A study of non-covalent protein complexes by means of electrospray...
advertisement

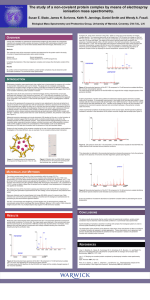
A study of non-covalent protein complexes by means of electrospray ionisation mass spectrometry. Susan E. Slade, James H. Scrivens, Keith R. Jennings, Daniel Smith, Andreas Kukol, Howard Dalton and Wendy A. Foxall. Biological Mass Spectrometry and Proteomics Group, University of Warwick, Coventry, CV4 7AL, U.K M2 protein OVERVIEW A study was undertaken to optimise the experimental conditions for the analysis of a number of intact biologically-important non-covalent protein complexes by means of nanoflow (NF) electrospray ionisation mass spectrometry (ESI-MS) on a Q-Tof Ultima Global (Waters MS Technologies, Manchester, U.K.) instrument. A transmembrane protein was investigated during the study reconstituted in membranemimic phospholipid bilayers. Methods The molecular mass of the monomeric species was determined for each of the proteins in the study. A number of experimental parameters were varied including: Solution pH Source temperature Source pressure Voltage applied to the 1st RF transport lens to facilitate the detection of the intact oligomeric complexes and increase the information content of the spectra. The largest protein studied was a component of the soluble methane monooxygenase (sMMO) enzyme complex. This consists of three proteins, a reductase and a regulatory protein B, together with the hydroxylase, which was selected for further investigation. The enzyme complex is involved in the catalytic conversion of methane to methanol in the bacterium Methylococcus capsulatus (Bath). The hydroxylase has an a2, b2, g2 subunit arrangement. The molecular masses of the monomers are approximately 60, 45 and 19.7 kDa respectively. Previous investigations of the intact hydroxylase protein by means of ESI-MS clearly identified the molecular masses of the b and g subunits but the a component was not evident in the spectrum obtained (Buzy et al.). It was proposed that the b and g subunits ionised preferentially during analysis of the intact hydroxylase. Molecular mass determination of the a subunit required liquid chromatographic separation of the subunits prior to mass spectrometric analysis. Initially this study aims to observe all three subunits in the intact hydroxylase protein and then, after optimisation of the experimental conditions, identify any multimeric complexes formed. Results By careful control of experimental conditions, protein-protein complexes may be observed under favourable conditions in a commercial mass spectrometer. MATERIALS AND METHODS Each of the proteins was diluted to a final concentration within the range 0.3 5 mM in a volatile aqueous buffer (10 mM ammonium acetate) and analysed by the use of ESI mass spectrometry on a Q-Tof Ultima Global instrument (Waters MS Technologies, Manchester, U.K.). The pH of each of the sample solutions was adjusted by the addition of 0.1% or 1% formic acid. Samples were infused using NF capillaries (Waters MS Technologies, Manchester, U.K.) maintained at 1.5 kV. In order to obtain a stable flow of sample from the capillary, a low backpressure of nitrogen gas was applied as necessary. The molecular masses of the monomeric species were determined with the first RF lens maintained at 45 V. During the optimisation experiments the RF lens voltage, source temperature and pressure were varied. Data were obtained over the mass/charge (m/z) range 500 8000 using a 2.4-second scan. Data collected over a 10 minute period were combined, smoothed and the background subtracted prior to processing by the deconvolution algorithm MaxEnt. The SLT-1 B component was further desalted by a 25-fold dilution into 10 mM ammonium acetate and concentrated using a Micron centrifugal filter device (Millipore, Billerica, U.S.A.) with a 3 kDa molecular weight exclusion limit. RESULTS INTRODUCTION SLT-1 B component Electrospray ionisation mass spectrometry has enabled one to demonstrate the presence of a number of weak protein interactions (see Loo for review). This study aims to investigate a number of non-covalent protein complexes by means of ESI-MS and observe the effects of varying the experimental conditions on the resulting spectra obtained. Three biologically-important proteins were selected for investigation. Previous observations suggest that all form non-covalent multimeric protein complexes in solution. Shiga-like toxins (SLTs), produced by enterohemorrhagic strains of Escherichia coli, are associated with disease in humans and animals. SLT-1 is an AB toxin consisting of a 32 kDa enzymatic A subunit that inhibits protein synthesis and a doughnut shaped Bcomponent that appears to be composed of five identical 7.7 kDa subunits (see Figure 1) based on crystallographic evidence (Ling et al.). One face of the pentameric Bcomponent is involved in toxin attachment to the host cell while the A-subunit is situated on the opposite face with its hydrophobic C-terminus anchored in the central pore. The Bcomponent was chosen for study as it is non-toxic and can be purified in the absence of the A-component by means of affinity chromatography. Elution from the column with a denaturant followed by dialysis results in the formation of the complex. A Western blot of the refolded B-component analysed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), probed with anti-SLT-1 antibody, clearly shows that even under denaturing conditions, the pentamer is still present (see Figure 2). Evidence for the formation of the pentamer complex by the use of mass spectrometry would then enable an investigation of the intact AB toxin, also an example of a non-covalent proteinprotein interaction. Figure 3. Crystal structure of the hydroxylase component of soluble methane monooxygenase from M. capsulatus (Bath). The smallest protein studied was the transmembrane M2 protein of Influenza A virus. The + M2 protein is a small 97 residue, hydrophobic protein that forms H -selective channels attributed to the formation of a homo-tetrameric transmembrane domain (see Figure 4, Kukol et al.). It participates in the virus uncoating process after uptake by endocytosis and modulates the pH of the Golgi cisternae during virus transport through the exocytic pathway. Analysis of similar transmembrane peptides in lipid vesicles has been successfully achieved (Demmers et al.). Experiments were carried out with synthetic peptides, which comprise the transmembrane domain of the protein to be studied. The key parameter to be determined is the state of oligomerisation of the peptide. Molecular mass measurements were performed for the SLT-1 B-component dissolved in ammonium acetate buffer containing 1% formic acid. Two major peaks were observed in the reconstructed mass spectrum (see Figure 5), the monomer (7,688 Da) and the less abundant dimer (15,374 Da). It was apparent from the data that the B component was multiply sodiated resulting from insufficient desalting during the purification step. At higher pH, peaks were observed in the 2700 - 4500 m/z range due to the formation of multiply charged species. The measured peak width at half height was >100 Da when the voltage on the first RF lens was 45 V. The peak widths were reduced considerably by increasing the voltage to 200 V but were still >10 Da at half height. The widths of the poorly resolved peaks were ascribed to the presence of sodiated species. After a 25-fold reduction in the NaCl concentration, the peak widths were reduced to < 2 Da. The reconstructed mass spectrum revealed three major peaks including the monomer, the pentamer complex (38,446 Da) and a third species of molecular mass 12,814 Da, which has yet to be identified (see Figure 6). It was not possible to increase the abundance of pentamer complex further by adjusting the source temperature, pressure and RF voltage. A sample of the AB complex is being purified and dialysed to reduce the concentration of NaCl to a minimum prior to analysis. work is in progress. Figure 6. Reconstructed spectrum of the SLT-1 Bcomponent in 10 mM ammonium acetate identifying the monomer and pentamer species. The inset shows the ESI spectrum for the 850-4000 m/z range with the multiply charged species of the pentamer identified When the sample was suspended in ammonium acetate buffer and 1% formic acid, the major peaks were due to singly charged lipid monomer and dimer (see Figure 9). In addition, a number of very low intensity peaks due to multiply charged ions were observed, including those from the peptide of interest. In the absence of formic acid, peaks due to the trimers and tetramers of the lipid were observed (see Figure 10) but no additional peaks due to peptide dimerisation were evident. Further work is planned with more concentrated samples. Figure 9. ESI spectrum of M2 protein in 10 mM ammonium acetate with 1% formic acid for the 600-6000 m/z range. The inset shows the triply charged monomer species of the M2 protein. Hydroxylase protein Molecular mass measurements were performed for the hydroxylase protein dissolved in ammonium acetate buffer containing 1% formic acid. The electrospray mass spectrum clearly reveals the presence of three series of multiply charged ions (see Figure 7). From the reconstructed mass spectrum, all three hydroxylase subunits have been observed in the intact protein. On expanding the reconstructed mass spectrum over the mass range 59,600 61,800 Da, it is evident that a series of peaks is observed indicating the presence of a number of truncates of the a subunit. Those at 59,774 and 59,971 have been reported previously (Buzy et al.) but at least five new truncates can be identified When the protein was analysed in the absence of formic acid with the RF lens maintained at 200 V, additional broad peaks were observed in the region of m/z 4000 4500 and 6750 8000 (see Figure 8). It is believed that these arise from higher mass complexes but because of the width of the peaks, it has not yet been possible to identify these. Further work is in progress. g b a Figure 7. Reconstructed spectrum of the hydroxylase protein in 10 mM ammonium acetate with 1% formic acid identifying the a, b and g subunits. The inset shows the ESI spectrum for the 1000-8000 m/z range. Figure 10. ESI spectrum of M2 protein in 10 mM ammonium acetate buffer for the 6006000 m/z range. The most abundant species have been identified as lipid complexes. The inset shows the triply charged monomer species of the M2 protein. CONCLUSIONS The above results demonstrate that by careful control of experimental conditions, proteinprotein complexes may be observed under favourable conditions in a commercial mass spectrometer. Careful desalting of samples is essential in working with samples from biological systems in order to minimize peak broadening fo high mass complexes. In the case of the SLT-1 B-component, the observation of the pentamer in the absence of the trimer and tetramer is taken as evidence of complex formation as opposed to simple aggregation. REFERENCES Buzy A., Millar, A. L., Legros, V., Wilkins, P. C., Dalton, H. and Jennings, K.R. The hydroxylase component of soluble methane monooxygenase from Methylococcus capsulatus (Bath) exists in several forms as shown by electrospray ionisation mass spectrometry. Eur. J. Biochem. 1998 (254) 602-609. Demmers, J.A.A., Haverkamp, J., Heck, A.J.R., Koeppe II, R.E., Killian, J.A. Electrospray ionization mass spectrometry as a tool to analyse hydrogen/deuterium exchange kinetics of transmembrane peptides in lipid bilayers. PNAS 2000, 97(7), 3189-94. Kukol, A., Adams, P. D., Rice, M. R., Brunger, A.T. and Arkin. I.T. Experimentally based orientational refinement of membrane protein models: a structure for the Influenza A M2 H+ channel. J. Mol. Biol. 1999 (286) 951-962. pentamer Ling, H., Boodhoo. A., Hazes, B., Cummings, M. D., Armstrong, G. D., Brunton, J. L. and Read, R.J. Structure of the Shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry 1998 (37) 1777-1788. + monomer Figure 1. Crystal structure of the pentameric B-component of Shiga-like toxin-1 from E. Coli. Figure 2. Western blot of a SDS-PAGE analysis of denatured SLT-1 B-component showing both the monomer and pentamer complex. Figure 4. Structure of the M2 H channels of Influenza A virus. Figure 5. Reconstructed mass spectrum of the SLT-1 B-component in 10 mM ammonium acetate with 1% formic acid identifying the monomer and dimer species. The inset shows the ESI spectrum for the 850-4000 m/z range with the multiply charged species of the monomer identified Figure 8. ESI spectrum of hydroxylase in 10 mM ammonium acetate for the 1000-8000 m/z range. Loo, J. A. Studying non-covalent protein complexes by electrospray ionisation mass spectrometry. Mass Spec. Reviews 1997 (16) 1-23.