Blowing Bubbles Experiment
advertisement

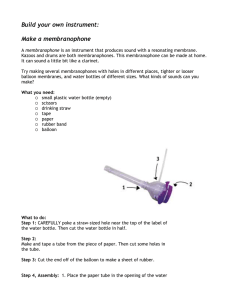
Blowing Bubbles Experiment Materials Glass bottles Bubble solution or liquid dish soap and water Balloons (if needed) 2 large containers (if needed) Water with ice (if needed) Warm water (if needed) Procedure Have students dip top of bottle into bubble or liquid dish soap solution. Make sure that the bottle top is sealed with the solution. Hold the bottle in one hand and watch to see how the solution expands or deflates. Guiding Questions for students Did anything happen to the solution? What caused the solution’s activity? How could you change this experiment to have a similar outcome? What other materials could you use to show the same result? Conclusion The warmth from the students’ hands will transfer to the glass bottle and increase the internal temperature of the bottle. The rise in temperature and pressure will cause bubble to “blow”. Supplementary Extension (If students are struggling with the concept of warm air molecules heating up and expanding, use the following demonstration.) Gather 2 containers of water, one hot and one cold with ice. Blow up 2 balloons to a medium size. Measure the diameter of each balloon and record. Place one balloon in the cold water and one in the warm water and leave for 10 minutes. Remove balloons and measure new diameter. Record data and changes that occurred. Students should see that the heat caused the air molecules to expand in the balloon in the hot water while the balloon in the cold water should have shrunk because it deflated.