Laboratory Quality System Documents & Control
LABORATORY QUALITY MANUAL
5. QUALITY SYSTEM STRUCTURE
The system documents are on three tiers or levels:
Section 5 – Quality System Documents
Version: 04
Page: 1 of 3
Issue Date: 12 January 1999
Authorised by : Marjorie Dickenson
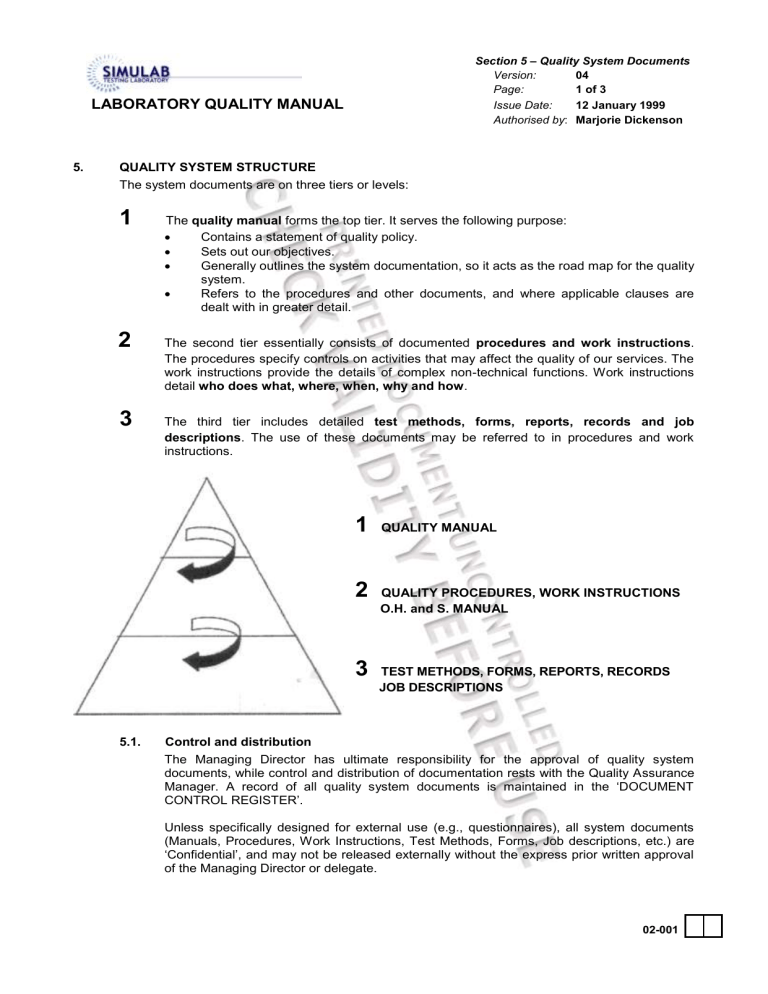
1
The quality manual forms the top tier. It serves the following purpose:
Contains a statement of quality policy.
Sets out our objectives.
Generally outlines the system documentation, so it acts as the road map for the quality system.
Refers to the procedures and other documents, and where applicable clauses are dealt with in greater detail.
2
The second tier essentially consists of documented procedures and work instructions .
The procedures specify controls on activities that may affect the quality of our services. The work instructions provide the details of complex non-technical functions. Work instructions detail who does what, where, when, why and how .
3
The third tier includes detailed test methods, forms, reports, records and job descriptions . The use of these documents may be referred to in procedures and work instructions.
1
QUALITY MANUAL
2
QUALITY PROCEDURES, WORK INSTRUCTIONS
O.H. and S. MANUAL
3
TEST METHODS, FORMS, REPORTS, RECORDS
JOB DESCRIPTIONS
5.1. Control and distribution
The Managing Director has ultimate responsibility for the approval of quality system documents, while control and distribution of documentation rests with the Quality Assurance
Manager. A record of all quality system documents is maintained in the ‘DOCUMENT
CONTROL REGISTER’.
Unless specifically designed for external use (e.g., questionnaires), all system documents
(Manuals, Procedures, Work Instructions, Test Methods, Forms, Job descriptions, etc.) are
‘Confidential’, and may not be released externally without the express prior written approval of the Managing Director or delegate.
02-001
LABORATORY QUALITY MANUAL
Section 5 – Quality System Documents
Version:
Page:
04
2 of 3
Issue Date: 12 January 1999
Authorised by : Marjorie Dickenson
All quality system documents are accessible in electronic form on the company’s intranet.
Only that version is considered to be a controlled copy. All printed documents are taken to be non-controlled copies, and their currency should be checked by reference to the electronic form.
Suitable security procedures are in place to avoid any unauthorized alteration or downloading of the quality system documentation.
Full details of the document control system in place at SimuLab P/L are described in Work
Instruction 03-003 Control of Documents .
5.2. Quality System Documents
Work Instructions (WI)
This m anual is a ‘methods manual’ for non-technical procedures such as methods preparation, analytical job registration, etc. Specific duties, such as Quality Assurance
Manager, etc, are detailed here also.
Document Control Register (DCR)
This document is the central record of all documents within the Quality System. The DCR also lists all reference books, maintenance logbooks and quality control data records.
Document Control Register details the location, identification, retention period, disposal method and responsible officer for controlled documents
Occupational Health and Safety Manual
This is the basic safe operating procedures manual for SimuLab P/L. Work Instructions and
Method Manuals safety procedures are based on those in the Occupation Health and Safety
Manual
Corrective and Preventive Actions Request (CAR) Register
All corrective actions are logged in the CAR Register.
Reference Material Register
Details of Certified Reference Materials and externally supplied standards are filed here.
Customer Complaint Register
All customer complaints whether formal or raised internally are logged on a Customer
Complaints Form (05-005) and filed in the Customer Complaint Register.
Audit Register
All reported audits are allocated a number and recorded in the Audit Register. This includes second and third party audits.
Equipment Calibration and Maintenance Manual
The Equipment Calibration and Maintenance Manual are intended as a reference to the calibration and maintenance of equipment and other instruments used within the SimuLab laboratory. All major items of laboratory equipment are listed in this manual.
Methods Manuals
These manuals give complete details of analytical procedures.
Worksheets and forms
All worksheets and forms used within the laboratory are part of the Quality System.
Worksheets and forms are available in PDF format on the SimuLab intranet.
02-001
LABORATORY QUALITY MANUAL
Section 5 – Quality System Documents
Version: 04
Page: 3 of 3
Issue Date: 12 January 1999
Authorised by : Marjorie Dickenson
Equipment Maintenance and Performance Log
All major items of equipment have a maintenance and performance log. Instrument performance parameters are either manually recorded in a log or an instrument printout is filed sequentially in a log. Maintenance on all equipment is recorded and filed in the log.
Standard Preparation Log
The preparation of all standard and calibration solutions are recorded on the appropriate worksheet and filed in the Standard Preparation Log.
02-001






