Skaggs Institute for Chemical Biology
advertisement

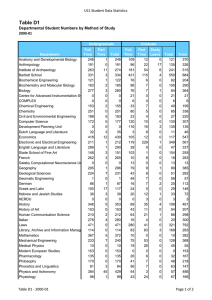
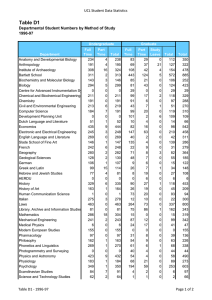
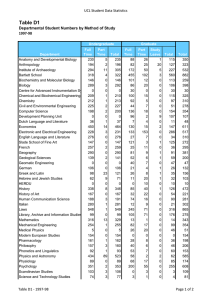
Skaggs Institute for Chemical Biology Wide-angle perspective: Looking down the barrel of the cylindrical capsule, we find n-tetradecane in a coiled, helical conformation. At first glance, this imposition of host on guest appears unfavorable, but in the end, adaptation maximizes contact between the two and results in an induced-fit match. Work for the image done by Michael P. Schramm, Ph.D., The Skaggs Institute for Chemical Biology. Trevor Dale Graduate Student, Chemistr y The Skaggs Institute for Chemical Biology THE SKAGGS INSTITUTE FOR CHEMICAL BIOLOGY THE SKAGGS INSTITUTE FOR CHEMICAL BIOLOGY S TA F F Julius Rebek, Jr., Ph.D.* Professor and Director Carlos F. Barbas III, Ph.D.** Professor Janet and W. Keith Kellogg II Chair in Molecular Biology Tamas Bartfai, Ph.D. Professor Chairman, Molecular and Integrative Neurosciences Department, Scripps Research Director, Harold L. Dorris Neurological Research Institute Ernest Beutler, M.D.*** Professor Chairman, Department of Molecular and Experimental Medicine, Scripps Research Dale L. Boger, Ph.D.* Richard and Alice Cramer Professor of Chemistry Geoffrey Chang, Ph.D.** Associate Professor Benjamin F. Cravatt, Ph.D.**** Professor Director, Helen L. Dorris Child & Adolescent NeuroPsychiatric Disorder Institute Philip Dawson, Ph.D.***** Associate Professor Gerald M. Edelman, M.D., Ph.D. † Professor Chairman, Department of Neurobiology, Scripps Research Albert Eschenmoser, Ph.D.* Professor Martha J. Fedor, Ph.D.** Associate Professor M.G. Finn, Ph.D.* Associate Professor Elizabeth D. Getzoff, Professor Ph.D.†† M. Reza Ghadiri, Ph.D.* Professor Kim D. Janda, Ph.D.* Professor Ely R. Callaway, Jr., Chair in Chemistry Director, The Worm Institute for Research and Medicine Gerald F. Joyce, M.D., Ph.D. ††† Professor Dean, Faculty Ehud Keinan, Ph.D.** Adjunct Professor Jeffery W. Kelly, Ph.D.* Lita Annenberg Hazen Professor of Chemistry Dean, Graduate and Postgraduate Studies Richard A. Lerner, M.D. ††† President, Scripps Research Lita Annenberg Hazen Professor of Immunochemistry Cecil H. and Ida M. Green Chair in Chemistry Stephen P. Mayfield, Ph.D.***** Professor Associate Dean, Kellogg School of Science and Technology K.C. Nicolaou, Ph.D.* Aline W. and L.S. Skaggs Professor of Chemical Biology Darlene Shiley Chair in Chemistry Chairman, Department of Chemistry, Scripps Research Paul R. Schimmel, Ph.D. ††† Ernest and Jean Hahn Professor and Chair in Molecular Biology and Chemistry 2006 THE SCRIPPS RESEARCH INSTITUTE Peter Schultz, Ph.D.* Professor Scripps Family Chair 11 Lionel Moisan, Ph.D. Andrew Myles, Ph.D. Severin Odermatt, Ph.D. K. Barry Sharpless, Ph.D.* W.M. Keck Professor of Chemistry Subhash C. Sinha, Ph.D.** Associate Professor John A. Tainer, Ph.D.** Professor James R. Williamson, Ph.D.††† Professor Associate Dean, Kellogg School of Science and Technology Ian A. Wilson, D.Phil.** Professor Chi-Huey Wong, Ph.D.* Ernest W. Hahn Professor and Chair in Chemistry Riccardo Salvio, Ph.D. Michael Schramm, Ph.D. Siddhartha Shenoy, Ph.D. Alessandro Volonterio, Ph.D. Felix Zelder, Ph.D. * Joint appointment in the Department of Chemistry ** Joint appointment in the Department of Molecular Biology *** Joint appointment in the Department of Molecular and Experimental Medicine **** Joint appointments in the Departments of Cell Biology and Chemistry ***** Joint appointment in the Department of Cell Biology † Peter E. Wright, Ph.D.** Professor Cecil H. and Ida M. Green Investigator in Biomedical Research Chairman, Department of Molecular Biology, Scripps Research Kurt Wüthrich, Ph.D. ††† Cecil H. and Ida M. Green Professor of Structural Biology RESEARCH A S S O C I AT E S †††† Dariush Ajami, Ph.D. Elizabeth Barrett, Ph.D. Sara Butterfield, Ph.D. Alexandre Carella, Ph.D. Naran Gombosuren, Ph.D. Clemens Haas, Ph.D. Junli Hou, Ph.D. Richard J. Hooley, Ph.D. Tetsuo Iwasawa, Ph.D. Enrique Mann, Ph.D. †† ††† †††† Joint appointment in the Department of Neurobiology Joint appointments in the Departments of Molecular Biology and Immunology Joint appointments in the Departments of Chemistry and Molecular Biology Research associates in the laboratories of staff other than Dr. Rebek are included in the lists of the respective departments in which the associates hold joint appointments. THE SKAGGS INSTITUTE FOR CHEMICAL BIOLOGY In 1996, The Scripps Research Institute established The Skaggs Institute for Chemical Biology, made possible by a gift of more than $100 million to The Skaggs Institute for Research from Aline W. and L.S. Skaggs. Scientific members of the Skaggs Institute hold dual appointments in various departments at Scripps Research. These scientists have broad expertise in areas including the structure of biological macromolecules, chemical and antibody catalysis, synthetic and combinatorial chemistry, molecular recognition, and molecular modeling methods. With the achievements of its staff, the Skaggs Institute has assumed its research identity in the United States and throughout the world at the interface of biology and chemistry. THE SKAGGS INSTITUTE FOR CHEMICAL BIOLOGY Julius Rebek, Jr., Ph.D. Director’s Overview he Skaggs Institute for Chemical Biology, established in 1996, is now completing its 1st decade. During this time, the generous endowment of the Skaggs family has supported the research of more than 30 principal investigators and some 500 postdoctoral fellows and graduate students. These researchers have produced more than 2,000 publications in the areas of chemistry, chemical biology, molecular biology, and immunology gaining the Skaggs Institute a worldwide reputation for excellence. Highlights of some of the research done this past year are discussed briefly below, and the individual reports of the principal investigators are presented elsewhere in this volume. Researchers in the laboratories of Geoffrey Chang and M.G. Finn have taken multidisciplinary measures to design inhibitors to biological molecules that cause cancer drug resistance. These measures take advantage of x-ray structures of drug “antiporters” and electron cryomicroscopy to study changes in the shapes of the molecules as they perform. Stephen Mayfield’s group is using algae as a system for production of therapeutic proteins. Specifically, they have developed a human anti-anthrax antibody by using samples obtained from soldiers who had been vac- T 2006 THE SCRIPPS RESEARCH INSTITUTE 13 cinated against anthrax. Other antibodies are targeted as anticancer agents, particularly those related to childhood lymphomas. Chi-Huey Wong and his group are using enzymes as reagents for organic synthesis; an ultimate target is to use these enzymes to modify proteins with sugars on their surfaces, leading to the development of vaccines against HIV, influenza, and breast cancer. This approach is complemented by a new ligation method that uses a sugar derivative to accelerate the coupling of protein fragments. Paul Schimmel’s research continues to be centered on the apparatus that converts genetic information into proteins. The enzymes that attach amino acids to tRNA were discovered to have an additional activity as cellsignaling proteins. These cytokines are needed for control of blood vessel growth and inflammation; controlling these signaling activities may eventually lead to therapies. Peter Wright, chairman of the Department of Molecular Biology, is studying protein-protein and protein–nucleic acid interactions in solution. Specific targets include the transcriptional coactivators that have been implicated in human diseases such as leukemia, cancer, and mental retardation. Dale Boger and his group are studying small-molecule nucleic acid binding agents; they have synthesized naturally occurring antitumor agents and have showed that the natural compound and its mirror image are equally effective at covalent binding to nucleic acids. Dr. Boger’s group is also working on redesigning the vancomycin antibiotic to overcome resistance. This exercise in molecular recognition has led to a new structure that is 100-fold more effective at binding to resistance peptides. Carlos Barbas and members of his laboratory are developing strategies to produce antibodies that officially form or break carbon-carbon bonds for use in organic synthesis. These antibodies are developed by using novel recombinant strategies. Dr. Barbas is also spearheading an effort to use small organic molecules that show enzyme-like activities for organic synthesis. Ernest Beutler, chairman of the Department of Molecular and Experimental Medicine, is studying mechanisms involved in the checkpoints that maintain genome stability. These are principle defense mechanisms against malignant phenotypes and forces for tumor growth. Elizabeth Getzoff characterizes the mechanisms of lightinduced protein activities, particularly in the cryptochrome flavoproteins that are components of the circadian clocks in animals and humans. Jamie Williamson’s group is using nuclear magnetic resonance to study very large 14 THE SKAGGS INSTITUTE FOR CHEMICAL BIOLOGY molecules such as viral capsid proteins in solution. The nuclear magnetic resonance spectra reveal protein segments that are sufficiently mobile so as not to be observable by using x-ray or electron microscopy. Ian Wilson also pursues the crystallographic characterization of influenza virus glycoproteins. Dr. Wilson and his group intend to improve the potency of antiviral drugs by solving the structure of the neuraminidase and the viral coat protein involved in the 1918 influenza pandemic. K.C. Nicolaou, chairman of the Department of Chemistry, has made great progress in the synthesis of several biologically active molecules. These include a number of antitumor agents from the cytoskyrin family, murinederived antibiotics, and marinomycins. K. Barry Sharpless and Valery Fokin continue to develop “click chemistry,” extremely versatile reactions that drive spontaneous, selective, and irreversible linkages between molecular building blocks. They and their researchers are using these reactions as a means for rapid exploration of chemical space. President Richard Lerner and Subhash Sinha are developing antibodies for selective chemotherapy. These involve drug conjugates and prodrugs that target cell-surface receptors and prostate-specific membrane antigen. The antibodies convert prodrugs into active molecules at the sites where they are most needed. Kim Janda’s group is exploring applications of botox in the treatment of multiple sclerosis, stroke, and migraine. Molecules that activate the toxin could ultimately allow lower doses of the agent to be effective and could reduce the immune response in these applications. Tamas Bartfai, chairman of the Molecular and Integrative Neuroscience Department, continues his studies on the temperature regulation of mammals. Dr. Bartfai and his group have used transgenic methods to develop an animal model, “the cool mouse,” whose thermo set has been effectively lowered, resulting in reduced energy expenditure and a longer lifetime for the animal. Jeffery Kelly, dean of the Graduate Program, is studying the protein folding and misfolding involved in Alzheimer’s, Parkinson’s, and Gaucher’s diseases. They have found that oxidative cholesterol metabolites can modify amyloid peptide and accelerate the precipitation associated with Alzheimer ’s disease. My own group continues the study of molecules in extremely small spaces, and how they interact when confined to close range. This has led to newly discovered stereochemical properties and a spring-loaded device for nanomachinery. All of us are thrilled to be associated with The Skaggs Institute for Research and are grateful for the research 2006 THE SCRIPPS RESEARCH INSTITUTE opportunities provided by the Skaggs family’s support. We look forward to our second decade. THE SKAGGS INSTITUTE FOR CHEMICAL BIOLOGY 2006 INVESTIGATOR’ S REPORT resembles that of an α-helical protein. We have built small libraries of these compounds that target a number of protein-protein interactions implicated in, for example, prolonged cancer states, chronic neuropathic pain, and epilepsy. Convex and Concave Recognition Surfaces J. Rebek, Jr., D. Ajami, E. Barrett, S. Biros, S. Butterfield, A. Carella, T.J. Dale, N. Gombosuren, C. Haas, R.J. Hooley, T. Iwasawa, E. Mann, L. Moisan, A. Myles, B. Purse, R. Salvio, M. Schramm, H. Van Anda, A. Volonterio, F. Zelder MIMETICS OF α-HELICES rotein-protein interactions are involved in the regulation of a wide variety of biological processes. These recognition events often occur between a large protein containing a well-defined binding site and a smaller protein with features complementary to the site. This relationship has been compared with that of a lock and its key: only a key with the correct grooves and notches will fit and elicit a response. Regulation of these events by small, synthetic molecules is a challenging but desirable goal in medicinal chemistry. Structures that can selectively enhance or antagonize protein-protein interactions have much promise as pharmaceuticals. We have constructed a series of molecules that target protein-protein interactions in which the smaller protein adopts an α-helical conformation (Fig. 1). The P F i g . 1 . Left, Line structure and skeletal model of a synthetic, scaffold-based α-helix mimetic. Right, Overlay of the scaffold (light gray) with an α-helical peptide (black tube); the side-chain functional groups used to recognize other proteins are shown as spheres. synthesis of these molecules is modular and readily amenable to combinatorial techniques. These structures act as scaffolds to project functional groups (the “grooves” and “notches”) in a manner that closely THE SCRIPPS RESEARCH INSTITUTE 15 C AV I TA N D S A S R E C E P T O R S Cavitands are concave hosts that bind small molecules of complementary size, shape, and chemical surface. Deepened cavitands enclose most of a small guest, but the open end reduces the selectivity and exposes part of the guest to the external medium. Exquisite selectivities can be achieved by using capsules that completely surround the guest. We recently prepared a water-soluble cavitand (Fig. 2) that coaxes hydropho- F i g . 2 . A water-soluble synthetic receptor extracts normal alkanes and other insoluble species into aqueous solution. Inside the cavity, the alkanes coil into a helix to maximize hydrophobic contacts with the receptor and tumble rapidly on the nuclear magnetic resonance timescale. bic guests into the cavity, where they are more or less shielded from the aqueous environment. These complexes are kinetically stable; that is, exchange of guests is slow on the nuclear magnetic resonance timescale. The guests are surrounded by surfaces made of aromatic subunits, allowing van der Waals interactions between host and guest. This attraction leads to conformational changes for normal hydrocarbons such as octane; the hydrocarbons coil to make better contacts with the inner lining of the receptor and reduce the surfaces exposed to the aqueous environment. A cavitand with doors that can be rotated over the open end has been synthesized and characterized. The doors shield guests from water and limit the size of guests that fit the space. The increase in selectivity for small guests allows cycloalkanes inside but excludes longer linear counterparts of cycloalkanes. Cyclopentane inside the cavitand is shown in Figure 3. The binding of n-hexane causes the doors to fully open and expose the guest to the aqueous surroundings. The closed doors 16 THE SKAGGS INSTITUTE FOR CHEMICAL BIOLOGY 2006 THE SCRIPPS RESEARCH INSTITUTE Menozzi, E., Onagi, H., Rheingold, A.L., Rebek, J., Jr. Extended cavitands of nanoscale dimensions. Eur. J. Org. Chem. 3633, 2005, Issue 17. Menozzi, E., Rebek, J., Jr. Metal directed assembly of ditopic containers and their complexes with alkylammonium salts. Chem. Commun. (Camb.) 5530, 2005, Issue 44. Purse, B.W., Gissot, A., Rebek, J., Jr. A deep cavitand provides a structured environment for the Menschutkin reaction. J. Am. Chem. Soc. 127:11222, 2005. Purse, B.W., Rebek, J., Jr. Functional cavitands: chemical reactivity in structured environments. Proc. Natl. Acad. Sci. U. S. A. 102:10777, 2005. F i g . 3 . Two views of a water-soluble cavitand with rotating doors on its upper rim. Two doors close access to the cavity and slow the uptake and release of guests. The guest shown is cyclopentane. also reduce the rate of motion as various small guests go in and out of the cavitand. Cavitands have also been outfitted for catalysis by introducing a metal complex fused to the upper rim. This complex features a deep cavity for driving guest recognition and a metal ion at the top of the cavity that is positioned to coordinate a phosphate group of the guest. These binding forces act simultaneously on smaller molecules that bear phosphocholine subunits as shown in Figure 4. Reactions that have been catalyzed involv- F i g . 4 . Left, Line drawing of a cavitand outfitted with a salen ligand with a zinc ion. Right, Energy-minimized structure of the complex of the cavitand and a simplified phosphocholine model (a wall of the cavitand has been removed for viewing clarity). ing a choline substrate include acylation, aminolysis, and ester cleavage. PUBLICATIONS Haas, C.H., Biros, S.M., Rebek, J., Jr. Binding properties of cavitands in aqueous solution: the influence of charge on guest selectivity. Chem. Commun. (Camb.) 6044, 2005, Issue 48. Hooley, R.J., Biros, S.M., Rebek, J., Jr. A deep, water-soluble cavitand acts as a phase-transfer catalyst for hydrophobic species. Angew. Chem. Int. Ed. 45:3517, 2006. Hooley, R.J., Biros, S.M., Rebek, J., Jr. Normal hydrocarbons writhe and tumble rapidly in a deep, water-soluble cavitand. Chem. Commun. (Camb.) 509, 2006, Issue 5. Hooley, R.J., Rebek, J., Jr. Deep cavitands provide organized solvation of reactions. J. Am. Chem. Soc. 27:11904, 2005. Hooley, R.J., Van Anda, H.J., Rebek, J., Jr. Cavitands with revolving doors regulate binding selectivities and rates in water. J. Am. Chem. Soc. 128:3894, 2006. Zelder, F.H., Rebek, J., Jr. Cavitand templated catalysis of acetylcholine. Chem. Commun. (Camb.) 753, 2006, Issue 7. Zelder, F.H., Salvio, R., Rebek, J., Jr. A synthetic receptor for phosphocholine esters. Chem. Commun. (Camb.) 1280, 2006, Issue 12.