Chapter 5 Basic Material Handling Techniques
advertisement

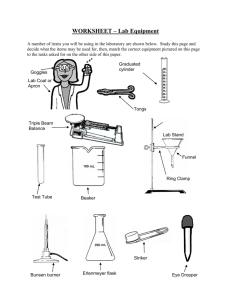
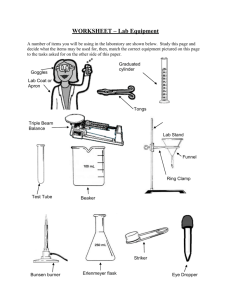
Intro Lab Methods Chapter 5 Basic Material Handling Techniques In any laboratory, certain techniques are regularly performed and are essential to most routine work. These techniques include the measurement and use of chemicals and other hazardous substances and the operation of laboratory equipment. A competent technician uses these basic techniques, confidently and without risk, in routine work and with any new procedures. Procedures include: • material transfer • weighing • volume measurement • solution preparation • filtration • heating, cooling and drying • temperature measurement • using gas cylinders • particle size reduction • cleaning and drying glassware. Material transfer Materials are routinely moved from one container to another during laboratory work. The transfer usually has to meet at least one of the following requirements: • safety with materials which are toxic, corrosive or dangerous in some way. • quantitative transfer where any loss will affect the accuracy of the task. • contamination control either from the surroundings to the material or vice versa. Weighing Weighing of materials is fundamental to most procedures. You should be competent at working with each of the balances found in the general laboratory. Weighing rules • all balances must be clean, level and zeroed • all chemicals must be weighed by difference in a container • don’t weigh hot or wet objects • clean up all spills immediately • check the balance has returned to zero and reset it before you leave. 28 | P a g e Intro Lab Methods Common terms used with weighing or the use of a balance include: • clean: nothing on the balance pan • level: the workbench and the balance need to be horizontal • balance reading: the mass on display • true mass: the mass of the sample after the container mass has been subtracted • reset: leave the balance clean, level and zeroed • weighing by difference: an empty container is weighed, the sample is added to it and the mass of the container and material recorded, the difference between the final mass and the empty container is the mass of the material • taring: the balance pan is adjusted to read zero with the empty container sitting on the balance pan, when the material to be weighed is added, the displayed weight is the weight of the added material only Volume measurement techniques The are many skills involved with working safely with liquids. Your teacher will demonstrate these skills and have you practice them. Liquid transfer involves transfer of all the liquid. Commonly losses occur with spillage, dribbles or failure to pour all of the liquid from one container to another. You will practice transferring liquids in the laboratory. The transfer of the correct volume also is dependent on having the correct volume to transfer. Measuring liquid volume requires you to read the position of the meniscus on a scale. The meniscus is the curvature or shape adopted by a liquid surface near the walls of any container. All volume readings are taken by lining up the flattest portion of the meniscus with the scale on the vessel. For water it is always the bottom but other liquids eg mercury have convex meniscus. Volume measurement may use quantitative or qualitative glassware. Quantitative glassware is highly accurate and reliable and includes: • pipettes • burettes • volumetric flasks Qualitative glassware is not very accurate or reliable and includes: • graduated beakers or flasks • measuring cylinders • droppers • an experienced guess in unscaled glassware 29 | P a g e Intro Lab Methods Pipetting techniques • perform a sacrificial rinse with the fill solution • overfill to above the mark • set the bottom of the meniscus on the line • allow to drain in a vertical position until no more liquid runs out • touch the tip on the side of the receiver to draw the final amount of liquid out • do not blow out, there must always be a little left behind, which is allowed for in the original calibration Burette techniques: • perform sacrificial rinse with the fill solution • overfill to above the mark • open tap fully and ensure all air bubbles are driven out of the tap and the tip of the burette • set the bottom of the meniscus on the chosen mark - usually zero • clamp in a vertical position at a convenient height above the receiver • touch a wastes receiver onto the side of the burette tip to draw any excess liquid off the tip • check your mark and record its value • run the required volume of liquid out and ensure any suspended drop on the burette tip is also transferred • read and record the new volume, perform any necessary subtractions General volumetric flask rules • perform a sacrificial rinse using the intended solvent • add the solute first, quantitatively, without loss • add some solvent to ensure the solute dissolves completely • mix well by inversion and swirling • solvent is added until the bottom of the meniscus is on the line, mix well and support the weight correctly • label with name, concentration, date and appropriate safety advice. Pipettors Also known as micropipettes, auto pipettes These are hand held filling devices to measure, very accurately, a very small volume of liquid or fluid. The device is operated via a plunger to provide the suction (the plunger moves up or out of the device) and delivery (the plunger moves down or into the device). The fluid or liquid only enters a disposable plastic tip, which may be yellow, white, green or blue. Different coloured tips have different capacities, but it is the pipettor that measures the actual volume. If you don’t fit the correct size you may overfill the tip and cause all sorts of problems. Generally the design of the device does NOT allow you to fit the wrong size tip. The device can be: • a fixed volume device eg 50 or 100 or 200 μL (microlitres) • a variable volume device across a wide range • multiheaded eg 5 or 10 or 20 tips may be fitted 30 | P a g e Intro Lab Methods Rules For Use TO FILL depress the plunger to the first stop, insert into your sample and allow the plunger to return SLOWLY to its rest position. TO EMPTY position tip over the receiver (as close as possible to the floor of the receiver to avoid splashes) and depress the plunger SLOWLY, past the first stop, all the way to the second stop. This blows all of your sample out of the tip. PROBLEMS accurate measurement and delivery is linked to an airtight seal. If you rush, you can force air or contents to escape. Do the operations slowly and smoothly. Preparation of solutions (common terms) Solute: the material which is being dissolved Solvent: the material which does the dissolving Solution: a solute dissolved in a solvent Concentration: the ratio of the quantity of solute to the quantity of solution Make up to volume: the required amount of solute is added to a container and solvent is added until the required final total volume is reached Dilution: making a weaker solution from a stronger one by addition of a solvent Serial dilution: making a more dilute solution by accurate dilution of an original solution and then repeating the procedure on the diluted solution to make even more diluted solution(s) whose concentration are accurately known with respect to the original solution. Cleaning and drying of glassware Laboratories have a huge variety of glassware and equipment, which needs to be clean, before it can be used. Equipment must be clean before use to prevent crosscontamination which may affect the analysis results Cleaning methods include the use of: • solvents • strong acids • abrasives • sanitising agents 31 | P a g e Intro Lab Methods Practical work 5.1 Basic material handling techniques All chemicals whether solid, liquid or gas must be handled with caution. This practical looks at the techniques to be employed when transferring solids and liquids. Practical work 5.2A Introductory weighing task The aim of the practical is to become familiar with the different types of balances available in a general laboratory. Your instructor will demonstrate the correct procedures to follow when using a balance. Practical work 5.2B Calibration of laboratory balances Practical work 5.3A Elementary volumetric measurements The practical is designed to provide the skills necessary to make volume measurements. It looks at the degree of accuracy of different measuring devices such as a beaker and volumetric flask. Practical work 5.3B Quantitative volumetric techniques Practical work 5.3C Calibration of volumetric glassware Practical work 5.4 Solution preparation You are required to prepare and validate known concentration solutions. Using an analytical balance weigh out accurately the mass of each solute indicated in the result sheet table. Quantitatively transfer the solid to a 100mL volumetric flask and make up to the mark with distilled water The teacher will show you the checks to be made on your sample and the previously prepared sample. Practical work 5.4B Advanced work with solution preparations A primary standard solution will be prepared followed by dilution to prepare a secondary standard solution. If the solutions do not meet the necessary tolerances, they will need to be remade. Using an analytical balance accurately weigh out the mass of each solute listed in the work sheet. (For hydrochloric acid, dilution by pipette is required). Quantitatively transfer the solid to a 100mL volumetric flask and make up to the mark Perform the dilutions and validity checks as indicated by the work sheet. 32 | P a g e Intro Lab Methods Practical work 5.5A Simple filtration Note: The chemicals in this practical are very toxic. Ensure that any spills are reported and also ensure that hands are properly washed if skin contact occurs and before leaving the laboratory. 1. Record the identity and quantity of each of the chemicals 2. Dispense an aliquot for each reagent bottle into a clean dry beaker 3. Carefully mix, using a glass stirring rod, the beaker contents over a steam bath for five minutes 4. Cool the beaker in an ice bath 5. Set up a filtration apparatus as shown by your teacher and using a labelled, preweighed filter paper filter your sample. 6. Wash the filter cake well with distilled water 7. Transfer the filter cake and filter paper to a labelled watch-glass and allow to dry 8. Reweigh and determine the amount of solid material. Practical work 5.5B Separation by filtration and centrifugation Practical work 5.6 Heating and temperature measuring equipment Practical work 5.7A Temperature calibration Practical work 5.7B Other laboratory temperature sensors Practical work 5.9 Manual methods of particle size reduction Practical work 5.10 Glass cleaning You will be given an amount of dirty glassware and equipment and a demonstration of various cleaning techniques. You will then practise the methods on your items. The teacher will inspect your efforts and may require you to attempt other methods or repeat the current process. Practical work 5 Application of heating equipment This practical looks at the workings of various types of heating equipment which are commonly in use in a general laboratory. (Bunsen burner, Meker burner, hotplate, steam-bath, etc) 33 | P a g e Intro Lab Methods Student Name: Practical: Weighing skills Practical Number: 5.2A Date Performed: Date Submitted: Text book References Procedure: Use the range of laboratory balances supplied as shown during your teacher’s demonstrations. You will perform repeated measurements which will be used to check your accuracy and that of the balances. Results: Object 1 code = Balance used Mass of Object 1 Object 2 code = Mass of Object 2 Object 3 code = Mass of Object 3 electronic top-pan 2 decimal place Brand name Range & sensitivity Electronic top-pan 3 decimal place Brand name Range & sensitivity Electronic analytical 4 decimal place Brand name Range & sensitivity Triple beam Brand name Range & sensitivity Questions: 1. Which type of balance do you prefer to use? Why? 2. Explain why you MUST keep using the same balance for a set of related mass measurements, from which the analysis results are calculated. 3. Outline the reproducibility of your readings for each object. 4. Which readings did your teacher reject (if any)? 5. Did improvements occur when they were repeated? Why? 34 | P a g e Intro Lab Methods Student Name: Practical: To become familiar with a range of laboratory balances and assess their performance using a range of unknown and standard masses Practical Number: Date Performed: Text book References Results: Balance specifications Mass (g) of 1.0000g Date Submitted: 5.2 B Mass (g) of 50.0000g Mass (g) of 100.0000g Other Mass Questions Write the answers to the textbook questions for this activity below. Please discuss your answers with your teacher / support staff if you are not sure of what to write. 35 | P a g e Intro Lab Methods Student Name: Practical: Practical Number: Date Performed: Elementary volume handling and measurements 5.3A + 4.3 Date Submitted: Text book References Procedure: Results: Read the quantity of liquid present and record in the table below: Vessel name Volume How accurate is your measurement? 36 | P a g e Intro Lab Methods Student Name: Practical: To become familiar with a range of laboratory volumetric devices and assess their performance using simple calibration procedures Practical Number: Date Performed: Date Submitted: Text book References see text book 5.3 B and 5.3 C Result table for 5.3B Name of volumetric device Sacrificial rinse Fill and adjust to zero / designated volume Deliver specified volume Pipette Burette Volumetric flask Not applicable Not applicable Teacher sign off 37 | P a g e Intro Lab Methods Result table for 5.3C Temperature = _______________ Density of water =________________ Name of volumetric device First attempt Second attempt Third attempt Fourth attempt First attempt Second attempt Third attempt Fourth attempt Measuring Cylinder Empty container mass (g) Container plus water mass (g) Mass of water (g) Volume of water (mL) Absolute error Relative error Stated tolerance Name of volumetric device Beaker Empty container mass (g) Container plus water mass (g) Mass of water (g) Volume of water (mL) Absolute error Relative error Stated tolerance 38 | P a g e Intro Lab Methods Name of volumetric device First attempt Second attempt Third attempt Fourth attempt First attempt Second attempt Third attempt Fourth attempt Volumetric Flask Empty container mass (g) Container plus water mass (g) Mass of water (g) Volume of water (mL) Absolute error Relative error Stated tolerance Name of volumetric device Burette Empty container mass (g) Container plus water mass (g) Mass of water (g) Volume of water (mL) Absolute error Relative error Stated tolerance 39 | P a g e Intro Lab Methods Name of volumetric device First attempt Second attempt Third attempt Fourth attempt Pipette Empty container mass (g) Container plus water mass (g) Mass of water (g) Volume of water (mL) Absolute error Relative error Stated tolerance 40 | P a g e Intro Lab Methods Calibrating an Autopipette Stated Vol (uL) = _______________ Temperature = _______________ Density of water =________________ Total Mass (g) Empty sample vial 1 Difference (g) ------ 2 3 4 5 6 7 8 9 10 Average (g) ------ Avg (mL) ------ Amount in uL (Avg x 1000) ------ 41 | P a g e Intro Lab Methods Student Name: Practical: Simple Solution Preparation Practical Number: 5.4A Date Performed: Date Submitted: Text book References Procedure: Results: Potassium Hydrogen Phthalate (Labelled as KHP) – 1.0g +- 0.1g Your Solution readings Standard Sample readings Sample mass: Sample volume: pH reading Conductivity Potassium chloride (labelled as KCl) – 5.0g +- 0.5g Your Solution readings Standard Sample readings Sample mass: Sample volume: RI Conductivity Potassium Dichromate (labelled K2Cr2O7) – 0.1g +- 0.01g - needs sulfuric acid added Your Solution readings Standard Sample readings Sample mass: Sample volume: Absorbance @ 450nm Questions: 1. Why is it important that an analytical balance is used to measure the mass? 2. Why is it important that the transfer of solid material is quantitative? 3. Why was a volumetric flask specified in each case? 42 | P a g e Intro Lab Methods Student Name: Practical: Advanced work with solution preparation – Serial dilution Practical Number: 5.4B Date Performed: Date Submitted: Text book References Procedure: follow the procedure outlined in the textbook Results: Sodium chloride Original solution Sample mass: (target 0.254g) Sample volume: 100mL Serial dilution details Volume taken for dilution Final diluted volume Solution concentration (ppm) Your Solution readings Standard Sample readings Cond. Cond. Your Solution readings Standard Sample readings 1000 Na Solution concentration (ppm) Dilution 1 100 Dilution 2 10 Dilution 3 1 Dilution 4 0.1 Hydrochloric Acid Original solution 1M Serial dilution details Sample mass: n/a Volume taken for dilution Sample volume: 100 mL Final diluted volume Solution concentration (molarity) pH Cond. pH Cond. 1M Solution concentration (molarity) Dilution 1 0.1M Dilution 2 0.01M Dilution 3 0.001M Dilution 4 0.0001M Conductivity tells you how well electricity can flow through the solution – how many ions and how able they are to carry a current. pH tells you how acidic the solution is – how many hydrogen ions are available. These measurements relate – in a linear fashion - to chemical concentration. 43 | P a g e Intro Lab Methods Questions: 1. What trends did you notice for your conductivity results. (hint: when an ionic solution is diluted to half its concentration, we expect to see half the conductivity) 2. What trends did you notice for your pH results. (hint: when an acidic solution is diluted to one tenth its concentration, we expect to see a full pH unit increase) 44 | P a g e Intro Lab Methods Student Name: Practical: Simple filtration Practical Number: 5.5A Date Performed: Text book References Procedure: Date Submitted: As per laboratory manual. Results: Identity of solution 1 Volume of solution 1 used Identify of solution 2 Volume of solution 2 used Mass of empty filter paper Mass of filter paper + precipitate Mass of precipitate Questions: 1. When is filtration a suitable method of separation? 2. One of the solutions you worked with contained lead. Why is it necessary to be extremely careful when working with this chemical? How would you find out the necessary safety requirements for working with lead solutions? 3. Why was it necessary to stir the solution over a water bath? 4. Why was it necessary to allow the solution to cool before filtration? 5. When filtering the solution is not filled to the top of the filter paper, why? 6. Draw a diagram to represent the simple filtration apparatus. List all the possible places where losses may have occurred. 45 | P a g e Intro Lab Methods Student Name: Practical: Comparison of types of Filtration and Centrifugation Practical Number: 5.5B Date Performed: Date Submitted: Text book References See p36-39 and p55-56 for overview of equipment and how to use it. Keywords for assessment purposes. Define: filtration, filtrate, residue, decantation, supernatant, Buchner funnel, Gooch funnel, Hirsch funnel, sintered glass crucibles, membrane filtration, centrifugation. Discuss; grades of paper, vacuum filtration, folding of papers, basic rules for centrifugation. Filter paper folding techniques Follow the teacher’s demonstration and supply one of each of the following for approval Conical fold approval Fluted paper approval Teacher’s signature Comparison of separation efficiencies You will be organised into teams to perform a study of different separation techniques for solid / liquid separations for at least two of the following systems. You will need to report on relative speed (use a stop watch to time the collection of 1mL of filtrate), effectiveness (assess the cloudiness or clarity of your filtrate) and efficiency (a combination of how well and how conveniently and how quick it worked) Calcium carbonate Copper hydroxide Muddy water Orange juice Conical folded qualitative paper Vacuum filtration Fluted paper Code… Fluted paper Code… Centrifuge appearance Ease of decantation after centrif. Ease of Pasteur pipette removal 46 | P a g e Intro Lab Methods Student Name: Practical: Application of heating equipment Practical Number: Date Performed: Date Submitted: Text book References Procedure: Task 1 1. Unscrew the stack on each of the supplied burners and examine the features of each burner. Note the gas jet or nipple and the rotatable sleeve which admits air and mixes it with the gas as they moves up the stack. 2. Attempt to light the gas at the nipple. Note - the gas supply may need to be very low for this. 3. Replace the stack and light the Bunsen as demonstrated by your teacher. 4. Note the effect of sleeve positioning on the appearance of the flame. 5. Using the blue flame, turn the gas pressure down slowly so that the flame gets smaller and strikes back down the stack to burn at the gas nipple. This condition is extremely dangerous because: • the Bunsen appears to be off and hence is a fire hazard • the stack will be heated by the flame and will burn anyone who touches it 6. Repeat the above five steps with the Meker and micro-burners. Results: Draw and label a Bunsen and discuss your observations below 47 | P a g e Intro Lab Methods Task 2 Use the supplied thermocouple to measure the temperature of various zones in both the ‘blue’ and ‘yellow’ versions of the Bunsen flame. Complete the following table: Zone of the flame being measured Yellow Flame temperature Blue Flame temperature Diagram to locate each zone Approx. 20cm above the tip of the flame At the tip of the flame 20 cm In the heart of the flame (top of blue cone) Level with the stack opening Task 3 This section is designed to investigate the changes which occur to the gas mixture in the flame. Following the demonstrations given by your teacher, examine the presence of zones of unburnt gases in a Bunsen burner as follows: 1. use a thin glass tube inserted directly into the bottom of the blue cone to tap off some of its contents – attempt to ignite the gases at the other end of the tube 2. pierce a live match with a pin inserted at right angles approximately half way along the match – suspend this match with its head pointing up, on the stack of an unlit Bunsen. Light the Bunsen, with the air hole open. Results: For parts 1 and 2 record your observations and your explanations of what is happening. 48 | P a g e Intro Lab Methods Task 4 This section tests the efficiency of heating water in various containers with different heating equipment. You are to report on: • the time taken for the water to boil • the amount of heating control you have • the safety of the method. Each of the containers should be about one third filled and heated as shown by your teacher. Container Heating device Time to boil (sec) Controllability or efficiency Safety hazards micro test tube Bunsen micro test tube micro burner test tube Bunsen test tube micro burner boiling tube Bunsen boiling tube micro burner Beaker (100 - 150mL) Beaker (100 - 150mL) Beaker (100 - 150mL) Beaker (100 150mL) Bunsen micro burner steam bath electric hotplate Questions: What is the major safety hazard when boiling liquids in test tubes? Which of the following would be suitable to heat water to boiling? • • • • heating mantle muffle furnace laboratory drying oven hair drier 49 | P a g e Intro Lab Methods Student Name: Practical: Practical Number: Date Performed: Temperature Calibration 5.7A Date Submitted: Thermometer 1 Thermometer 2 Identification (if any) Temperature Range Reading in ice water ( 0 degrees C) Reading in Boiling water ( 100 degrees C) Reading in dry ice/ acetone mixture ( -78 degrees C) 50 | P a g e