implants Worldwide Ltd, Stroke Association, Sue Harris Bone Marrow Trust, Summit plc, Supreme Biotechnologies Ltd, Susan G Komen Breast Cancer Foundation, Swiss National Science Foundation, Syngenta, Sysmex Ltd, Takeda Cambridge Ltd, Takeda Europe Research and Development Centre Ltd, Takeda Pharmaceutical Co. Ltd, Tana Trust, Target Ovarian Cancer, Tavistock and Portman NHS Trust, Tavistock Trust for Aphasia, Technology and Medicine, Technology Strategy Board, Teenage Cancer Trust, Thomas Pocklington Trust, Thrombosis Research Institute, Tissue Regenix Group Plc, Tourette Syndrome Association Inc., Toyota Motor Europe, Tuberous Sclerosis Association of Great Britain, UBS AG, UCB Pharma SV, UCB S.A, UCLH/UCL Comprehensive Biomedical Research Centre, UK Clinical Research Collaboration, UK Human Tissue Bank, UK Stem Cell Foundation, Unilever UK Central Resources Ltd, United Kingdom Continence Society, United Therapeutics Corporation, University College London Hospitals, University College London Hospitals Charities, University Medical Center Hamburg–Eppendorf, University of Alabama at Birmingham, University of California, University of Coimbra, University of Iowa, University of Kansas Medical Center, University of Kwazulu-Natal, University of London, University of Oulu, University of Oxford, University of Rochester, University of Southampton, University of Sussex, University of Washington, University of Western Australia, Varian Ltd, Ventana Medical Systems Inc., Veterinary Laboratories Agency, Vitaflo International Ltd, Vital Therapies Inc., Vitol Charity Fund, Wayne State University, Weight Concern, Weizmann UK, Wellbeing of Women, Wellchild, Wellcome Trust, Welton Foundation, Wockhardt UK Ltd, Wolfson Foundation, World Cancer Research Fund, World Health Organization, World Vision International, Wyeth Laboratories and Wyeth Pharmaceuticals Inc. CREDITS Cover: Dr Patrick Wingfield Digby; p. 2: Dr Jay Patel; p. 3: Dr Tom Hawkins; Dr Arantza Barrios; Professor Gabriel Waksman; p. 4: Professor Gabriel Waksman; p. 5: Dr Amanda Price, Dr Leo James; pp. 6, 7: Professor Gabriel Waksman; p. 7: Dr Claudia Bauer; p. 8: Dr Amanda Price, Dr Leo James; p. 8: Professor Finn Werner; p. 9: David Bishop; p. 12: Mr Paul Mondragonteran; p. 13: Dr Alessandro Fantin; p. 14: Steve Gschmeissner/SPL; p. 14: Professor Michael Duchen; p. 15: Dr Yaron Silberberg; p. 16: Dr Sion Lewis; p. 17: Dr Caroline Dalton; p.17: Professor Dan Cutler; p. 18: Dr Katharina Seiferth; p. 18: David Bishop; p. 19: Dr Sang-bing Ong; p. 19: Dr Lisa Clayton; p. 20: Dr Andreas Charidimou; p. 21: Dr Rachael Pearson; p. 21: Professor Alison Lloyd; p. 22: Manos Protonotarios; p. 24: Dr Sayandip Mukherjee; p.25: Professor Derek Yellon; p. 26: Professor Roberto Mayor; p. 26: Michel Delarue, ISM/SPL; p. 27: Dr Emily Richardson; pp. 28, 29: Dr Kara Cerveny; p. 29: Professor Jeremy Brockes; p. 30: Professor Patricia Salinas; p. 30 Professor Kostas Kostarelos; p. 31: Dr David Greenberg; p. 31: Dr Monica Folgueira p. 32: Dr Karli Montague; p. 33: Dr Kate Harris; p. 34: Professor Jürg Bähler; p. 34: webphotographer/iStockphoto; p. 35: Dr Laura Mantoan; p. 36: Gannet77/iStockphoto; p. 37: Professor Alexander Gourine; p. 37: Dr David Gems; p. 39: Heritage Images/Corbis; p. 41: Pablo Rojas/Wellcome Images; p. 42: Grant Museum of Zoology; p. 42 David Bishop; p. 43: Dr Bernhard Egger; p. 43: David Bishop; p. 44: Pohopetch. Text: Ian Jones, Jinja Publishing Ltd Design: Jag Matharu, Thin Air Productions Ltd © UCL. Text may not be reproduced without permission. The UCL ‘dome’ logo and the letters ‘UCL’ are the registered trademarks of UCL and may not be used without permission. TAP1559/08-03-13/V9 BASIC LIFE SCIENCES UCL School of Life and Medical Sciences 49 About UCL UCL is one of the world’s top universities. Based in the heart of London it is a modern, outward-looking institution. At its establishment in 1826 UCL was radical and responsive to the needs of society, and this ethos – that excellence should go hand-in-hand with enriching society – continues today. UCL’s excellence extends across all academic disciplines; from one of Europe’s largest and most productive hubs for biomedical science interacting with several leading London hospitals, to world-renowned centres for architecture (UCL Bartlett) and fine art (UCL Slade School). UCL is in practice a university in its own right, although constitutionally a college within the federal University of London. With an annual turnover exceeding £800 million, it is financially and managerially independent of the University of London. UCL’s staff and former students have included 21 Nobel prizewinners. It is a truly international community: more than one-third of our student body – around 25,000 strong – come from nearly 140 countries and nearly one-third of staff are from outside the UK. www.ucl.ac.uk UCL Gower Street London WC1E 6BT Tel: +44 (0)20 7679 2000