Thermal Physics II – Solutions for Problem Sheet 1
advertisement
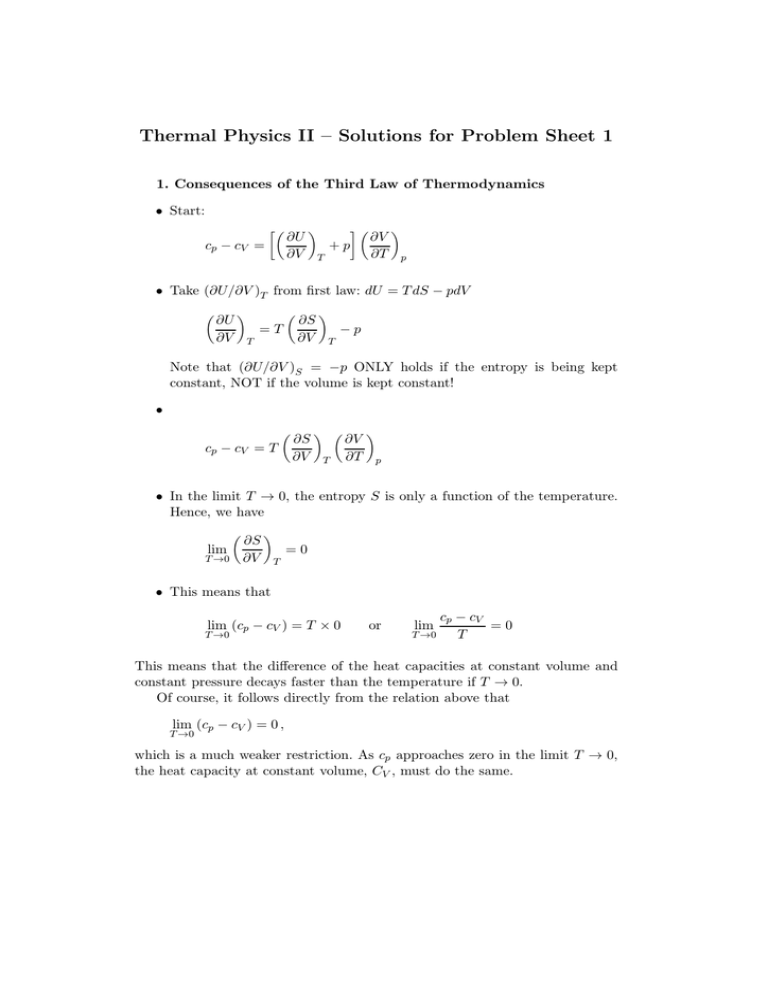
Thermal Physics II – Solutions for Problem Sheet 1 1. Consequences of the Third Law of Thermodynamics • Start: cp − cV = ∂U ∂V +p T ∂V ∂T p • Take (∂U/∂V )T from first law: dU = T dS − pdV ∂U ∂V =T T ∂S ∂V −p T Note that (∂U/∂V )S = −p ONLY holds if the entropy is being kept constant, NOT if the volume is kept constant! • cp − cV = T ∂S ∂V T ∂V ∂T p • In the limit T → 0, the entropy S is only a function of the temperature. Hence, we have lim T →0 ∂S ∂V =0 T • This means that lim (cp − cV ) = T × 0 T →0 or cp − cV =0 T →0 T lim This means that the difference of the heat capacities at constant volume and constant pressure decays faster than the temperature if T → 0. Of course, it follows directly from the relation above that lim (cp − cV ) = 0 , T →0 which is a much weaker restriction. As cp approaches zero in the limit T → 0, the heat capacity at constant volume, CV , must do the same. 2. Maxwell Relation • Start from first law: dU = T dS − pdV + µdN • Then the first derivatives are ∂U ∂S =T, V,N ∂U ∂V = −p , S,N ∂U ∂N =µ S,V • The mixed derivatives of U must be equal as the internal energy is a total differential. Hence, we have ∂ ∂N ∂U ∂S V,N ! ∂ ∂S = S,V ∂U ∂N S,V ! V,N • Now we insert the first derivatives from above (the first on the left side, the last on the right side) and obtain as required ∂T ∂N S,V = ∂µ ∂S V,N . 3. Stability of the Equilibrium • We start from the fundamental law of thermodynamics: dU = T dS − pdV or dS = i 1h dU + pdV . T • Now we take into account that entropy, internal energy and volume are extensive quantities (T and p can be different in both subsystems): dS = dS1 + dS2 = i i 1h 1h dU1 + p1 dV1 + dU2 + p2 dV2 . T1 T2 • Total internal energy and total volume are conserve (we only allow energy transfer between the two subsystems and replacement of one gas with the other). This means dU1 = −dU2 and dV1 = −dV2 . We then have dS = dS1 + dS2 = h 1 T1 − hp 1i p2 i 2 − dU1 + dV1 T2 T1 T2 • In equilibrium, the entropy reaches its maximum and we find ∂S ∂U1 = V1 h1 T1 − 1i =0 T2 and ∂S ∂V1 U1 = hp 2 T1 − p2 i =0 T2 These conditions can only be fulfilled if T1 = T2 and p1 = p2 as required.