Efficient microwave-assisted solid phase coupling of nucleosides, small library
advertisement

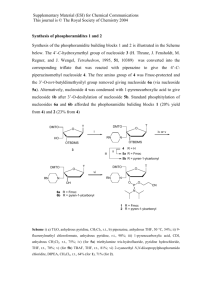
Tetrahedron Letters 54 (2013) 1869–1872 Contents lists available at SciVerse ScienceDirect Tetrahedron Letters journal homepage: www.elsevier.com/locate/tetlet Efficient microwave-assisted solid phase coupling of nucleosides, small library generation, and mild conditions for release of nucleoside derivatives Hanumantharao Paritala a, Yuta Suzuki b, Kate S. Carroll a,⇑ a b Department of Chemistry, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, USA Department of Chemistry, University of Michigan, Ann Arbor, MI 48109, USA a r t i c l e i n f o Article history: Received 22 January 2013 Accepted 24 January 2013 Available online 1 February 2013 Keywords: Solid phase synthesis Microwave assisted Nucleoside Combinatorial library a b s t r a c t Nucleosides are essential bio-molecules that participate in a wide array of biological processes involved in maintaining physiologic homeostasis. Recent efforts geared toward the synthesis of nucleoside analogues and development of nucleoside combinatorial libraries using solid phase synthesis have contributed invaluable information toward drug design and development. These studies have provided information concerning the structural requirements of substrate binding pockets of enzymes and evaluation of enzyme kinetics. However, the synthesis of nucleosides and its corresponding analogues remains a challenging and time consuming process. Herein, we report an efficient, microwave assisted solid phase coupling of nucleosides, combinatorial chemistry on the coupled nucleosides to generate small library, and mild cleavage conditions to release nucleoside derivatives from its solid support. We anticipate these findings will accelerate the development of synthetic methods or combinatorial library design of nucleoside analogues in similar settings. Ó 2013 Elsevier Ltd. All rights reserved. Introduction Nucleosides and its derivatives are important components and hold key roles in numerous biological processes and signal transduction. These bio-molecules are utilized as genomic building blocks, enzyme substrates, and cellular energy sources.1 Additionally, nucleoside analogues have been successfully utilized in anticancer, -bacterial, and—viral therapeutics.2 In this context, the synthesis and testing of bio-mimetic nucleoside derivatives has emerged as a widely used approach in drug discovery campaigns. The design of nucleoside analogues and combinatorial libraries using solid phase based techniques has garnered large momentum due to the importance of their therapeutic applications.3 However, the synthesis of nucleosides remains a challenge due to long reaction times, harsh cleavage conditions, and poor product yields.4 Redwan et al. recently reported a novel solid phase strategy that enables the synthesis of structurally different 5-O-[N-(acyl)-sulfamoyl] adenosines from a protected ribo-purine starting material. This approach applied cleavage conditions using trifluoroacetic acid (TFA) and ammonium formate, which resulted in poor yields of the nucleoside derivatives.5 In a separate study, Caroline et al. reported the synthesis of solid phase poly-phosphorylated nucleoside analogues with decent yields and mild cleaving conditions, but extremely slow reaction times.6 Therefore, we have explored microwave assisted solid phase nucleoside synthesis in order to ⇑ Corresponding author. Tel.: +1 561 228 2460; fax: +1 561 228 2919. E-mail address: kcarroll@scripps.edu (K.S. Carroll). 0040-4039/$ - see front matter Ó 2013 Elsevier Ltd. All rights reserved. http://dx.doi.org/10.1016/j.tetlet.2013.01.109 optimize reaction conditions and to create a platform to perform combinatorial chemistry on solid phase nucleosides. Herein, we describe an efficient microwave assisted solid phase coupling of nucleosides, small chemical library generation on the resin bound nucleosides, and the mild cleavage conditions to release the nucleoside derivatives from its resin support. Results and discussion For the synthesis of the adenosine scaffold, we initially used an existing protocol developed for generating inosine and uridine analogues3a. This procedure required purification after formation of the benzylidene intermediate 4, gave low yields of the scaffold (10–25%), and was insufficient for further solid phase synthesis of adenosine analogues. Collectively, this synthetic procedure was time consuming and required 8–10 days to achieve the final product. Optimization of the reported literation conditions would result in a facile synthesis of nucleoside derivatives with improved yield and decreased reaction times. The following key factors were included during the development of our approach: (1) employ microwave conditions to enhance reaction rates, and (2) utilize mild reaction conditions (Scheme 1). Generation of the poly styrene bound nucleoside scaffold started with the synthesis of Aldehyde 2 as previously reported3a, and activated by the formation of dimethylacetal 3. Compound 3 was immediately converted into benzylidene 4 via transacetylation to prevent reversion back to compound 2. The acetal forming reaction produced a mixture of diastereomers due to non-selective formation of the acetal 1870 H. Paritala et al. / Tetrahedron Letters 54 (2013) 1869–1872 Scheme 1. Synthesis of solid-supported nucleoside derivatives. Table 1 Optimization of reaction conditions for alkylation of aromatic amine on the nucleosides a b S. No Microwave temperature (°C) Time (min) Equivalents of propargylbromide Equivalents of base (DIEA)b Yielda (%) 1 2 3 4 80 100 150 150 10 15 10 15 1 1 1 2 1 1 1 2 0 0 45 90 % yield was determined by NMR using alanine as an internal standard. N,N-Diisopropylethylamine. carbon.7 Compound 4 was hydrolyzed to the carboxylic acid 5 with 48% overall yield in four steps. Finally, compound 5 was coupled to aminomethyl resin using O-(Benzotriazol-1-yl)-N,N,N0 ,N0 -tetramethyluroniumtetrafluoroborate (HBTU) activation to obtain the corresponding solid phase nucleoside scaffolds 6. When compared to solution chemistry, MW irradiation provides an overall higher yield and shorter reaction times. Using the resin bound nucleosides 6 as platform we started exploring possible chemistry on the aromatic amine and 50 -hydroxyl functionalities to construct four member library of resin bound nucleosides. In order to achieve this 50 -hydroxyl group of the resin bound nucleosides 6 was protected by using tert-butyldimethylsilyl chloride and imidazole with quantitative yields and this protection allowed us to selectively explore the alkylation reactions on the aromatic amine of 7. We have tested variety of conditions to install propargyl group on the aromatic amine as shown in Table 1. The ideal conditions to conduct this reaction using microwave are achieved by using two equivalents of propargylbromide and two equivalents of N,N-diisopropylethylamine at 150 °C with reasonable yields. Using the alkyne functional group on 8 we have H. Paritala et al. / Tetrahedron Letters 54 (2013) 1869–1872 1871 Scheme 2. Click chemistry on the solid-supported adenosine derivative and simultaneous 50 -TBDMS deprotection and release of nucleoside from solid phase. Scheme 3. Click chemistry on the solid-supported cytidine derivative and simultaneous 50 -TBDMS deprotection and release of nucleoside from solid phase. conducted click reaction with benzyl azide and methyl 2-azioacetate to create a four member library of solid phase nucleoside derivatives 9a, 10a, 9b, and 10b, as shown in scheme 2 and scheme 3. This chemistry demonstrated the facile synthesis of solid phase nucleoside derivatives using microwave conditions. Our next objective was to determine the mildest conditions required to cleave the polystyrene bound adenosine moiety (Table 2). We envisioned the development of cleavage conditions that would efficiently release the resin-bound nucleoside, but be able to tolerate the stability of nucleoside derivatives. This approach would contribute to the development of combinatorial libraries composed of nucleoside analogues with diverse structural motifs. We tested various cleavage conditions against resin 6a and 7a to yield adenosine as shown in Table 2. The product yield of each reaction condition was determined by NMR using alanine as an internal standard, which was chosen based on its solubility and stability.8 HPLC was used to analyze the integrity of the nucleoside after cleavage since adenosine can undergo decomposition to adenine and ribose sugar3a. In order to identify optimal cleavage conditions, we screened various conditions beginning with TFA as 1872 H. Paritala et al. / Tetrahedron Letters 54 (2013) 1869–1872 Table 2 Cleavage conditions to cleave adenosine from solid phase adenosine scaffold Type Acidic Acidic Acidic Oxidation Oxidation Oxidation Catalyst Catalyst Catalyst Catalyst Catalyst Catalyst Catalyst Catalyst Acidic Acidic a b c d e Condition c TFA 80%, 15 min, RT TFA 80%, 30 min, RTc TFA 5%, 30 min, RTc DDQ 1.5 equiv, 3 h, RTc DDQ 3 equiv, 3 h, RTc DDQ 3 equiv, 24 h, RT 8 mol % In(OTf)3, 6 min, 100 °C (MW assisted)c 8 mol % In(OTf)3, 30 min, 100 °C (MW assisted)c 8 mol % In(OTf)3, 30 min, 130 °C (MW assisted)c 8 mol % In(OTf)3, 30 min, 150 °C (MW assisted) c 40 mol% In(OTf)3, 30 min, 100 °C (MW assisted)c 8 mol% In(OTf)3, 1 h, 50 °C (MW assisted)c 8 mol% In(OTf)3, 24 h, RTc 40 mol% In(OTf)3, 24 h, RTc Acetic acid/TBAF (1:1), DMSO/water, rt, 2 he Acetic acid 10% v/v, 2 h, RTe Yielda (%) (%) Of adenosine7b 91 67 24 11 14 41 2 6 NAd NAd 15 6 5 22 88 89 88 80 90 78 77 93 99 99 — — 91 99 99 98 82 99 % Yield was determined by NMR using alanine as an internal standard. % Of adenosine is the ratio between adenosine and adenine (decomposed product from adenosine). R = OH. NA: adenosine was not observed. R = TBDMS. shown in Table 2. TFA is commonly used as a reagent to cleave the ribose acetal linker, and was found to efficiently cleave the nucleoside in 15 min with 80% TFA. Extending the reaction time resulted in cleavage of the glycosidic bond and formation of free adenine and ribose moieties. Mild acidic conditions (5% TFA, 24 h) were tested in hopes of improving adenosine cleavage while maintaining the glycosidic bond. These conditions reduced product yield and did not prevent adenosine decomposition. Oxidative cleavage conditions (e.g., 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)9, 3 equiv) afforded 41% yield of nucleoside coupled with the formation of adenine. Furthermore, catalytic cleavage of the acetal linker with indium trifluoromethanesulfonate (In(OTf)3)10 did not prove as efficient as acidic or oxidative conditions, but preserved the glycoside bond. Finally, nucleoside release from the resin was tested using acetic acid to cleave the acetal bond. Acetic acid (10% v/v) in water cleaved the nucleoside with simultaneous 50 -TBDMS deprotection with 89% yield without any detectable decomposition. In conclusion, we have developed a microwave-assisted synthetic route for coupling of nucleosides to aminomethylated polystyrene resin and showed using microwave conditions generations of four member solid phase nucleoside library coupled with mild cleavage conditions. This procedure offers decent product yields, shorter reaction times, and bypasses lengthy purification steps in comparison to previous reports. We anticipate that the present synthetic scheme should increase the overall efficiency of ribosebased combinatorial nucleoside libraries and is amendable to the synthesis of other derivatives. Acknowledgement This work was supported by the National Institutes of Health (GM087638 to K.S.C.). Supplementary data Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tetlet.2013.01. 109. References and notes 1. Berg, J. M.; Tymoczko, J. L.; Stryer, L. Biochemistry; W.H. Freeman: Basingstoke, 2012. 2. (a) Damaraju, V. L.; Damaraju, S.; Young, J. D.; Baldwin, S. A.; Mackey, J.; Sawyer, M. B.; Cass, C. E. Oncogene 2003, 22, 7524–7536; (b) De Clercq, E. J. Clin. Virol. 2004, 30, 115–133; (c) Winn, M.; Goss, R. J. M.; Kimura, K.-I.; Bugg, T. D. H. Nat. Prod. Rep. 2010, 27, 279–304. 3. (a) Epple, R.; Kudirka, R.; Greenberg, W. A. J. Comb. Chem. 2003, 5, 292–310; (b) de Champdore, M.; De Napoli, L.; Di Fabio, G.; Messere, A.; Montesarchio, D.; Piccialli, G. Chem. Commun. 2001, 10, 2598–2599; (c) Crimmins, M. T.; Zuercher, W. J. Org. Lett. 2000, 2, 1065–1067; (d) Bozzoli, A.; Kazmierski, W.; Kennedy, G.; Pasquarello, A.; Pecunioso, A. Bioorg. Med. Chem. Lett. 2000, 10, 2759–2763. 4. (a) Aucagne, V.; Berteina-Raboin, S.; Guenot, P.; Agrofoglio, L. A. J. Comb. Chem. 2004, 6, 717–723; (b) Ramasamy, K. S.; Amador, R. B.; Habib, Q.; Rong, F.; Han, X.; Li, D. Y.; Huang, J.; Hong, Z.; An, H. Nucleosides Nucleotides Nucleic Acids 2005, 24, 1947–1970; (c) Rodenko, B.; Detz, R. J.; Pinas, V. A.; Lambertucci, C.; Brun, R.; Wanner, M. J.; Koomen, G.-J. Bioorg. Med. Chem. 2006, 14, 1618–1629; (d) Oliviero, G.; Amato, J.; D’Errico, S.; Borbone, N.; Piccialli, G.; Mayol, L. Nucleosides Nucleotides Nucleic Acids 2007, 26, 1649–1652; (e) Poon, K. W. C.; Liang, N.; Datta, A. Nucleosides Nucleotides Nucleic Acids 2008, 27, 389–407. 5. Redwan, I. N.; Ingemyr, H. J.; Ljungdahl, T.; Lawson, C. P.; Grøtli, M. Eur. J. Org. Chem. 2012, 2012(19), 3665–3669. 6. Tonn, V. C.; Meier, C. Chem. Eur. J. 2011, 17, 9832–9842. 7. (a) Douglass, J. G.; Patel, R. I.; Yerxa, B. R.; Shaver, S. R.; Watson, P. S.; Bednarski, K.; Plourde, R.; Redick, C. C.; Brubaker, K.; Jones, A. C.; Boyer, J. L. J. Med. Chem. 2008, 51, 1007–1025; (b) Bakthavachalam, V.; Lin, L.-G.; Cherian, X. M.; Czarnik, A. W. Carbohydr. Res. 1987, 170, 124–135. 8. Hamper, B. C.; Kolodziej, S. A.; Scates, A. M.; Smith, R. G.; Cortez, E. J. Org. Chem. 1998, 63(3), 708–718. 9. (a) McDonald, C. E.; Dugger, R. W. Tetrahedron Lett. 1988, 29, 2413–2415; (b) Oikaw, Y.; Yoshioka, T.; Yonemitsu, O. Tetrahedron Lett. 1982, 23, 889–892. 10. Gregg, B. T.; Golden, K. C.; Quinn, J. F. J. Org. Chem. 2007, 72(15), 5890–5893. Efficient microwave-assisted solid phase coupling of nucleosides, small library generation and mild conditions for release of nucleoside derivatives. Hanumantharao Paritalaa, Yuta Suzukib, Kate S. Carrolla,* a Department of Chemistry, The Scripps Research Institute, Jupiter, Florida, 33458, USA Department of Chemistry, University of Michigan, Ann Arbor, Michigan, 48109, USA * Corresponding author b Address: Department of Chemistry, The Scripps Research Institute, 130 Scripps Way, Jupiter, Florida, 33458, USA email: kcarroll@scripps.edu Phone: (561) 228-2460 Fax: 561-228-2919 Supplementary Information General Experimental: Reagents and solvents were purchased from Sigma or other commercial sources and used without further purification. Aminomethyl polystyrene HL (100 – 200 mesh) and 4- benzyloxybenzaldehyde polystyrene HL were purchased from Novabiochem. Microwave reactions were conducted in sealed vessels using a microwave synthsizer (Initiator One Point, Biotage) with a power output ranging from 0 to 300W. Experiments were performed in 0.5-2 mL, 2-5 mL, or 10-20 mL glass vials with constant mixing. NMR spectra were obtained on a bruker 400 MHz for 1H and 13 C. 1H and 13 C NMR chemical shifts are reported in parts per million (ppm) referenced to the residual solvent peak. To determine percent yield of adenosine, the molarity of adenosine was calculated from the 1H NMR integral of the nucleoside pentose sugar ring relative to alanine (10 mg). Mass spectra were obtained with a agilent LC/MS mass spectrometer. Reverse-phase HPLC was performed on a Agilent HPLC system with a UV detector (λ = 260 nm) using a C18 (4.6 × 250 mm) Beckman Coulter Ultrasphere with a gradient of 0.1% TFA in H2O and CH3CN as the mobile phase. 6-(4-dimethoxymethyl-phenoxy)-hexanoic acid ethyl ester (3). A mixture of 2 (6.0 g, 25.4 mmol), trimethylorthoformate (8.78 mL, 80.2 mmol), and PTSA (216 mg, 1.14 mmol) in MeOH (3.15 mL) was irradiated by MW for 25 min at 60 oC. Triethylamine (158 µL, 1.14 mmol) was added to neutralize the reaction mixture. The reaction was concentrated in vacuo, diluted with EtOAc (30 mL), and washed with H2O (2 × 30 mL) and brine (1 × 30 mL). The organic layer was dried over Na2SO4, filtered, and concentrated in vacuo to provide a white solid (7.05 g, 25.4 mmol, 100%). The analytical data matched previously reported data3a. General procedure for the synthesis of (4). To a solution of adenosine/cytidine (7.5 mmol) in DMF (15 mL), 3 (5.17 g, 16.7 mmol) was added, followed by PTSA (144 mg, 0.757 mmol). The reaction mixture was subjected to MW irradiation for 1 hr at 130 oC, concentrated in vacuo, washed with methanol, and filtered to give a white solid. 4a: (2.06 g, 53.0 %). 1H NMR ((CD3)2SO, 400 MHz): δ = 8.37 (s, 1H), 8.36 (s, 1H´), 8.16 (s, 1H), 8.16 (s, 1H´), 7.48 (d, J = 9.0, 2H), 7.41 (d, J = 8.4, 2H´), 7.35 (s, 2H, 2H´), 6.98 (d, J = 9.0, 2H), 6.94 (d, J = 9.0, 2H´), 6.28 (d, J = 2.4, 1H), 6.25 (d, J = 3.0, 1H´), 6.17 (s, 1 H´), 5.96 (s, 1H), 5.46 (m, 1H, 1H´), 5.25 (t, J = 6.3, 1H), 5.13 (t, J = 5.7, 1H´), 5.05 (m, 1H, 1H´), 4.36 (q, J = 2.4, 1H), 4.26 (q, J = 4.8, 1H´), 4.03 (m, 2H, 2H´), 3.98 (m, 2H, 2H´), 3.58 (m, 2H, 2H´), 2.30 (q, J = 6.6, 2H, 2H´), 1.71 (m, 2H, 2H´), 1.60 (m, 2H, 2H´), 1.41 (m, 2H, 2H´), 1.16 (m, 3H, 3H´).13C NMR ((CD3)2SO, 400 MHz): δ = 172.8, 159.8, 159.6, 156.1, 156.1, 152.7, 152.6, 149.0, 148.8, 139.8, 139.7, 128.5 (2C), 128.4 (2C), 128.0, 127.9, 119.1, 119.0, 114.2 (2C), 114.1 (2C), 106.5, 102.9, 89.5, 87.9, 86.3, 84.4, 83.6, 82.8, 82.5, 80.4, 67.4, 67.3, 61.5, 59.6, 33.4, 28.3, 25.0, 24.2, 14.1. Mass calculated for C25H31N5O7 is 513.5429, found (M+H) = 514.62. 4b:(1.5 g, 42.5 %) 1H NMR ((CD3)2SO, 400 MHz): δ = 9.16 (d, J = 8.5, 1H), 7.28 (d, J = 9.0, 2H), 7.28 (d, J = 8.4, 2H´), 7.35 (s, 2H, 2H´), 6.17 (d, J = 9.0, 2H), 6.94 (d, J = 9.0, 2H´), 6.28 (d, J = 2.4, 1H), 6.25 (d, J = 3.0, 1H´), 6.17 (s, 1 H´), 5.96 (s, 1H), 5.46 (m, 1H, 1H´), 5.25 (t, J = 6.3, 1H), 5.13 (t, J = 5.7, 1H´), 5.05 (m, 1H, 1H´), 4.36 (q, J = 2.4, 1H), 4.26 (q, J = 4.8, 1H´), 4.03 (m, 2H, 2H´), 3.98 (m, 2H, 2H´), 3.58 (m, 2H, 2H´), 2.30 (q, J = 6.6, 2H, 2H´), 1.71 (m, 2H, 2H´), 1.60 (m, 2H, 2H´), 1.41 (m, 2H, 2H´), 1.16 (m, 3H, 3H´).13C NMR ((CD3)2SO, 400 MHz): δ = 173.5, 160.2, 158.6, 154.1, 155.2, 151.8, 151.1, 149.5, 147.6, 139.8, 139.7, 127.5 (2C), 129.4 (2C), 128.0, 127.9, 119.1, 119.0, 114.2 (2C), 114.1 (2C), 106.5, 102.9, 89.5, 87.9, 86.3, 84.5, 83.6, 83.1, 82.8, 80.9, 67.5, 67.2, 61.2, 59.7, 33.6, 28.3, 25.2, 24.1, 14.2. Mass calculated for C24H31N3O8 is 489.5182. Found (M+H) = 490.34. General procedure for synthesis of (5). A solution of NaOH (195 mg, 4.87 mmol) in H2O (1 mL) and MeOH (3 mL) was added to a suspension of 4a/4b (1.0 mmol) in MeOH (4.5 mL), irradiated by MW for 25 min at 100 oC, and concentrated in vacuo. The remaining residue was dissolved in H2O, and was treated dropwise with 1 M HCl to pH 4. The white precipitate was filtered and dried in vacuo to yield a white powder. 5a: (461 mg, 97.6 %); 1H NMR (CDCl3, 400 MHz): δ = 8.38 (s, 1H), 8.37 (s, 1H´), 8.16 (s, 1H), 8.15 (s, 1H´), 7.47 (d, J = 8.4, 2H), 7.40 (d, J = 8.4, 2H´), 7.34 (s, 2H, 2H´), 5.98 (d, J = 8.4, 2H), 6.94 (d, J = 8.4, 2H´), 6.28 (d, J = 3.0, 1H), 6.26 (d, J = 3.6, 1H´), 6.16 (s, 1H), 5.94 (s, 1H), 5.45 (m, 1H, 1H´), 5.06 (m, 1H, 1H´), 4.36 (m, J = 2.4, 1H), 3.96 (m, 2H, 2H´), 3.57 (m, 2H, 2H´), 2.15 (q, J = 6.6, 2H, 2H´), 1.70 (m, 2H, 2H´), 1.53 (m, 2H, 2H´), 1.40 (m, 2H, 2H´).13C NMR (CDCl3, 400 MHz): δ = 175.1, 159.8, 159.7, 156.1, 156.1, 152.7, 152.6, 149.0, 148.8, 139.8, 139.7, 128.5 (2C), 128.4 (2C), 128.0, 127.9, 119.1, 119.0, 114.2 (2C), 114.1 (2C), 106.5, 102.9, 89.5, 87.9, 86.3, 84.5, 83.6, 82.8, 82.6, 80.5, 67.5, 67.4, 61.5, 34.7, 28.5, 25.3, 24.7. Mass calculated for C23H27N5O7 is 485.4898, found (M+H) 486.54. 5b: (461 mg, 97.6 %); 1H NMR (CDCl3, 400 MHz): δ = 9.16 (d, J= 6.9, 1H), 8.6 (s, 1H), 8.16 (s, 1H), 8.15 (s, 1H´), 7.27 (d, J = 8.4, 2H), 7.29 (d, J = 8.4, 2H´), 6.92 (d, J= 8.2, 2H, 2H´), 5.98 (d, J = 8.4, 2H), 6.94 (d, J = 8.4, 2H´), 6.28 (d, J = 3.0, 1H), 6.26 (d, J = 3.6, 1H´), 6.16 (s, 1H), 5.94 (s, 1H), 5.45 (m, 1H, 1H´), 5.06 (m, 1H, 1H´), 4.37 (m, J = 2.4, 1H), 3.96 (m, 2H, 2H´), 3.57 (m, 2H, 2H´), 2.17 (q, J = 6.6, 2H, 2H´), 1.72 (m, 2H, 2H´), 1.54 (m, 2H, 2H´), 1.41 (m, 2H, 2H´).13C NMR (CDCl3, 400 MHz): δ = 178.4, 165.5, 159.8, 155.1, 143, 129.6, 127.4, 115.1, 113.1, 100.2, 95.2, 88.1, 87.1, 84.8, 69.6, 62.3, 34.2, 28.7, 25.3, 24.3. Mass calculated for C22H27N3O8 is 461.4651, found (M+H) 462.54. General procedure for resin-bound nucleoside scaffold (6). Aminomethyl resin (864 mg, 0.50 mmol) was swelled in DMF (2 mL) for 20 minutes. A solution of 5a/5b (0.6 mmol), HBTU (198 mg, 0.62 mmol), DIEA (108 µL, 0.62 mmol) in DMF (6 mL) was added to the swollen resin in DMF and subjected to MW irradiation for 1.5 hr at 60 oC. Then the Resin was washed with DMF and MeOH, and lyophilized to remove excess residual solvent. Nucleoside coupling to solid phase resin was confirmed by LC/MS analysis. Resin bound nucleoside (6a/6b) 20mg was taken and washed with 10%V/V acetic acid in water for 2 hours and the solid phase resin was filtered with help of glass wool. Then the filtrate was lyophilized, added with 10mg alanine as internal standard, solubilised in 1ml of CD3OD and NMR spectra were recorded (6a, 75%; 6b, 63%). General procedure for synthesis of (7). Solid phase nucleoside (6a/6b) (0.4 mmol) was dried under high vacuum and swelled in DMF (5 ml) for 20 minutes. Imidazole (68 mg, 1 mmol), TBDMS-Cl (150mg, 1mmol) added to the reaction at 0C. The contents were stirred for 15 min then switched to microwave and conducted the reaction at 45C, for 20min. Then the resin was washed with DMF followed by methanol to remove residual imidazole and unreacted TBDMSCl. The resin was dried and under high vaccum. Resin bound 5’-TBDMS protected nucleoside (7a/7b) 20mg was taken and washed with 10%V/V acetic acid in water for 2 hours and the solid phase resin was filtered with help of glass wool. Then the filtrate was lyophilized, added with 10mg alanine as internal standard, solubilised in 1ml of CD3OD and NMR spectra were recorded. 7a (85%) 1H NMR (CD3OD, 400 MHz): δ = 8.58 (s, 1H), 8.35 (s, 1H), 6.12 (s, 1H), 4.71 (m, 1H), 4.52(m, 1H), 4.40 (m, 1H), 1.01(s, 9H), 0.26(s, 6H.13C NMR (CD3OD, 400 MHz): δ = 156.2, 152.4, 149.8, 140.3, 119.4, 97.5, 88.0, 74.2, 70.1, 63.2, 30.6, 25.9, 3.2. Mass calculated for C16H27N5O4Si is 381.5022, found (M+H) 382.27; 7b (79%) 13 C NMR (CD3OD, 400 MHz): δ = 9.11(d, J=8.2, 1H), 5.93 (s, 1H), 4.52(s, 1H), 4.40(m, 1H), 0.99(s, 9H), 0.24(s, 6H.13C NMR (CD3OD, 400 MHz): δ= 165.5, 155.2, 143.8, 100.3, 95.2, 86.7, 73.2, 70.6, 63.1, 30.6, 25.9, 2.4. Mass calculated for C15H27N3O5Si is 357.4775, found (M+H) 358.53. General procedure for synthesis of (8). Resin bound 5’-TBDMS nucleoside (7a/7b) (0.35 mmol) was dried under high vacuum and swelled in DMF (6ml) for 20 minutes, added propargyl bromide(238 mg, 2 mmol), N,N-Diisopropylethylamine (260mg, 2mmol) at room temperature. The contents were stirred for 5 min then switched to microwave and conducted the reaction at 150 oC, for 15minutes. Then the resin was washed with DMF followed by methanol to remove residual N,N-Diisopropylethylamine and un reacted prpargylbromide. The resin was dried and under high vaccum. The resin bound 5’-TBDMS, N-alkylated nucleoside (8a/8b) 20mg was taken and washed with 10%V/V acetic acid in water for 2 hours and the solid phase resin was filtered with help of glass wool. Then the filtrate was lyophilized, added with 10mg alanine as internal standard, solubilised in 1ml of CD3OD and NMR spectra were recorded. 8a (65%) 1H NMR (CD3OD, 400 MHz): δ = 8.35 (s, 1H), 8.16 (s, 1H), 6.16 (d, J=6.3, 1H), 4.75 (m, 1H), 4.51 (m, 1H), 4.40 (m, 1H), 3.8 (s, 2H), 2.65 (t, J=5.8, 1H), 1.01(s, 9H), 0.26(s, 6H.13C NMR (CD3OD, 400 MHz): δ = 154.7, 152.4, 149.8, 140.3, 119.4, 97.3, 87.0, 79.5, 73.7, 73.2, 70.5, 63.0, 30.7, 30.4, 25.9, 3.2. Mass calculated for C19H29N5O4Si is 419.5502, found (M+H) 420.41.8b (54%) 1H NMR (CD3OD, 400 MHz): δ = 9.16 (d, J=8.2, 1H), 5.93 (d, J=5.7, 1H), 5.4 (d, J=8.4, 1H), 4.51(m, 1H), 4.40 (m, 1H), 4.28 (m, 1H), 4.04 (m, 2H), 3.66 (m, 2H), 2.65 (s, 1H), 1.01(s, 9H), 0.26(s, 6H.13C NMR (CD3OD, 400 MHz): δ =158.9, 155.1, 143.8, 100.2, 95.7, 86.7, 73.4, 73.2, 70.6, 63.0, 25.9, 5.1. Mass calculated for C18H29N3O5Si is 395.5255, found (M+H) 396.73. General procedure for synthesis of (9 and 10). Solid supported 5’O-TBDMS, N-alkyl nucleoside, 8a/8b (0.1 mmol), was swelled in DMF for 20 mins. Added CuI (19mg, 0.1 mmol), N,N-Diisopropylethylamine (130mg, 1mmol) and Benzyl azide/methyl 2-azidoacetate, (134mg, 1mmol/115mg, 1mmol respectively) to the reaction mixture and stirred for 10 min at room temperature. Then the reaction was transferred to microwave reactor heated at 100oC for 15 minutes. Then the resin was washed with DMF followed by methanol to remove residual reagents. The resin was dried and under high vaccum. The resin bound 5’-TBDMS, N-alkyl trazolyl nucleoside (9a/10a/9b/10b) 20mg was taken and washed with 10%V/V acetic acid in water for 2 hours and the solid phase resin was filtered with help of glass wool. Then the filtrate was lyophilized, added with 10mg alanine as internal standard, solubilised in 1ml of CD3OD and NMR spectra were recorded. Optimization of conditions for the cleavage of nucleoside derivatives from the resin (11 and 12). 1. Cleavage under acidic or catalytic conditions: The resin bound scaffold (100 µmol) was treated TFA, In(OTf)32,or acetic acid in D2O (total volume: 2 mL) and subjected to MW irradiation under the conditions indicated in Table 1. The reaction was then filtered through glass wool and washed with its respective solution (1mL). The filtrates were combined and transferred to a NMR tube for direct evaluation of the cleaved product yield as described in the general experimental section. The sample was evaporated in vacuo and/or lyophilized overnight. The sample was characterized using analytical RP-HPLC and ESI-LRMS. 2. Cleavage under oxidative conditions: The resin bound adenosine scaffold (0.1 mmol) was treated with DDQ3 in 1:1 H2O/acetonitrile (2 mL) and subjected to the conditions indicated in Table 1. The reaction was then filtered through glass wool and washed with 1:1 H2O/acetonitrile (1mL). The sample was evaporated in vacuo and lyophilized overnight. The residue was dissolved in D2O (2 mL) and analyzed by NMR as described in the general experimental section. The sample was characterized using analytical RP-HPLC and ESI-LRMS. Optimized procedure for the release of nucleoside derivative from resin (11 and 12). The resin bound 5’-TBDMS, N-alkyl trazolyl nucleoside (9a/10a/9b/10b) (0.1mmol) was taken and washed with 10%V/V acetic acid in water for 2 hours and the solid phase resin was filtered with help of glass wool. Then the filtrate was lyophilized, added with 10mg alanine as internal standard, solubilised in 1ml of CD3OD and NMR spectra were recorded. 11a (45%) 1H NMR (CD3OD, 400 MHz): δ = 8.35 (s, 1H), 8.16 (s, 1H), 7.63 (s, 1H), 7.33 (m, 5H), 6.16 (d, J=8.2, 1H), 5.48 (s, 2H), 4.75 (s, 1H), 4.51 (m, 1H), 4.40 (m, 1H), 3.79 (m, 2H). 13 C NMR (CD3OD, 400 MHz): δ = 154.7, 152.4, 149.8, 140.3, 133.7, 130.7, 128.6, 125.7, 122.9, 119.4, 97.3, 87.4, 73.7, 70.5, 61.6, 57.3, 42.3. Mass calculated for C20H22N8O4 is 438.4399, found (M+H) 439.51. 12a (52%) 1H NMR (CD3OD, 400 MHz): δ = 8.35 (s, 1H), 8.16 (s, 1H), 7.5 (s, 1H), 6.16 (d, J= 6.4, 1H), 5.12 (s, 2H), 4.75 (m, 1H), 4.51 (m, 1H), 4.4 0 (m, 1H), 4.36 (s, 2H), 3.79 (m, 2H), 3.68 (s, 3H). 13 C NMR (CD3OD, 400 MHz): δ = 166.1, 154.7, 152.4, 149.8, 140.3, 130.7, 122.9, 119.4, 97.3, 87.4, 73.7, 70.5, 61.6, 52.9, 51.6, 42.3. Mass calculated for C16H20N8O6 is 420.38, found (M+H) 421.22. 11b (65%) 1H NMR (CD3OD, 400 MHz): δ = 9.16 (d, J=8.2, 1H), 7.63 (s, 1H), 7.23 (m, 5H), 5.93 (d, J=5.3, 1H), 5.48(s, 2H), 5.39 (d, J=7.9, 1H), 4.52 (m, 1H), 4.4(m, 1H), 4.28(s, 1H), 3.8 (m, 2H), 3.59 (s, 2H). 13 C NMR (CD3OD, 400 MHz): δ = 158.9, 155.1, 143.8, 133.7, 130.7, 128.6, 127.6, 125.7, 122.9, 100.2, 95.7, 87.0, 73.2, 70.6, 61.6, 57.3, 43.1. Mass calculated for C19H22N6O5 is 414.4152, found (M+H) 415.38. 12b (62%) 1H NMR (CD3OD, 400 MHz): δ = 9.16 (d, J=7.8, 1H), 7.5 (s, 1H), 5.93 (d, J=5.4, 1H), 5.4 (d, J=8.1, 1H), 5.12 (s, 2H), 4.51 (m, 1H), 4.40 (m, 1H), 4.28 (m, 1H), 3.79 (m, 2H), 3.68 (s, 3H). 13 C NMR (CD3OD, 400 MHz): δ = 170.1, 158.9, 155.1, 143.8, 130.7, 123.1, 100.5, 96.1, 87.3, 73.2, 70.6, 2.9, 51.6, 44.1.Mass calculated for C15H20N6O7 is 396.3553, found (M+H) 397.42.