Spatial variation in the degradation rate of the pesticides
advertisement

Environmental Pollution 139 (2006) 279e287
www.elsevier.com/locate/envpol
Spatial variation in the degradation rate of the pesticides
isoproturon, azoxystrobin and diflufenican in soil and
its relationship with chemical and microbial properties
Gary D. Bending*, Suzanne D. Lincoln, Rodney N. Edmondson
Warwick HRI, University of Warwick, Wellesbourne, Warwick CV35 9EF, UK
Received 19 November 2004; accepted 13 May 2005
Spatial variation determines risk assessment for pesticides in soil.
Abstract
The extent of within field variability in the degradation rate of the pesticides isoproturon, azoxystrobin and diflufenican, and the
role of intrinsic soil factors and technical errors in contributing to the variability, was investigated in sites on sandy-loam and clayloam. At each site, 40 topsoil samples were taken from a 160!60 m area, and pesticides applied in the laboratory. Time to 25%
dissipation (DT25) ranged between 13 and 61 weeks for diflufenican, 5.6 and 17.2 weeks for azoxystrobin, and 0.3 and 12.5 weeks
for isoproturon. Variability in DT25 was higher in the sandy-loam in which there was also greatest variability in soil chemical and
microbial properties. Technical error associated with pesticide extraction, analysis and lack of model fit during derivation of DT25
accounted for between 5.3 and 25.8% of the variability for isoproturon and azoxystrobin, but could account for almost all the
variability for diflufenican. Azoxystrobin DT25, sorption and pH were significantly correlated.
Ó 2005 Elsevier Ltd. All rights reserved.
Keywords: Soil; Persistent pesticides; Degradation; Spatial variability; pH
1. Introduction
A large proportion of any pesticide application reaches
the soil where it interacts with organic and mineral
constituents and undergoes biological and chemical
transformations. Microbial degradation is the primary
route for loss, and is therefore the key process affecting
the dynamics of pesticide residues in the environment,
including their persistence in soil, and their susceptibility
to leaching (Aislabie and Lloyd-Jones, 1995).
A number of studies have shown that there can be
significant spatial variation in the degradation rate of
* Corresponding author. Tel.: C44 24 76575057; fax: C44 24 7657
4500.
E-mail address: gary.bending@warwick.ac.uk (G.D. Bending).
0269-7491/$ - see front matter Ó 2005 Elsevier Ltd. All rights reserved.
doi:10.1016/j.envpol.2005.05.011
pesticides within agricultural fields, with implications for
patterns of pesticide leaching from fields. Walker and
Brown (1983) showed that the rapidly metabolised
herbicides metribuzin and simazine showed coefficients
of variation (CV) for time to 50% dissipation (DT50) of
21 and 7%, respectively, in samples taken from an
80!80 m plot from within a field, indicating that there
could be variation in degradation rates at the local scale.
The pesticides used in the study of Walker and Brown
(1983) showed first order degradation consistent with
cometabolism, in which biodegradation does not involve
proliferation of degradative organisms.
For many pesticides, degradation occurs by growthlinked metabolism (Aislabie and Lloyd-Jones, 1995).
However, the occurrence of microbes adapted to use
pesticides as energy sources can be localised at the field
scale (Bending et al., 2003). Mixtures of degradation
280
G.D. Bending et al. / Environmental Pollution 139 (2006) 279e287
mechanisms within fields can result in extreme variation
in degradation rates. Parkin and Shelton (1992) showed
that variability in DT50 in a field area in which there
were sites showing either growth-linked or cometabolic
degradation of carbofuran was significantly higher than
areas in which degradation occurred by growth-linked
metabolism only, with CVs of 25e49% and 9e17%,
respectively. Similarly, Walker et al. (2001a) found high
variability in isoproturon DT50 in a field which
possessed sites showing cometabolic and growth-linked
metabolism, with a CV of 41%.
Variability in degradation rates across fields in some
studies has been linked to variation in soil properties. The
variability in degradation rate shown by Walker et al.
(2001a) was shown to be associated with pH, which was
found to control the extent to which isoproturon degrading
organisms could proliferate (Bending et al., 2001, 2003).
However, in other studies, no relationship between
variability in degradation rate and soil characteristics has
been established (e.g. Cullington and Walker, 1999).
Investigations on spatial variation in pesticide degradation have focussed on compounds with high to
moderate mobilities in soil and which are degraded
relatively quickly, with DT50 of up to 3 months (Price
et al., 2001; Walker et al., 2001a,b; Parkin and Shelton,
1992; Muller et al., 2003). Much less is known of the
spatial variability in the degradation rate of persistent
pesticides with DT50 of up to 6 months or more, or of
those pesticides that become tightly sorbed to soil
components. A number of such compounds are widely
used in Europe, including the triazole fungicides
(Bromillow et al., 1999) and the herbicide diflufenican
(Conte et al., 1998; Rouchard et al., 1994). For these
compounds, variability in degradation rate has implications for the persistence of the compound in soil, and
particularly the possibility that they may accumulate on
repeated use at the same site.
Massey and Lenoir (2003) suggested that variability
in pesticide degradation rates arises from errors
associated with technical error resulting from variability
in pesticide application, soil sampling and sample
analysis, in addition to variability associated with
abiotic and biotic degradation processes. Further contributions to technical error could also occur through
lack of fit of the model used to derive DT50 values.
However the contribution of these sources of variability
to measured in-field spatial variability of pesticide
dissipation have not previously been considered (Walker
and Brown, 1983; Price et al., 2001; Walker et al.,
2001a,b; Parkin and Shelton, 1992; Muller et al., 2003).
It is unclear therefore, whether the variability in
pesticide DT50 reported by these authors is due to
variability in the rate of soil degradation processes
alone, as has been assumed, or whether technical error
and model lack of fit could have contributed to the
observed variability.
The aim of the current study was to characterise the
extent of within-field variability in the degradation rate
of three pesticides with a range of persistence and
bioavailabilities in soil. The contribution of technical
error and model lack of fit to the variability in
degradation rate was elucidated. We also determined
whether variability in degradation rate was linked to
variability in key chemical and microbial soil properties.
2. Materials and methods
2.1. Pesticides
Studies focussed on the persistent, highly sorbed
pesticide diflufenican (2#,4#-difluoro-2-(a,a,a,-trifluoro-mtolyloxy)nicotinanilide), the relatively rapidly degraded,
moderately mobile compound isoproturon (3-(4-isopropylphenyl)-1,1-dimethylurea) and the intermediate compound
azoxystrobin (methyl (E )-2-{2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl}-3-methoxyacrylate). Characteristics
of the pesticides are given in Table 1.
2.2. Field sites and pesticide treatment history
Two sites with contrasting soil types were sampled
during March 2002. The first site was Long Close field
on the farm at Warwick HRI, Wellesbourne, Warwickshire, UK. This soil is a sandy loam of the Wick series
(Whitfield, 1974). Over the 5 years prior to sampling, the
field had a history of diflufenican (1998, 1999, 2001) and
isoproturon use (1999, 2001). The second site was
Asplands 2 field at the Warwick HRI site at Kirton,
Lincolnshire, UK. The soil is a silt-loam of the Wisbech
series (Hodge et al., 1984), and the field had received
a previous application of azoxystrobin (2001).
2.3. Soil collection
At each site, 40 samples of soil were taken at 20 m
intervals on a 160!60 m grid within each field. At each
sampling site the top 20 cm of soil was collected with
a trowel which was sterilised with ethanol between each
site. The soils were sieved (!3 mm), with the sieve
sterilised with ethanol between processing of each
Table 1
Pesticide characteristics
Compound
Pesticide class
DT50a
Kow log Pb
Isoproturon
Azoxystrobin
Diflufenican
Phenylurea herbicide
Strobilurin fungicide
Pyridinecarboxamide
herbicide
6e28 days
1e8 weeks
15e30 weeks
2.5
2.5
4.9
a
b
Tomlin (2000).
Ware (1994).
G.D. Bending et al. / Environmental Pollution 139 (2006) 279e287
sample. Soils were air-dried to approximately 8%
moisture content prior to experimental use.
2.4. Soil chemical and biological characterisation
Total organic C and N were determined using an
automated C/N analyser (CN-2000, Leco Corporation,
Michigan, USA). Microbial biomass-N was extracted
using the chloroform fumigationeextraction technique
(Joergensen and Brookes, 1990). Ninhydrin-reactive N
released by fumigation was converted to biomass-N
using a conversion factor of 3.1 (Amato and Ladd, 1988).
Dehydrogenase activity was measured using the method
of Tabatabai (1994). Soil pH was measured using a glass
electrode in a 1:2.5 suspension of distilled H2O.
2.5. Pesticide application
Commercial formulations of isoproturon (Aventis
Crop Science, Lyon, France), azoxystrobin (Syngenta
Crop Protection, Bracknell, UK) and diflufenican
(Bayer Crop Science, Monheim, Germany) were diluted
with distilled H2O and applied to 500 g dw equivalent
portions of soil to give a concentration of 5 mg kg1
soil, except for isoproturon which received 15 mg kg1
soil. These concentrations correspond to the recommended field dose of the compounds when applied to
a 1 cm depth of soil. Additional distilled H2O was added
as necessary to bring the soil matric potential to
33 kPa. Each soil sample was mixed by hand using
a pair of disposable latex gloves. The soil was transferred to screw-top polypropylene bottles and incubated
in the dark at 15 C.
Sterile controls were prepared for soil from 10
locations at each site for each pesticide. Analytical grade
pesticide (British Greyhound, Birkenhead, UK) dissolved in ethanol was added to 500 g dw autoclave
sterilised soil to give the pesticide concentrations described above. Distilled H2O was added to give a matric
potential of 33 kPa and the soils were incubated in
sterile polypropylene bottles, as described above.
2.6. Pesticide analysis
Initial studies compared GC and HPLC methods for
determining amounts of azoxystrobin and diflufenican
extracted from soil. Azoxystrobin and diflufenican were
spiked into a single non-sterile soil sample taken from Long
Close field at concentrations between 0.1 and 5.0 mg kg1
soil, and residues extracted after 24 h using methanol,
as described below. Using GC conditions similar to
those described in Conte et al. (1998), and the HPLC conditions described below, recoveries of azoxystrobin and
diflufenican were 89.0 and 90.2%, respectively, by HPLC
and 84.3 and 78.6%, respectively, by GC. Furthermore, the
GC method could not detect azoxystrobin residues at
281
concentrations below 1 mg kg1 soil. Errors associated
with the GC method were also higher, with the standard
deviation associated with pesticide recovery 7.9 and 6.7%,
respectively, for azoxystrobin and diflufenican when
measured by HPLC, and 11.6 and 15.2%, respectively,
when measured by GC. HPLC was therefore chosen for
analysis of pesticide residues.
Amounts of parent compound in the non-sterile
samples were determined at monthly intervals, except
for isoproturon, which was assessed weekly. For the sterile
soils, samples of soil were aseptically removed after 0, 3,
6 and 9 months and the amounts of pesticide determined.
To extract pesticides, 20 g of soil was shaken with
20 ml of 90:10 v/v methanol:H2O for 1 h. After allowing
the soil to settle, pesticide concentrations were determined by HPLC using a Kontron Series 300 HPLC
with a Lichrosorb RP18 column (250!4.6 mm, Merck).
The pesticides were eluted with a mobile phase of
acetonitrile:H2O:orthophosphoric acid of 85:15:0.25 at
a flow rate of 1 ml min1, with diflufenican and
azoxystrobin detected by absorbance at 220 nm, and
isoproturon at 240 nm. In order to calculate the
technical error attributable to the combined effects of
sampling from bottles and the extraction and analysis of
pesticides, pesticide recovery from duplicate samples
taken from each bottle of soil was determined at the first
sampling. Analyses were not corrected for recovery.
2.7. Azoxystrobin sorption
Immediately after application of azoxystrobin, and
after 1 and 8 months, amounts of the compound in the soil
solution were determined in a sub-sample of 10 soil
samples selected from each location. These 10 samples
were chosen to span the range of C and pH values found
within each field. The method of Walker (2000) was used
to assess sorption in the soil samples. Ten grams of soil was
placed into a syringe body and centrifuged at 500!g for
5 min. The soil solution spun from the soil was collected
and azoxystrobin concentration measured by HPLC, as
described above. The amounts recovered in the soil
solution were expressed as a % of the total azoxystrobin
remaining in the sample (as determined in Section 2.6).
2.8. Statistical analysis
Diflufenican was degraded slowly, with 50% degradation not being reached in most soil samples.
Consequently, time to 25% degradation (DT25) was
calculated for each compound. For azoxystrobin and
diflufenican, degradation followed first-order kinetics,
and DT25 was determined by linear regression analysis
of the logarithm of concentration against time of
incubation. For isoproturon, degradation did not follow
first-order kinetics. Cubic regression analysis of the
logarithm of concentration against time was found to
282
G.D. Bending et al. / Environmental Pollution 139 (2006) 279e287
provide the best fit to isoproturon degradation, and was
used to generate DT25 values.
The components of variability of the DT25, which
incorporated technical errors associated with sampling
and pesticide analysis and model lack of fit, were
determined by inverse estimation using the reciprocal of
the decay rate constant (b), using a first-order Taylor
series approximation (Stuart and Ord, 1994). An
approximate expression for the variance of the DT25
was given by:
2
VarianceðDT25ÞZ
ðloge ðDT25ÞÞ varðbÞ
b4o
This expression was used to compute approximate
components of variability of DT25 by substituting the
estimated decay rate variance components var(b) and
using the average rate constant (bo) of the 40 soil
samples.
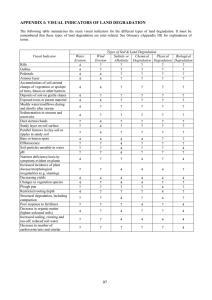
(Table 2). At Wellesbourne, organic-C was lower and
more variable, with a range of 0.89e2.31% (Fig. 1a),
a mean of 1.37% and a CV of 20.0% (Table 2). At
Kirton pH ranged from 7.1 to 8.1 (Fig. 1b) with an
average of 7.8 and a CV of 2.5% (Table 2). pH at
Wellesbourne was lower and more variable, with a range
of 5.7e7.7 (Fig. 1b), a mean of 6.8 and a CV of 6.2
(Table 2). Biomass at Kirton ranged from 3.4 to 9.2 mg
kg1 soil (Fig. 1c) with a mean of 6.2 and a CV of 21.4
(Table 2). Biomass was lower at Wellesbourne, ranging
from 1.0 to 6.3 mg C kg1 (Fig. 1c) with a mean of
4.0 mg kg1 with a CV of 25.0 (Table 2). Dehydrogenase
activity was also higher at Kirton relative to Wellesbourne, with mean values of 19.7 and 5.0 mg TPF g1
soil, respectively (Table 2). Variability of dehydrogenase
activity was higher at Wellesbourne relative to Kirton,
with CV of 31.0 and 27.6%, respectively.
3.2. Pesticide degradation
3. Results
3.1. Soil chemical and microbial characteristics
Soil organic-C at Kirton ranged from 1.47 to 1.87%
(Fig. 1a) with a mean of 1.60% and a CV of 6.9%
3.2.1. Degradation in sterile samples
There was no measurable degradation of any of the
compounds at either site, with recovery of pesticide
added to the sterile soil ranging from 98.9 to 106.3%
after 8 months.
a. Organic-C
b. pH
8.5
2.5
8.0
1.5
K
7.5
K
W
pH
% Organic-C
2.0
7.0
W
1.0
6.5
0.5
6.0
0.0
5.5
c. Biomass
d. Dehydrogenase
10
35
30
6
4
K
W
µg TPF g-1 soil
mg kg-1 soil
8
25
20
K
15
10
2
5
0
W
0
Fig. 1. Box and whisker plot showing distribution of soil chemical and microbial properties at Kirton (K) and Wellesbourne (W). Line within box
represents median; top and bottom box boundaries represent 75th and 25th percentiles, respectively; error bars indicate 90th and 10th percentiles.
283
G.D. Bending et al. / Environmental Pollution 139 (2006) 279e287
Table 2
Soil chemical and biological characteristics at Asplands 2 field, Kirton
and Long Close field, Wellesbourne
120
Parameter
100
Wellesbourne
Average % Coefficient Average % Coefficient
of variation
of variation
Organic-C (%)
1.60
pH
7.8
Biomass-C
6.2
(mg kg1 soil)
Dehydrogenase
19.7
(mg TPFa g1 soil)
a
6.9
2.5
21.4
1.37
6.8
4.0
20.0
6.2
25.0
27.6
5.0
31.0
Extractable isoproturon
(as % applied)
Kirton
a. Kirton
80
60
40
20
Triphenyl formazen.
0
0
5
10
15
20
25
Time (weeks)
b. Wellesbourne
120
100
Extractable isoproturon
(as % applied)
3.2.2. Pesticide degradation
Degradation of isoproturon in most soil samples
from Kirton showed kinetics typical of growth-linked
metabolism, with a lag phase which lasted for between 8
and 18 weeks, followed by a period of rapid degradation
(Fig. 2a). Several samples showed an exponential, with
no change in the rate of decline over time. Such
degradation kinetics are usually referred to as cometabolic degradation, and indicate that those organisms
responsible do not proliferate during degradation
(Torstensson, 1980). After 22 weeks, amounts of isoproturon remaining varied from 1.4 to 7.6% of the
amount applied (Fig. 2b). At Wellesbourne, degradation
was extremely rapid in all samples. Most samples
showed no lag phase before a period of exponential
decline. However, several samples showed a lag phase of
up to 18 d before a phase of rapid exponential decline.
After 74 d, amounts of residual isoproturon ranged
between 0.6 and 10.7% of the amount applied.
Isoproturon was degraded faster in Wellesbourne soil
than from Kirton, with DT25 of 0.56 and 4.4 weeks,
respectively (Table 3). Variation in DT25 was higher at
Wellesbourne than Kirton, with ranges of 0.34e1.16
and 2.51e7.18 weeks, respectively (Fig. 5a) with CV of
39.34 and 29.95%, respectively (Table 3). Degradation
of azoxystrobin showed cometabolic degradation, with
no change in the rate of decline over time at either site
(Fig. 3a,b). After 8 months, the amount of azoxystrobin
remaining ranged from 15.4 to 50.9% at Wellesbourne,
and between 29.9 and 49.3% at Kirton. The average
degradation rate was higher at Wellesbourne relative to
Kirton, with DT25 of 10.5 and 12.8 weeks, respectively
(Table 3). However, the range of DT25 was higher
at Wellesbourne than Kirton (Fig. 5b), with DT25 of
5.6e17.2 and 8.7e15.7 weeks, respectively (Fig. 5b).
CV for DT25 was 26.1 and 14.4% at Wellesbourne and
Kirton, respectively (Table 3).
Diflufenican was degraded very slowly in soil from both
locations, with no change in the rate of degradation over
time (Fig. 4a,b). The herbicide was degraded at almost the
same rate in the two soils. After 28 weeks, average
amounts of diflufenican remaining were 73.6 and 72.8%
80
60
40
20
0
0
20
40
60
80
Time (days)
Fig. 2. Isoproturon degradation. (a) Kirton: , average of 40 samples;
;, sample showing lag phase of 8 weeks prior to phase of rapid
degradation; :, sample showing lag phase of 14 weeks before period
of rapid degradation; -, sample showing no period of rapid
degradation. Bars represent G standard error of the mean. (b)
Wellesbourne: , average of 40 samples; ;, sample showing lag phase
of 18 days prior to phase of rapid degradation; :, sample showing no
lag phase prior to period of rapid degradation.
of the amount added in the Kirton and Wellesbourne soils,
respectively. The average DT25 was 29.5 weeks in the
Kirton and Wellesbourne soils (Table 3). The range of
DT25 was 13.0e60.6 weeks at Kirton and from 13.0 to
106.0 weeks at Wellesbourne (Fig. 5b). However, the
highest DT25 at Wellesbourne was almost twice that of the
Table 3
Pesticide DT25 in soil from Asplands 2 field, Kirton and Long Close
field, Wellesbourne
Pesticide
Isoproturon
Azoxystrobin
Diflufenican
Kirton
Wellesbourne
Average
DT25
(weeks)
% Coefficient
of variation
Average
DT25
(weeks)
% Coefficient
of variation
4.40
12.76
29.49
29.95
14.40
34.62
0.56
10.51
29.45
39.34
26.14
55.88
284
G.D. Bending et al. / Environmental Pollution 139 (2006) 279e287
a. Kirton
a. Kirton
110
100
Extractable diflufenican
(as % applied)
Extractable azoxystrobin
(as % applied)
100
90
80
70
60
50
80
60
40
40
30
20
0
10
20
30
40
0
5
10
15
20
Time (weeks)
Time (weeks)
b. Wellesbourne
b. Wellesbourne
25
30
35
110
100
Extractable diflufenican
(as % applied)
Extractable azoxystrobin
(as % applied)
100
90
80
70
60
50
80
60
40
40
30
0
10
20
30
40
Time (weeks)
20
0
5
10
15
20
25
30
Time (weeks)
Fig. 3. Azoxystrobin degradation. Bars represent G standard error of
the mean.
Fig. 4. Diflufenican degradation. Bars represent G standard error of
the mean.
second highest value (56.8 weeks). Variability was higher
at Wellesbourne than Kirton with CV of 55.9 and 34.6%,
respectively (Table 3).
the soil solution of 1.05e1.18 in Wellesbourne soil, and
1.24e1.40% of the total remaining in Kirton soil (Table 4).
After 8 months, amounts of azoxystrobin in the soil
solution had declined to 0.56 and 0.74% of the amount
remaining in Wellesbourne and Kirton soil, respectively.
3.2.3. Variability in DT25 attributed to
technical error and model lack of fit
Variability in pesticide recovery resulting from
technical error associated with soil sampling and residue
analysis, and model lack of fit, accounted for 22.2 and
25.8% of the variability in isoproturon DT25 at Kirton
and Wellesbourne, respectively. For azoxystrobin,
technical error and model lack of fit accounted for 5.2
and 24.6% of the variability in DT25 at Kirton and
Wellesbourne, respectively. However, in the case of
diflufenican, technical error and model lack of fit could
account for 97.0 and 93.4% of the variability in DT25 at
the Kirton and Wellesbourne sites, respectively.
3.3. Azoxystrobin sorption
Sorption of azoxystrobin remained constant over the
first month following addition, with average amounts in
3.4. Relationships between pesticide degradation and
soil characteristics
At Kirton, soil pH was significantly correlated with
DT25 for azoxystrobin (rZ0.38, P!0.05). At Wellesbourne, DT25 of azoxystrobin was correlated with soil
pH (rZ0.65, P!0.001), organic C (rZ0.42, P!0.01)
and organic N (rZ0.39, P!0.05). Regression analysis
indicated that each unit increase in pH gave a reduction
in time to DT25 of 4.25 (SE mean 0.808) and 3.6
(SE mean 1.43) weeks at Wellesbourne and Kirton,
respectively. There were no significant correlations
between DT25 of the other pesticides and the soil
parameters.
In soil from Wellesbourne, the % of azoxystrobin
residues in the soil solution was significantly correlated
285
G.D. Bending et al. / Environmental Pollution 139 (2006) 279e287
a. Isoproturon
b. Azoxystrobin
20
8
18
16
DT 25 (weeks)
DT 25 (weeks)
6
K
4
14
K
12
10
W
8
6
2
4
2
W
0
0
c. Diflufenican
120
DT 25 (weeks)
100
80
60
40
K
W
20
0
Fig. 5. Box and whisker plot showing distribution of DT25 values at Kirton (K) and Wellesbourne (W). Box structure as described in Fig. 1.
with pH after 0 and 1 month following addition
(rZ0.61 and 0.84, respectively, significant at
P!0.05 and 0.01, respectively) and with % organic C
after 1 month only (rZ0.63, P!0.05). DT25 was
significantly correlated with % azoxystrobin residues in
the soil solution after 0 and 1 month (rZ0.81 and 0.83,
respectively, P!0.01). There were no significant relationships between any of these parameters in soil from Kirton.
4. Discussion
Variability in degradation differed between the pesticides and between sites. Several previous studies using
Table 4
Percentage of total azoxystrobin residues in the water extractable
fraction in soil from Asplands 2 field, Kirton and Long Close field,
Wellesbourne, immediately following addition to soil, and after 1 and
8 months from application
Site
% azoxystrobin in soil solution
Time 0
1 month
8 months
Wellesbourne
Kirton
1.05 (0.10)
1.24 (0.11)
1.18 (0.08)
1.40 (0.04)
0.56 (0.05)
0.74 (0.05)
Figures in parentheses give G standard error of the mean.
readily degraded pesticides with low persistence have
demonstrated significant within-field variability in degradation rates, with CV of pesticide DT50 between 7 and
49% recorded (Walker and Brown, 1983; Parkin and
Shelton, 1992; Walker et al., 2001a,b). The variability
shown in our study, in which CV varied between 14.4 and
55.9%, is in the same range as that in the previous studies.
Massey and Lenoir (2003) suggested that withinsite variability in pesticide dissipation can arise from
variability in application, soil sampling and sample
analysis in addition to variability caused by factors
affecting biotic and abiotic degradation processes. We
determined combined CV associated with the technical
errors associated with bottle sampling and pesticide
residue analysis, and model lack of fit during derivation
of DT25 values, which was found to vary according to
site and pesticide. For isoproturon and azoxystrobin,
these sources of error could account for between 5 and
25.8% of the total variability, indicating that most of the
variability in degradation rates within the fields could
be accounted for by variation in the biotic processes
associated with pesticide biodegradation. However for
diflufenican, technical errors and model lack of fit
contributed between 93.4 and 97.0% of the variability,
suggesting that very little of the variation in degradation
286
G.D. Bending et al. / Environmental Pollution 139 (2006) 279e287
rates across the fields could be attributed to variation in
biotic degradation processes.
The % CV for pesticide degradation at Kirton was
lower than at Wellesbourne, in which variation in the
major soil and microbial properties was higher, suggesting
that variability in pesticide degradation rates was linked
to variability in soil properties. However, with the
exception of azoxystrobin, there were no clear relationships between the variation in soil properties and pesticide
degradation. Azoxystrobin degradation was strongly
linked to pH, with a stronger relationship at Wellesbourne, where there was a 2 unit variation in pH, than
at Kirton where there was only a 1 unit variation in pH.
The degradation rate of several pesticides has been
found to be related to pH. In a study of Canadian and
French soils, Houot et al. (2000) found that soils with a
pH over 6.5 typically showed growth-linked metabolism
of atrazine and those with pH lower than 6.5 showed
slower cometabolic degradation. Further, Bending et al.
(2003) showed that pH was a key factor controlling the
induction of growth-linked metabolism of isoproturon,
and thereby spatial patterns of degradation within
a field. In the current study, azoxystrobin degradation
occurred by cometabolism. Soil pH is known to play
a key role in controlling the structure of soil microbial
communities, including the fungus:bacteria ratio and the
distribution of functional and taxonomic groups (Baath
and Anderson, 2003). The identity and characteristics of
organisms contributing to cometabolic pesticide metabolism are unclear, and it is therefore uncertain whether
the regulation of azoxystrobin degradation by pH
reflects impacts of pH on the size or activity of
azoxystrobin degrading organisms. Additionally, pH is
known to affect the extent to which some pesticides sorb
to soil organic matter, and therefore to determine the
bioavailability of such pesticides to the degradative
community.
There is currently uncertainty as to how to quantify the
bioavailability of xenobiotics in soil. The amount of total
pesticide residues extractable in water may represent
a portion of the bioavailable fraction but does not provide
a measure of the total bioavailable fraction (Reid et al.,
2000). Our results indicate that in soil from Wellesbourne,
azoxystrobin sorption was related to pH, with sorption
decreasing as pH declined. This was unexpected, since
azoxystrobin is not an ionic pesticide. Further, there was
a strong correlation between azoxystrobin sorption and
DT25, with degradation rate decreasing as sorption
increased. This suggests that pH induced differences in
pesticide bioavailability could have had a role in
controlling the degradation rate of azoxystrobin.
There was no relationship between isoproturon
degradation and pH at Kirton or Wellesbourne. Isoproturon degradation has been studied extensively in the
field Deep Slade, which is next to Long Close, Wellesbourne. Deep Slade field has a pH gradient identical to
that in Long Close, and the two fields have the same soil
type, and similar cropping and isoproturon application
histories. However, a number of studies have shown clear
associations between isoproturon DT50 and pH in Deep
Slade (Walker et al., 2001a; Price et al., 2001; Bending
et al., 2003). In these previous studies, sites within the
field showing slow degradation of the compound were
associated either with cometabolic degradation, or an
extended phase of cometabolic degradation prior to
a period of rapid degradation. This was attributed to
direct effects of soil pH on the growth of isoproturon
degrading strains of Sphingomonas spp. (Bending et al.,
2003), with pH over 7.0 required for rapid degradation.
However, rapid growth-linked metabolism was recorded
in some low pH sites. In the current study, all sites within
Long Close showed rapid degradation, and there were no
extended periods of cometabolic degradation in any
samples. Comparisons between the Long Close and Deep
Slade fields highlight the problems in extrapolating the
characteristics of degradation processes to different
geographical locations.
Diflufenican was highly persistent in the soils studied,
with the extrapolated average DT50 in excess of a year
in most soil samples. Previous studies of diflufenican
metabolism in field experiments have found lower
persistence than in our study, with DT50 ranging
between 14 and 215 days (Rouchard et al., 1994;
Rouchard et al., 2000; Conte et al., 1998). However,
pesticide degradation in the field can occur more rapidly
than in laboratory experiments (Bromillow et al., 1999;
Muller et al., 2003). Despite a history of diflufenican
application at the Wellesbourne site, the kinetics of
degradation indicated that there had been no growthlinked metabolism of the compound. Similarly, Conte
et al. (1998) and Rouchard et al. (1999) found no
growth-linked metabolism of diflufenican despite repeated application at the same site for up to 4 years.
5. Conclusions
This study has shown that within single fields there can
be significant spatial variability in the degradation rate of
pesticides. This has implications for the potential for
pesticides to leach from soil and contaminate ground- and
surface-water, and to accumulate within agricultural soil
following repeated year-on-year application. However,
technical errors associated with sampling and analysis and
model lack of fit can make a significant contribution to
measured within-field variability in pesticide degradation,
and clearly need to be measured in studies which assess
spatial variability in pesticide fate. Current pesticide fate
models and degradation assays used in the pesticide
registration process to assess the environmental behaviour
of pesticides take no account of spatial variability in
degradation rate or fate. However, accurate assessment of
G.D. Bending et al. / Environmental Pollution 139 (2006) 279e287
the environmental risks associated with pesticide use will
require the extent and significance of spatial variability in
the environmental fate of pesticides to be considered.
Acknowledgements
We are grateful to the late Professor Allan Walker for
valuable discussion. We thank the Pesticides Safety
Directorate and the Department for Food, Environment
and Rural Affairs for funding.
References
Aislabie, J., Lloyd-Jones, G., 1995. A review of bacterial degradation
of pesticides. Australian Journal of Soil Research 33, 925e942.
Amato, M., Ladd, J.N., 1988. Assay for microbial biomass based
on ninhydrin-reactive nitrogen in extracts of fumigated soils.
Soil Biology and Biochemistry 20, 107e114.
Baath, E., Anderson, T.H., 2003. Comparison of soil/bacterial ratios
in a pH gradient using physiological and PLFA-based techniques.
Soil Biology and Biochemistry 35, 955e963.
Bending, G.D., Shaw, E., Walker, A., 2001. Spatial heterogeneity in
the metabolism and dynamics of isoproturon degrading microbial
communities in soil. Biology and Fertility of Soil 33, 484e489.
Bending, G.D., Lincoln, S.D., Sørensen, S.R., Morgan, J.A.W.,
Aamand, J., Walker, A., 2003. In-field spatial variability in the
degradation of the phenyl-urea herbicide isoproturon is the result
of interactions between degradative Sphingomonas spp. and soil
pH. Applied and Environmental Microbiology 69, 827e834.
Bromillow, R.H., Evans, A.A., Nicholls, P.H., 1999. Factors affecting
degradation rates of five triazole fungicides in two soil types: 2.
Field studies. Pesticide Science 55, 1135e1142.
Conte, E., Morali, G., Galli, M., Imbroglini, G., Leake, C.R., 1998.
Long-term degradation and potential plant uptake of diflufenican
under field conditions. Journal of Agriculture and Food Chemistry
46, 4766e4770.
Cullington, J.E., Walker, A., 1999. Rapid biodegradation of diuron
and other phenylurea herbicides by a soil bacterium. Soil Biology
and Biochemistry 31, 677e686.
Hodge, C.A.H., Burton, R.G.O., Corbett, W.M., Evans, R., Seale,
R.A., 1984. Soils and Their Use in Eastern England, Soil
Survey of England and Wales Bulletin No. 13, Harpenden,
Herts, UK.
Houot, S., Topp, E., Yassir, A., Soulas, G., 2000. Dependence of
accelerated degradation of atrazine on soil pH in French and
Canadian soils. Soil Biology and Biochemistry 32, 615e625.
Joergensen, R.G., Brookes, P.C., 1990. Ninhydrin-reactive measurements of microbial biomass in 0.5 M K2SO4 soil extracts. Soil
Biology and Biochemistry 22, 1023e1027.
Massey, J.H., Lenoir, J.S., 2003. Sources and magnitudes of variability
in the terrestrial field dissipation of pesticides. Terrestrial field
287
dissipation studies: purpose, design and interpretation. ACS
Symposium Series 842, 73e87.
Muller, K., Smith, R.E., James, T.K., Holland, P.T., Rahman, A.,
2003. Spatial variability of atrazine dissipation in an allophanic
soil. Pest Management Science 59, 893e903.
Price, O.R., Walker, A., Wood, M., Oliver, M.A., 2001. Using
geostatistics to evaluate spatial variation in pesticide/soil interactions. In: Walker, A. (Ed.), BCPC Symposium Proceedings No.
78: Pesticide Behaviour in Soils and Water. British Crop Protection
Council Symposium, Farnham, Surrey, pp. 233e238.
Parkin, T.B., Shelton, D.R., 1992. Spatial and temporal variability of
carbofuran degradation in soil. Journal of Environmental Quality
21, 672e678.
Reid, B.J., Jones, K.C., Semple, K.T., 2000. Bioavailability of
persistent organic pollutants in soils and sediments e a perspective
on mechanisms, consequences and assessment. Environmental
Pollution 108, 103e112.
Rouchard, J., Gustin, F., Callens, D., Vanhimme, M., Bulcke, R.,
1994. Effects of organic fertilizer treatment on herbicide diflufenican soil metabolism in winter-wheat crops. Toxicological and
Environmental Chemistry 42, 191e198.
Rouchard, J., Neus, O., Bulcke, R., Cools, K., Eelen, H., Dekkers, T.,
2000. Soil dissipation of diuron, chlorotoluron, simazine, propyzamide and diflufenican herbicides after repeated applications in
fruit tree orchards. Archives of Environmental Contamination and
Toxicology 39, 60e65.
Stuart, A., Ord, K., 1994. Distribution Theory. Kendall’s Advanced
Theory of Statistics, sixth ed., vol. 1. Edward Arnold, London.
Tabatabai, M.A., 1994. Soil enzymes. In: Weaver, R.W. (Ed.),
Methods of Soil Analysis: Part 2 Microbiological and Biochemical
Properties. Soil Science Society of America, Madison, WI,
pp. 903e947.
Tomlin, C.D.S., 2000. The Pesticide Manual, 11th ed. British Crop
Protection Council, Surrey, UK.
Torstensson, N.T.L., 1980. Role of microorganisms in decomposition.
In: Hance, R.J. (Ed.), Interactions Between Herbicides and the
Soil. EWRS, Academic Press, London.
Walker, A., 2000. A simple centrifugation technique for the extraction
of soil solution to permit direct measurement of aqueous phase
concentrations of pesticides. In: Jamet, P., Cornejo, J. (Eds.),
Pesticide/Soil Interactions: Some Current Research Methods.
INRA Publications, Paris, pp. 173e177.
Walker, A., Brown, P.A., 1983. Spatial variability in herbicide
degradation rates and residues in soil. Crop Protection 2,
17e25.
Walker, A., Jurado-Exposito, M., Bending, G.D., Smith, V.J.R.,
2001a. Spatial variability in the degradation rate of isoproturon in
soil. Environmental Pollution 111, 417e427.
Walker, A., Bromilow, R.H., Nicholls, P.H., Evans, A.A.,
Smith, V.J.R., 2001b. Spatial variability in the degradation rates of
isoproturon and chlortoluron in a clay soil. Weed Research 42, 39e44.
Ware, G.W., 1994 In: Reviews of Environmental Contamination and
Toxicology, vol. 137. Springer-Verlag, New York, USA.
Whitfield, W.A.D., 1974. The soils of the National Vegetable Research
Station, Wellesbourne. Report of the National Vegetable Research
Station for 1973, 21e30.