Ch-3: Tutorial Crystallographic Directions & Crystallographic Plans
advertisement

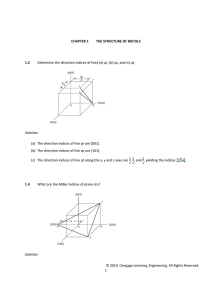
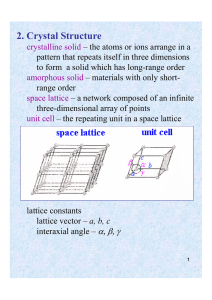
Ch-3: Tutorial Crystallographic Directions & Crystallographic Plans Chose the correct answer: 1- The number of atoms present in the unit cell of HCP structure is: (1) 2 (2) 4 (3) 6 (4) 12 2- The unit cell has a = 5 Å, b = 8 Å, c = 3 Å, α = 90°, β= 65° and γ = 54°. The space lattice for this unit cell is: (1) monoclinic (2) rhombohedral (3) triclinic (4) orthorhombic 3- The lattice constant of a BCC unit cell with atomic radius of 1.24 Å is: (1) 1.432 Å (2) 2.864 Å (3) 1.754 Å (4) 1.432 Å 4- The correct order of coordination number in BCC, FCC and HCP unit cells is: (1) 12, 8, 6 (2) 8, 12, 12 (3) 6, 8, 12 (4) 12, 8, 24 5- The Miller indices of the plane parallel to Y and Z axes are: (1) (0 0 1) (2) (1 1 1) (3) (0 1 0) (4) (1 0 0) 6- A plane in a unit cell is described by its Miller indices (632).The plane intersects x, y, z respectively at points whose distances from origin are: (1) 6, 3 and 2 units (2) 1/3, 2/3 and 1 units (3) 1/3, 1/2 and 1/1 units (4) 1/6, 1/3 and 1/2 units Q: What are the three relatively simple crystal structures in which most common metals exist? Ans. FCC, BCC and HCP.

![Lecture 6 & 7 NCE101[1]](http://s2.studylib.net/store/data/027190486_1-78da1c9733f62f95836cf1c7498ebdc1-300x300.png)