Absorption of Radiant Energy LabQuest

Name ____________________________________ Date __________________
LabQuest
Absorption of Radiant Energy
PRELIMINARY QUESTION
Does color affect the absorption of radiant energy? If it does, why would that matter to you? Write this question in your journal and take 5 minutes to respond to it.
BACKGROUND INFORMATION
After conducting your investigation, research the topic of solar or radiant energy transfer and take notes in your journal as time permits.
OBJECTIVES
In this experiment, you will
•
•
•
Monitor temperature change due to radiant energy absorption.
Calculate temperature changes.
Interpret your results.
MATERIALS
LabQuest
LabQuest App
2 Temperature Probes lamp and bulb piece of white paper piece of black paper tape
Science Journal
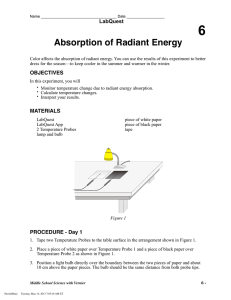
Figure 1
PROCEDURE
1. Tape two Temperature Probes to the table surface in the arrangement shown in Figure 1.
Modified from Middle School Science with Vernier
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
6 - 1
Name ____________________________________ Date __________________
LabQuest
2. Place a piece of white paper over Temperature Probe 1 and a piece of black paper over Temperature
Probe 2 as shown in Figure 1.
3. Position a light bulb directly over the boundary between the two pieces of paper and about 10 cm above the paper pieces. The bulb should be the same distance from both probe tips.
4. Connect Temperature Probe 1 to Channel 1 and Temperature Probe 2 to Channel 2 of LabQuest. C hoose
New from the File menu. If you have older sensors that do not auto-ID, manually set up the sensors .
5. On the Sensor screen, tap Rate. Change the data-collection rate to 0.1
samples/second and the data collection length to 600 seconds. Data collection will last 10 minutes .
6. Start data collection, then sw itch on the light bulb. Data collection will end automatically after 600 seconds (10 minutes).
7. When data collection is complete, turn the light bulb off and return all materials to the places directed by your teacher.
8. Record your beginning and final temperatures.
a. After data collection is complete, a graph of temperature vs . time will be displayed. To examine the data pairs on the displayed graph, tap any data point. As you tap each data point, the temperature values of both probes are displayed to the right of the graph.
b. Identify the beginning and final temperatures for both Probe 1 and Probe 2. Record these values to the nearest 0.1°C in your data table.
9.
Sketch or print the graph as directed by your teacher.
DATA
Final temperature
Beginning temperature
Temperature change
Probe 1
White
°C
°C
°C
Probe 2
Black
°C
°C
°C
PROCESSING THE DATA
1. In the space provided in the data table, subtract to find the temperature changes.
2. Which color had the larger temperature increase?
3. Which color had the smaller temperature increase?
6 - 2 Modified from Middle School Science with Vernier
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
4. Would it matter if you wore dark clothing in the summertime? Why or why not?
5. Do you think clothing designers are concerned with heat transfer? Which decisions should a designer make that are about heat transfer?
6. Solar collectors can be used to absorb the sun’s radiation and change it to heat. What color would work best for solar collectors? Explain.
EXTENSION
1. Design an experiment to compare the radiant heat absorption of different colors. Perform the experiment you designed. Remember to include the questions needed to process the data.
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
6 - 3
Absorption of Radiant Energy LabQuest 6
TEACHER NOTES
This activity was modified from Vernier's Middle School Science LabQuest 6.
Goals and Outcomes:
NC Essential Standard: 6.P.3.1
Content Outcomes: Students will learn that different colors absorb radiant heat at different rates.
Content Integration: ELA: Students write and draw in journals.
Math: Students will collect and organize, and graph data to compare difference.
Process Skills: infer observe collect and organize data analyze and interpret data evaluate choices create (the extension for students who finish quickly)
Assessment:
Pre-assessment for this activity is simply to have students answer the preliminary question: Does color affect how much heat a material will absorb?
Post assessment: students will journal using the ICE format of ideas, claims, and evidence. The teacher may request students to use a 3-column table or paragraph format for this according to teacher preference. Students should reflect upon their response to the preliminary question and make changes as needed.
The teacher facilitates a short, whole class sharing of what students learned and how their ideas changed.
Materials: (listed in the student sheet)
The number of students in a class and in each group will dictate how many light bulbs and sheets of paper will be needed.
The number of Labquest stations available will dictate how many students are assigned to each group. It is advisable not to exceed 4 students per group. 2-3 would be best.
Students will need access to their journals and the ICE format checklist as well as research notetaking guidelines. 2-column note-taking may offer an efficient format for research notes.
6 - 4
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
Middle School Science with Vernier
Homework:
- Students could create a diagram or cartoon showing the process of transfer by radiant energy, being sure to title, label, date, and name, with dialogue boxes explaining the process.
- Extension: Students can begin to design their wardrobe and describe the materials they would use for each piece of clothing.
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
6 - 5
Absorption of Radiant Energy
Reading Selection from Rader's Geography4Kids.com
http://www.geography4kids.com/files/en_solarrad.html
LabQuest 6
6 - 6
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
Middle School Science with Vernier
Radiant Energy From The Sun
Radiant energy is also called electromagnetic energy.
Almost every reaction that happens on the surface of the
Earth is the result of energy coming to the Earth from the
Sun. Radiant energy is also called electromagnetic energy because it is made up of two combined fields. One of the fields is electrical and the other is magnetic.
One other thing to remember: when we talk about incoming radiation, we don't always mean radiation like X-rays, or radioactivity. Light is radiation of some kind. All light is one form of radiation or another. Just keep that in mind.
Constant Output Of Energy
Solar radiation is light energy from the Sun. So you've got the
Sun. Millions of kilometers away from the Earth it sits there with all sorts of nuclear reactions going on. It's constantly giving off a huge amount of energy and radiation. By the time the energy and light reach Earth, there isn't much energy left.
Scientists have figured out something called a solar constant . The constant is the amount of radiation that actually hits the Earth. They say it's about 29.4 MJ
(Megajoules, a unit of energy) for every square meter for each day.
Constant Input Of Energy
The flow of energy to the Earth is constant. That constant flow is just the amount of EM radiation hitting the outside of the atmosphere. The energy from the Sun has changed by the time it hits the surface of the Earth. At the top of the atmosphere you have the 29.4 MJ. We only see about 17 MJ of energy at the surface of the Earth. Something had to happen to the EM radiation between the top of the atmosphere and the surface of the Earth. Energy and certain types of EM radiation ( Infrared and Ultra Violet ) have been filtered away.
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
6 - 7
Not Always Constant At The Surface
Several factors determine the amount of radiant energy hitting the Earth. Our planet is a specific distance from the
Sun. Even though we have a slightly elliptical orbit around the Sun, we can expect a certain amount of energy to hit our planet. If we were closer there would be more energy hitting our atmosphere and if we were further away there would be less energy. We also have a Sun with special characteristics.
As our Sun gets older, it will begin to expand. That increase in size is going to affect the amount of radiant energy hitting our atmosphere. The solar constant is based on the Sun's activity now.
Eruptions in 3-D
Coronal Mass Ejections, or CMEs, are huge solar eruptions that hurl billions of tons of electrified gas out into space. When Earth happens to be in the path of a CME, our power systems, radio communications and satellites can be affected. (NASA/KSC)
- View Video (Real-cc)
Tracking Solar Flares
NASA's SOHO Observatory and TRACE satellite transmitted spectacular images and data from a solar eruption on October 28, 2003. (NASA/
KSC)
- View Video (Real)
Absorption of Radiant Energy LabQuest 6
6 - 8
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
Middle School Science with Vernier
Reading Selection from Rader's Chem4Kids.com
http://www.chem4kids.com/files/react_thermo.html
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
6 - 9
Absorption of Radiant Energy LabQuest 6
Heat and Cold
What are heat and cold? It's a pretty simple idea. When you think of heat , you probably think of fire. When you think of cold , you might think of an ice cube. It all has to do with kinetic energy in atoms . Heat has a lot of kinetic energy and gives it away. The cold doesn't have much energy and absorbs it from the surrounding area. Chemists measure heat in units called Joules . You may also hear about sinks and sources . If the temperature of an object is higher than the surrounding area, it is considered a heat source. If the temperature of an object is lower than the surrounding area, it is considered a heat sink.
Thermochemistry
There are two kinds of heat in chemistry. The first is caused by physical activity. As you get more kinetic energy, there is more activity in the system. This extra activity makes more molecular collisions occur. The collisions create the heat.
This happens when you increase the pressure in a system.
Chemical processes cause the second type of heat. Instead of exciting a system and feeling the heat, chemical bonds are made and broken, and the energy is then released. A release of energy charges up the system and the molecules bounce around faster, resulting in that physical activity we just explained. The opposite can also happen. Sometimes bonds are made and broken and energy is absorbed. The system then gets colder as the temperature goes down. Those emergency icepacks you see when people hurt their ankles are good examples of chemical reactions that absorb energy.
There is energy all around us. Just as matter is all around us, energy is always there. Usually, you will feel this energy as heat. Let's say it's really hot out today. Why is it hot? One big reason is that there is a lot of heat/energy coming from the
Sun . The Sun is a big furnace, and that furnace heats the
6 - 10 Middle School Science with Vernier Earth. When a lot of the Sun's radiant energy makes it to
Earth, it transmits energy to the atoms and molecules in the
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time air and ground. Those molecules heat up. The Sun makes your molecules more excited because of the energy hitting you. You should remember that only a small percentage of the Sun's energy makes it to Earth. We're talking about millionths of a percent. The Sun gives off more energy than you can imagine, and it doesn't end there. There are also millions of stars that are bigger than our Sun. There's a lot of energy in the Universe.
Energy in Chemical Bonds
We just talked about energy in a star. There is also energy stored in the bonds between atoms. How about when you burn a piece of wood? When you burn something, you release the energy from the chemical bonds in the wood.
Where did the energy come from? The Sun. A plant needs the Sun to grow. Light hits the plant and is used by a process called photosynthesis . The plant captures the Sun's energy and stores it in the chemical bonds. You have probably heard of glucose (C
6
H
12
O
6
), which is one of the smallest sugar building blocks made by plants. The plant uses glucose to power certain processes, to manufacture the cellulose , and as a building block in the cellulose itself. When you burn a piece of wood, you are releasing all of the energy stored up.
You experience that energy as heat and light (fire).
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
6 - 11
Absorption of Radiant Energy
Reading Selection from Rader's Physics4Kids.com
http://www.physics4kids.com/files/thermo_intro.html
LabQuest 6
6 - 12
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
Middle School Science with Vernier
Heat and Thermal Energy
When scientists originally studied thermodynamics, they were really studying heat and thermal energy . Heat can do anything: move from one area to another, get atoms excited, and even increase energy. Did we say energy ? That's what heat is. When you increase the heat in a system, you are really increasing the amount of energy in the system. Now that you understand that fact, you can see that the study of thermodynamics is the study of the amount of energy moving in and out of systems.
Heat of Atoms
Now all of this energy is moving around the world. You need to remember that it all happens on a really small scale.
Energy that is transferred is at an atomic level. Atoms and molecules are transmitting these tiny amounts of energy.
When heat moves from one area to another, it's because millions of atoms and molecules are working together. Those millions of pieces become the energy flow throughout the entire planet.
Heat Movement
Heat moves from one system to another because of differences in the temperatures of the systems. If you have two identical systems with equal temperatures, there will be no flow of energy. When you have two systems with different temperatures, the energy will start to flow. Air mass of high pressure forces large numbers of molecules into areas of low pressure. Areas of high temperature give off energy to areas with lower temperature. There is a constant flow of energy throughout the universe. Heat is only one type of that energy.
Increasing Energy and Entropy
Another big idea in thermodynamics is the concept of energy that changes the freedom of molecules. For example, when
6 - 13 you change the state of a system (solid, liquid, gas), the atoms and/or molecules have different arrangements and
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time degrees of freedom to move. That increase in freedom is called entropy . Atoms are able to move around more and there is more activity. That increase in freedom (also called randomness) is an increase in entropy.
Absorption of Radiant Energy http://www.physics4kids.com/files/thermo_heat.html
LabQuest 6
6 - 14
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
Middle School Science with Vernier
Making Heat
How do you make heat? You could burn things ( chemical reactions ), or you could rub things together ( friction ).
When you burn things, thermal energy is released. Thermal energy is measured in calories . For example, when you burn wood, you release 3000 calories for each gram of wood. When you burn an apple, it creates only 600 calories.
The amount of energy released is directly related to the chemical bonds that are broken and formed. If you use that idea, there is more energy available when you break and rebond the atoms in wood, than when you do the same to an apple.
Losing Energy
We just talked about friction. Heat is also created because of inefficiency. When a car engine runs, a lot of heat is given off. Much of that heat is the result of the friction and inefficiency in the running motor. When you lift something and your muscle contracts, you are only 25% efficient.
Seventy-five percent of the energy is lost to heat.
More Transfer of Energy
Heat is the thermal energy transported from one system to another because of a t emperature difference . The transfer of that energy stops when the temperature balances out in the entire environment. Scientists use the unit of a calorie to measure heat. You might be saying, "I've heard of calories.
Are those like the ones in food?" The answer is "Yes." One calorie is measured as the amount of energy needed to raise the temperature of one gram of water, one degree
Celsius . When you “burn” food (this happens VERY slowly in your body), you release energy.
Specific Heat Capacity
There is also something totally important called specific heat capacity. It is the amount of energy required to raise the temperature of one gram of a substance by one degree
Celsius. The specific heat capacity for water is one. As we said, heat is a form of thermal energy. Because it's energy, scientists also use the units of Joules to measure the energy. One calorie equals 4.186 Joules which also equals
4.186 Watts seconds (Ws). Does that mean you can measure the amount of energy you make in your body in one second and express that in terms of an electric value
(Watts)? Yes, the rate at which energy is created or used in your body can be expressed as electrical power.
6 - 15
Absorption of Radiant Energy http://www.physics4kids.com/files/thermo_transfer.html
LabQuest 6
6 - 16
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
Middle School Science with Vernier
Energy Likes to Move
If there is a temperature difference in a system, heat will naturally move from high to low temperatures. The place you find the higher temperature is the heat source . The area where the temperature is lower is the heat sink . When examining systems, scientists measure a number called the temperature gradient . The gradient is the change in temperature divided by the distance. The units are degrees per centimeter. If the temperature drops over a specific distance, the gradient is a negative value. If the temperature goes up, the gradient has a positive value. The greater the gradient, the more energy will be exchanged.
Ever Hear of Convection Ovens?
Convection is the way heat is transferred from one area to another when there is a "bulk movement of matter." It is the movement of huge amounts of material, taking the heat from one area and placing it in another. Warm air rises and cold air replaces it. The heat has moved. It is the transfer of heat by motion of objects. Convection occurs when an area of hot water rises to the top of a pot and gives off energy. Another example is warm air in the atmosphere rising and giving off energy. They are all examples of convection. The thing to remember is that objects change position.
Radiating Energy
When the transfer of energy happens by radiation , there is no conductive medium (such as in space). That lack of medium means there is no matter there for the heat to pass through. Radiation is the energy carried by electromagnetic waves (light). Those waves could be radio waves, infrared, visible light, UV, or Gamma rays. Heat radiation is usually found in the infrared sections of the EM spectrum. If the temperature of an object doubles (in Kelvin), the thermal radiation increases 16 times. Therefore, if it goes up four times, it increases to 32 times the original level.
Scientists have also discovered that objects that are good at giving off thermal radiation are also good at absorbing the same energy. Usually the amount of radiation given off by an object depends on the temperature. The rate at which you absorb the energy depends on the energy of the objects and molecules surrounding you.
6 - 17
Conducting Energy and Heat
Conduction is a situation where the heat source and heat sink are connected by matter. As we discussed before, the heat flows from the source down the temperature gradient to the sink. It is different from convection because there is no movement of large amounts of matter, and the transfers are through collisions. The source and the sink are connected.
If you touch an ice cream cone, the ice cream heats up because you are a warmer body. If you lie on a hot sidewalk, the energy moves directly to your body by conduction. When scientists studied good thermal radiators, they discovered that good thermal conductors are also good at conducting electricity. So when you think of a good thermal conductor, think about copper, silver, gold, and platinum.
Absorption of Radiant Energy LabQuest 6
6 - 18
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
Middle School Science with Vernier
http://www.kapili.com/index_press.html
About Andrew Rader
Two of the most asked questions we receive are "Who makes these sites?" and "Why do you make these sites?" There is one driving force behind all of the sites created by Andrew Rader
Studios... Andrew Rader.
Andrew has a background in both science and computers. While he graduated with a degree in Physiology and Cell Biology, he found success working with computers in the corporate world. For several years, he worked as a freelance designer and then moved to Los Angeles. He found work in the entertainment industry as an
Associate Producer for an interactive television network. At that time, the Internet was starting to grow in popularity and he began producing small web sites. As he gained experience, he moved on to work as Producer for auto web sites. Returning to entertainment, he was also Senior Producer. In 2005, he returned to developing his projects full-time.
During the time he produced commercial sites he was able to maintain his pet projects with a small group of volunteers. The most notable of these projects is the web site Chem4Kids.com. As the years passed, the original chemistry site blossomed into several companion sites. Biology4Kids, Geography4Kids,
Cosmos4Kids, and Physics4Kids eventually joined Chem4Kids.
The group also launched the math activity site NumberNut.com.
6 - 19
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
Absorption of Radiant Energy LabQuest 6
Resources
Volz, D. L. & Sapatka, S. (2007) Middle school science with Vernier. Beaverton, OR: Vernier
Software & Technology.
http://www.geography4kids.com/files/en_solarrad.html
http://www.chem4kids.com/files/react_thermo.html
http://www.physics4kids.com/files/thermo_intro.html
http://www.physics4kids.com/files/thermo_heat.html
http://www.physics4kids.com/files/thermo_transfer.html
http://www.kapili.com/index_press.html
http://www.wisc-online.com/Objects/ViewObject.aspx?ID=sce304 An interactive describing and illustrating the three processes of heat transfer.
6 - 20
StienbargerGregory Tuesday, May 7, 2013 2:43:37 PM Eastern Daylight Time
Middle School Science with Vernier