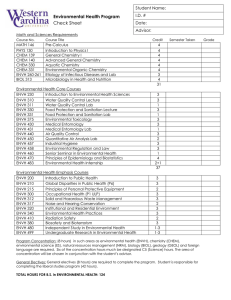
Chemistry Published by TSRI Press . Copyright 2005,
advertisement

Chemistry Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. M. Reza Ghadiri, Ph.D., Professor, Department of Chemistry Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. CHEMISTRY 2005 DEPAR TMENT OF CHEMISTRY Hartmuth Kolb, Ph.D.** University of California Los Angeles, California Chi-Huey Wong, Ph.D.* Ernest W. Hahn Professor and Chair in Chemistry Jung-Mo Ahn, Ph.D.** University of Texas Dallas, Texas Ramanarayanan Krishnamurthy, Ph.D. Associate Professor Andrew Bin Zhou, Ph.D. Assistant Professor Dariush Ajami, Ph.D. Richard A. Lerner, M.D.**** President, Scripps Research Lita Annenberg Hazen Professor of Immunochemistry Cecil H. and Ida M. Green Chair in Chemistry S TA F F S C I E N T I S T S 63 S TA F F K.C. Nicolaou, Ph.D.* Chairman Aline W. and L.S. Skaggs Professor of Chemical Biology Darlene Shiley Chair in Chemistry Phil Baran, Ph.D. Assistant Professor Dale L. Boger, Ph.D.* Richard and Alice Cramer Professor of Chemistry Bruce Clapham, Ph.D.** Abbott Laboratories Abbott Park, Illinois Tobin Dickerson, Ph.D. Assistant Professor Albert Eschenmoser, Ph.D.* Professor Sheng Ding, Ph.D. Assistant Professor M.G. Finn, Ph.D.* Associate Professor Valery Fokin, Ph.D. Assistant Professor Lital Alfonta, Ph.D. Byeong D. Song, Ph.D. Toru Amaya, Ph.D.** Osaka University Osaka, Japan Lubica Supekova, Ph.D. Rajesh Ambasudhan, Ph.D. I N S T R U M E N TAT I O N / Masayuki Matsushita, Ph.D. Assistant Professor Evan Powers, Ph.D. Assistant Professor Julius Rebek, Jr., Ph.D.* Professor Director, The Skaggs Institute for Chemical Biology Ed Roberts, Ph.D. Professor SERVICE FACILITIES Stellios Arseniyadis, Ph.D. Raj K. Chadha, Ph.D. Director, X-Ray Crystallography Facility Gonen Ashkenasy, Ph.D. Dee H. Huang, Ph.D. Director, Nuclear Magnetic Resonance Facility Masato Atsumi, Ph.D. Gary E. Siuzdak, Ph.D. Director, Mass Spectrometry Facility Christoph Behrens, Ph.D. Floyd E. Romesberg, Ph.D. Assistant Professor SENIOR RESEARCH Peter G. Schultz, Ph.D.* Professor Scripps Family Chair M. Reza Ghadiri, Ph.D.* Professor K. Barry Sharpless, Ph.D.* W.M. Keck Professor of Chemistry Inkyu Hwang, Ph.D. Assistant Professor Anita Wentworth, Ph.D. Assistant Professor Kim D. Janda, Ph.D.*** Professor Ely R. Callaway, Jr., Chair in Chemistry Paul Wentworth, Jr., Ph.D. Professor Narendra B. Ambhaikar, Ph.D. A S S O C I AT E S Ashraf Brik, Ph.D. Yanping Chen, Ph.D. Tobin Dickerson, Ph.D. Michael Meijler, Ph.D. Nurit Ashkenasy, Ph.D. Elizabeth Barrett, Ph.D. Clay Bennett, Ph.D. Michael Best, Ph.D.** University of Tennessee Knoxville, Tennessee Jan Bieschke, Ph.D. Babu Boga, Ph.D. Anthony Boitano, Ph.D. Brant Boren, Ph.D. Daryl Bosco, Ph.D. Jeffery W. Kelly, Ph.D.* Vice President, Academic Affairs Dean, Kellogg School of Science and Technology Lita Annenberg Hazen Professor of Chemistry Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Peter Wirsching, Ph.D.***** R E S E A R C H A S S O C I AT E S Ramzey Abujarour, Ph.D. Mohua Bose, Ph.D.** Stanford University Stanford, California S E C T I O N C O V E R F O R T H E D E P A R T M E N T O F C H E M I S T R Y : A single rotaxane struc- ture created by threading and capturing a DNA-poly(ethylene glycol) strand inside an α-hemolysin transmembrane pore protein. This supramolecular system constitutes the basis of a research program to design a rapid pore-mediated single-molecule DNA-sequencing technology. Work done in the laboratory of M. Reza Ghadiri, Ph.D. 64 CHEMISTRY 2005 Patrick Braun, Ph.D.** University of Minnesota Minneapolis, Minnesota Antonella Converso, Ph.D. Rebecca Fraser, Ph.D. Zhangyong Hong, Ph.D. Jeromy Cottell, Ph.D. Graeme Freestone, Ph.D. Richard Hookey, Ph.D. Andy Brogan, Ph.D. James Crawford, Ph.D. Yanwen Fu, Ph.D. Daniel Horne, Ph.D. Adrian Brunkhorst, Ph.D. Matthew Cremeens, Ph.D. Jim Fuchs, Ph.D. Paul Bulger, Ph.D. Ashton Cropp, Ph.D.** University of Maryland College Park, Maryland Carmen Galan, Ph.D.** Massachusetts Institute of Technology Cambridge, Massachusetts Che-Chang (Jeff) Hsu, Ph.D.** EMD Biosciences, Inc. San Diego, California Kevin Bunker, Ph.D. Mark Bushey, Ph.D. Sara Butterfield, Ph.D. Edelmira Cabezas, Ph.D.** Intel Corp. Santa Clara, California Francesco De Riccardis, Ph.D.** Università di Salerno Baronissi (SA), Italy Jianmin Gao, Ph.D. Tsui-Ling Hsu, Ph.D. Qihong Huang, Ph.D.** Wistar Institute Philadelphia, Pennsylvania Mu-yun Gao, Ph.D. Zheng-Zheng Huang, Ph.D. Konstantinos Dellios, Ph.D.** Chemistry Laboratory of the Government Larisa, Greece Nathan Gianneschi, Ph.D. Amy Hurshman, Ph.D. David Diaz-Diaz, Ph.D. Arnaud Gissot, Ph.D.** Université Victor Segalen Bordeaux II Bordeaux, France Christine Dierks, Ph.D. Rajesh Grover, Ph.D. Hayato Ishikawa, Ph.D. Ross Denton, Ph.D. Shen Gu, Ph.D.***** Tetsuo Iwasawa, Ph.D. Romyr Dominque, Ph.D.** Hoffmann-La Roche, Inc. Nutley, New Jersey Sayam Sen Gupta, Ph.D. Michael Jahnz, Ph.D. Clemens Haas, Ph.D. Wei Jin, Ph.D. Song Byeong Doo, Ph.D. Young Wan Ham, Ph.D.** Molecular Therapeutics, Inc. Ann Arbor, Michigan Florian Kaiser***** Akiyuki Hamasaki, Ph.D. Gyungyoun Kim, Ph.D. David Edmonds, Ph.D. Wooseok Han, Ph.D. Greg Elliott, Ph.D. Nile Emre, Ph.D. Christophe Hardouin, Ph.D.** Oril Industry, SA Bolbec, France Sang Jick Kim, Ph.D.** Korean Research Institute of Bioscience and Biotechnology Taejon, Korea Simon Eppacher, Ph.D. Frank Hauke, Ph.D. Youhoon Chong, Ph.D.** Konkuk University Seoul, Korea Lisa Eubanks, Ph.D. Mark Hixon, Ph.D. Raffaella Faraoni, Ph.D. Benoit Colasson, Ph.D.** University of Pavia Pavia, Italy Laura Flatauer, Ph.D. Rebecca Holmberg, Ph.D.** Ionian Technologies, Inc. Upland, California Alexandre Carella, Ph.D. Giacomo Carenzi, Ph.D.** Nerviano Medical Sciences Nerviana, Italy Michael Cassidy, Ph.D. Aileen Chang, Ph.D.** Kresge Library Scripps Research Shuo Chen, Ph.D. Heng Cheng, Ph.D.** FibroGen, Inc. South San Francisco, California Jodie Chin, Ph.D. Charles Cho, Ph.D. Younggi Choi, Ph.D.** Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, Connecticut Kevin Cole, Ph.D. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Wu Du, Ph.D.** Merck Research Laboratories Rahway, New Jersey Der-Ren Hwang, , Ph.D. Giltae Hwang, Ph.D. Michael Kelso, Ph.D. Yoonkyung Kim, Ph.D.***** James Fletcher, Ph.D.** Creighton University Omaha, Nebraska Sukwon Hong, Ph.D. Wang Hong, Ph.D. Theocharis Koftis** Pharmathen Pharmaceuticals Thessaloniki, Greece Ravinder Reddy Kondreddi, Ph.D. Antoni Krasinski, Ph.D.** ChemoCentryx, Inc. Mountain View, California CHEMISTRY 2005 65 Andreas Krebs, Ph.D.** BASF Ludwigshafen, Germany Tao Tao Ling** Nereus Pharmaceuticals, Inc. San Diego, California Robert Milburn, Ph.D. Tülay Polat, Ph.D. Kyung-Hoon Min, Ph.D. Sreenivas Punna, Ph.D. Grover Rajesh Kumar, Ph.D. Jun Liu, Ph.D. Christos A. Mitsos, Ph.D. Jane Kuzelka, Ph.D. Junjie Liu, Ph.D.** AMSI San Diego, California Gopi Kumar Mittapalli, Ph.D. Longwu Qi, Ph.D.** Prolexys Pharmaceuticals, Inc. Salt Lake City, Utah Lionel Moisan, Ph.D. Daniela Radu, Ph.D. Lei Liu, Ph.D. Ann Montero, Ph.D. Nicole Rahe, Ph.D. Wenshe Liu, Ph.D. Shai Rahimipour, Ph.D. Surakattula Murali Mohan Reddy, Ph.D. Carolina Martinez Lamenca, Ph.D. Eltepu Laxman, Ph.D.** MediVas, LLC San Diego, California Ying (Cindy) Liu, Ph.D. Yasutaka Morita, Ph.D.** Kinki University Fukuoka, Japan Byong Se Lee, Ph.D.** Asian Medical Center Seoul, Korea Dimitrios Lizos, Ph.D. Mridul Mukherji, Ph.D. Hing Ken Lee, Ph.D.***** Jon Loren, Ph.D.** Ligand Pharmaceuticals, Inc. San Diego, California Oscar Munoz, Ph.D.** Universidad Veracruzana Veracruz, México Jinq-Chyi Lee, Ph.D. Jongkook Lee, Ph.D. Hendrick Luesch, Ph.D.** University of Florida Gainesville, Florida Ki-Bum Lee, Ph.D. Hongzheng (Eric) Ma, Ph.D. Lac Lee, Ph.D** Novartis Institutes for Biomedical Research Inc. Cambridge, Massachusetts Sang Hyeup Lee, Ph.D.** Korean Research Institute of Bioscience and Biotechnology Taejon, Korea Sunil Mandal, Ph.D.** Acenta Discovery, Inc. Tucson, Arizona Nello Mainolfi, Ph.D. Roman Manetsch, Ph.D. Enrique Mann, Ph.D. Sanghyup Lee, Ph.D. Andrew Myles, Ph.D. Joonwoo Nam, Ph.D. Sridhar Narayan, Ph.D. Daniel Nicoletti, Ph.D. Alain Noncovich, Ph.D. Yasuo Norikane, Ph.D. Mehdi Numa, Ph.D. Barun Okram, Ph.D. Dalit Rechavi-Robinson, Ph.D. Yosup Rew, Ph.D.** Amgen, Inc. Thousand Oaks, California Sebastien Richeter, Ph.D.** Université Montpellier II Montpellier, France Stefanie Roeper, Ph.D. F. Anthony Romero, Ph.D. Youngha Ryu, Ph.D. Riccardo Salvio, Ph.D. Pradip Sasmal** Dr. Reddy’s Laboratories, Ltd. Hyderabad, India Felix Marr, Ph.D. Chunmei Li, Ph.D. ** Stephen F. Austin State University Nacogdoches, Texas Ke Li, Ph.D. Yongkai Li, Ph.D.** Ligand Pharmaceuticals, Inc. San Diego, California Antonietta M. Lillo, Ph.D.** Los Alamos National Laboratory Los Alamos, New Mexico Yeon-Hee Lim, Ph.D. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Hideki Onagi, Ph.D.***** Yazmin Osornio, Ph.D. Alessandro Scarso, Ph.D.** Università Cà Foscari di Venezia Venice, Italy Charles Papageorgiou, Ph.D. Patrick Schanen, Ph.D. Laxman Pasunoori, Ph.D. Stefan Schiller, Ph.D. Andrew McPherson, Ph.D.** TargeGen, Inc. San Diego, California Goran Petrovic, Ph.D. Edoardo Menozzi, Ph.D. Steven Pfeiffer, Ph.D.** Gilead Foster City, California Daniel Schlawe, Ph.D.** Syncom BV Groningen, the Netherlands Shigeo Matsuda, Ph.D. Laura McAllister, Ph.D. Kathleen McKenzie, Ph.D. Sergio Meth, Ph.D.** Federal University of Rio de Janeiro Rio de Janeiro, Brazil Jared Piper, Ph.D. Suresh Pitram, Ph.D. Michael Schramm, Ph.D. Akira Shigenaga, Ph.D.** Tokushima University Tokushima, Japan 66 CHEMISTRY 2005 Dongwoo Shin, Ph.D.** Samsung Seoul, Korea Gregory Watt, Ph.D.** Nature Chemical Biology Cambridge, Massachusetts Junhwa Shin, Ph.D. Lisa Whalen, Ph.D. Sebastian Steiniger, Ph.D. Matthew Whiting, Ph.D. Xiuwen Zhu, Ph.D. Joerg Zimmermann, Ph.D. Makoto Yamashita, Ph.D. Takeda Chemical Industries, Ltd. Osaka, Japan V I S I T I N G I N V E S T I G AT O R S Shula Stokols, Ph.D. Aarron Willingham, Ph.D. Ji Young Suk, Ph.D. R. Luke Wiseman, Ph.D. Daniel Summerer, Ph.D. Xiowen Sun** Lanzhou University Lanzhou, China Leo Takaoka, Ph.D. Margarita Wuchrer, Ph.D. Hui Xiong, Ph.D.** University of Pennsylvania Philadelphia, Pennsylvania Trond Vidar Hansen, Ph.D.** University of Oslo Oslo, Norway Wen Xiong, Ph.D. Bumpei Hatano, Ph.D.** Yamagata University Yonezawa, Japan Chung-Yi Wu, Ph.D. Masamichi Yamanaka, Ph.D.** Shizuoka University Shizuoka, Japan Eric Tippmann, Ph.D. Ryu Yamasaki, Ph.D. Laurent Trembleau, Ph.D.** University of Aberdeen Aberdeen, Scotland Shuyuan Yao, Ph.D. Meng-Lin Tsao, Ph.D. Yong Sik Yoo, Ph.D. Craig Turner, Ph.D. Ninghui Yu, Ph.D.** Serono, Inc. Rockland, Massachusetts Elke Ullrich, Ph.D.** Syncom BV Groningen, the Netherlands Jan Van Maarseveen,** Universiteit van Amsterdam Amsterdam, the Netherlands Juraj Velcicky, Ph.D. David Vodak, Ph.D.** Bend Research, Inc. Bend, Oregon Robert M. Yeh, Ph.D. Tomoyasu Hirose, Ph.D.** The Kitasato Institute Tokyo, Japan Jiayu Liao, Ph.D. Genomics Institute of the Novartis Research Foundation San Diego, California Jie Li, Ph,D.***** Dawei Yue, Ph.D. Huaqiang Zeng, Ph.D. Qing-Hai Zhang, Ph.D.** Department of Molecular Biology Scripps Research Masayuki Oda, Ph.D. Kyoto Prefectural University Kyoto, Japan Masaaki Sawa, Ph.D. Dainippon Pharmaceuticals Co., Ltd. Osaka, Japan Alexandro Volonterio, Ph.D. Yuanxiang Zhao, Ph.D. Masakazu Sugiyama, Ph.D. Ajinomoto Co., Inc. Kawasaki-shi, Japan Jiangyun Wang, Ph.D. LianXing Zheng, Ph.D.** Models of Disease Center Cambridge, Massachusetts Shin-Ichi Takanashi, Ph.D. Mitsubishi Pharma Corporation Osaka, Japan Xiaolong Wang, Ph.D. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Qisheng Zhang, Ph.D. Suresh Mahajan, Ph.D. * Joint appointment in The Skaggs Institute for Chemical Biology ** Appointment completed; new location shown *** Joint appointments in The Skaggs Institute for Chemical Biology and the Department of Immunology **** Joint appointments in The Skaggs Institute for Chemical Biology and the Department of Molecular Biology ***** Appointment completed Jiyong Hong, Ph.D. Genomics Institute of the Novartis Research Foundation San Diego, California Zhanqian Yu, Ph.D. Felix Zelder, Ph.D. Jon Ashley Maria-Teresa Dendle Masakazu Fujio, Ph.D. Mitsubishi Pharma Corporation Yokohama, Japan Wenjun Tang** Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, Connecticut Jim Turner, Ph.D. S C I E N T I F I C A S S O C I AT E S Luda Bazhenova, M.D. Moores Cancer Center La Jolla, California CHEMISTRY 2005 67 K.C. Nicolaou, Ph.D. Chairman’s Overview s the “central science,” chemistry stands between biology and medicine and between physics and materials science and provides the crucial bridge for drug discovery and development. But chemistry has a much more profound and useful role in science and society. It is the discipline that continually creates the myriad of new materials that we all encounter in our everyday lives: pharmaceuticals, high-tech materials, polymers and plastics, insecticides and pesticides, fabrics and cosmetics, fertilizers, and vitamins—basically everything we can touch, feel, and smell. Chemists at Scripps Research focus on chemical synthesis and chemical biology, the most relevant areas to biomedical research and materials science. The members of our faculty are distinguished teacher-scholars who maintain highly visible and independent research programs in areas as diverse as biological and chemical catalysis, synthesis of natural products, combinatorial chemistry, molecular design, supramolecular chemistry, chemical evolution, materials science, and chemical biology. The chemistry graduate program attracts some of the best-qualified candidates from the United States and abroad. Our major research facilities, under the direction of Dee H. Huang (nuclear magnetic resonance), Gary Siuzdak (mass spectrometry), and Raj Chadha A Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. (x-ray crystallography), are second to none and continue to provide crucial support to our research programs. In addition, the Mabel and Arnold Beckman Center for the Chemical Sciences constantly receives high praise from visitors from around the world for its architectural design and operational aspects, both highly conducive to research. Research in the Department of Chemistry goes on unabated, establishing international visibility and attracting attention as evidenced by numerous lecture invitations, visits by outside scholars, and headline news in the media. As of 2004, the Institute for Scientific Information ranked 4 members of the department as highly cited researchers (in the top 100 worldwide); 2 of the 4 are among the top 35. Dr. Lerner and his group continue to make advances in catalytic antibodies, with new antibodies that catalyze important synthetic and biological reactions and novel applications in chemical synthesis. The research of the group recently was expanded to include the fundamental chemistry of polyoxygen species. Members of the Sharpless group continue endeavors to discover and develop better catalysts for organic synthesis and to construct, through innovative chemistry and biology, libraries of novel compounds for biological screening. Scientists in the La Jolla–based Eschenmoser group advance in experimental studies on the chemical etiology of nucleic acid structure by investigating nucleic acid alternatives that have novel backbone structures unrelated to the canonical phosphodiester-based oligonucleotide systems. Members of my group continue explorations of chemical synthesis and chemical biology, focusing on the total synthesis of new anticancer agents, antibiotics, marine-derived neurotoxins, antimalarial compounds, antifeedant agents, other biologically active natural products, solid-phase synthesis, and combinatorial chemistry. Members of the Rebek group devise biomimetic receptors for studies in molecular recognition. These include molecules that bind neurotransmitters and membrane components. Larger host receptors can surround 3 or more molecular guests and act as chambers where the chemical reactions of the guests are accelerated. Scientists in the Schultz laboratory continue to expand the genetic code. Using unique triplet and quadruplet codons, they have genetically encoded more than 30 novel amino acids in bacteria, yeasts, and mammalian cells. Dr. Wong and his group further advance the fields of chemoenzymatic organic synthesis, chemical glycobiology, and the development of enzyme inhibitors. A new 68 CHEMISTRY 2005 strategy for the synthesis of glycoproteins has been developed. The programmable 1-pot synthesis of oligosaccharides developed by this group has been further used in the assembly of glycoarrays in microtiter plates for study of saccharides and aminoglycosides that bind to proteins and RNA, respectively. This group also developed new inhibitors of glycosyltransferases, sulfotransferases, and the HIV protease. Members of the Boger group continue their work on chemical synthesis; combinatorial chemistry; heterocycle synthesis; anticancer agents, such as fostriecin and yatakemycin; and antibiotics, such as vancomycin, teicoplanin, and ramoplanin. Scientists in the Janda laboratory focus on the impact of organic chemistry in specific biological systems. Their targeted programs span a wide range of interests, from drug addiction to biological and chemical warfare agents to catalytic antibodies to combinatorial chemistry. Their recent achievements include the discovery that a secondary nicotine metabolite can inhibit the formation of the fibrils characteristic of Alzheimer’s disease, the biological validation of a common quorumsensing molecule, and a high-throughput assay based on a blue fluorescent antibody sensor. Dr. Ghadiri and members of his laboratory are making significant contributions in the design and study of a new generation of antimicrobial agents, based on selfassembling peptide nanotube architecture, to combat multidrug-resistant infections. In addition, they continue to make novel contributions in several ongoing basic research endeavors, such as design of biosensors, molecular computation, design of self-reproducing systems, understanding the origins of life, and design of emergent chemical systems. Dr. Finn and his group have pioneered the use of virus particles as chemical reagents and building blocks for nanochemical structures. This effort is directed toward the development of new diagnostics for disease and catalysts for organic reactions. Members of the Finn laboratory also develop and investigate new organic and organometallic reactions and use these processes to synthesize biologically active compounds. Research by members of the Kelly group emphasizes the role of protein conformational changes in neurodegenerative disease and the alteration of these processes through the design and synthesis of small molecules. These scientists also take advantage of the power of chemistry and biology to study β-sheet folding. An emerging interest is self-assembling biomaterials made from peptides and proteins. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Researchers in the Romesberg laboratory are using diverse techniques ranging from bioorganic and biophysical chemistry to bacterial and yeast genetics to understand and manipulate evolution. Major efforts include the design of unnatural base pairs and the directed evolution of DNA polymerases to efficiently synthesize unnatural DNA containing the base pairs; using spectroscopy to understand biological function and how it evolves; and understanding how induced and adaptive mutations contribute to evolution in eukaryotic and prokaryotic cells. Dr. Baran and his group have made extraordinary contributions in synthetic organic chemistry. In only 2 years, they have invented practical chemical solutions to several long-standing synthetic challenges of great interest, such as the biologically active natural products pyrrole-imidazole alkaloids, stephacidins, welwitindolinones, and chartellines. The Frontiers in Chemistry Lecturers (17th Annual Symposium) for the 2004–2005 academic year were Carolyn Bertozzi, University of California, Berkeley; EiEichi Negishi, Purdue University; Thomas Steitz, Yale University; and David Liu, Harvard University. Jean-Marie Lehn, ISIS, Université Louis Pasteur, Strasbourg, and Collège de France, Paris, also visited Scripps this year as the 2004 Merck lecturer. CHEMISTRY 2005 INVESTIGATORS’ R EPORTS Practical Total Synthesis of Natural Products P.S. Baran, N.B. Ambhaikar, C.A. Mitsos, K. Li, R.A. Shenvi, D.P. O’Malley, N.Z. Burns, M.P. DeMartino, C.A. Guerrero, B.D. Hafensteiner, D.W. Lin, J.M. Richter rom penicillin to paclitaxel (Taxol), natural products have an unparalleled track record in the betterment of human health. In fact, 9 of the top 20 best-selling drugs were either inspired by or derived from natural products. Even the best-selling drug of all time, atorvastatin (Lipitor), was based on a natural product lead. Total synthesis, the art and science of recreating these entities in the laboratory, invariably leads to fundamental discoveries in chemistry, biology, and medicine. We focus on solving interesting challenges in the total synthesis of natural products and on bridging gaps in synthetic capabilities by inventing new reactions. Through judicious target selection and creative retrosynthetic analyses, total synthesis becomes an engine for discovery that drives organic chemistry to new levels of sophistication and practicality. Synthetic organic chemistry requires tremendous ingenuity, artistic taste, experimental acumen, persistence, and character. Not surprisingly, drug development relies on the expertise of researchers who have these characteristics. Although we focus entirely on educating students in fundamental chemistry, we also collaborate with expert biologists to explore the medicinal potential of newly synthesized natural products and the products’ analogs. Recently completed total syntheses (Fig. 1) include the anticancer agents stephacidins A and B and avrainvillamide, the antibacterial agents sceptrin and ageliferin, and several members of the bioactive fischerindole and hapalindole indole alkaloid family. Current natural product targets (Fig. 2) include chartelline C, welwitindolinone A, haouamine A, strictamine, axinellamine, and sarcodonin. F PUBLICATIONS Baran, P.S., Guerrero, C.A., Ambhaikar, N.B., Hafensteiner, B.D. Short, enantioselective total synthesis of stephacidin A. Angew. Chem. Int. Ed. 44:606, 2005. Baran, P.S., Guerrero, C.A., Hafensteiner, B.D., Ambhaikar, N.B. Total synthesis of avrainvillamide (CJ-17,665) and stephacidin B. Angew. Chem. Int. Ed. 44:3892, 2005. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. F i g . 1 . Recently completed total syntheses. 69 70 CHEMISTRY 2005 bioorganic and medicinal chemistry, the study of DNAagent interactions, and the chemistry of antitumor antibiotics. We place a special emphasis on investigations to define the structure-function relationships of natural or designed organic agents. SYNTHETIC METHODS Central to much of our work are investigations to develop and apply the hetero Diels-Alder reaction, including the use of heterocyclic and acyclic azadienes (Fig. 1), the thermal reactions of cyclopropenone ketals, intermolecular and intramolecular acyl radical–alkene addition reactions, medium- and large-ring cyclization technology, and solution-phase combinatorial chemistry. In each instance, the development of the methods represents the investigation of chemistry projected as a key element in the synthesis of a natural or designed agent. F i g . 1 . N-Sulfonyl-1-aza-1,3-butadiene Diels-Alder reaction. F i g . 2 . Ongoing natural product total syntheses. Baran, P.S., Richter, J.M., Lin, D.W. Direct coupling of pyrroles with carbonyl compounds: short, enantioselective synthesis of (S)-ketorolac. Angew. Chem. Int. Ed. 44:609, 2005. Baran, P.S., Shenvi, R.A., Mitsos, C.A. A remarkable ring contraction en route to the chartelline alkaloids. Angew. Chem. Int. Ed. 44:3714, 2005. Synthetic and Bioorganic Chemistry D.L. Boger, S.B. Boga, K. Bunker, K. Capps, H. Cheng, Y. Choi, Y. Chong, R. Clark, J. Cottell, B. Crowley, J. DeMartino, R. Dominique, W. Du, G. Elliott, J. Fuchs, J. Garfunkle, Y. Ham, A. Hamasaki, W. Han, N. Haq, C. Hardouin, S. Hong, D. Horne, I. Hwang, H. Ishikawa, W. Jin, D. Kastrinsky, M. Kelso, G. Kim, B. Lawhorn, S. Lee, Y. Li, K. MacMillan, J. Nam, S. Pfeiffer, Y. Rew, A. Romero, M. Schnermann, D. Shin, C. Slown, L. Takaoka, H. Tao, M. Tichenor, J. Trzupek, J. Velcicky T he research interests of our group include the total synthesis of natural products, development of new synthetic methods, heterocyclic chemistry, Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. T O TA L S Y N T H E S I S O F N AT U R A L P R O D U C T S Efforts are under way on the total synthesis of a number of natural products that constitute agents in which we have a specific interest. Representative agents currently under study include (+)-CC-1065 and functional analogs; the duocarmycin class of antitumor antibiotics, including yatakemycin; tropoloalkaloids; prodigiosin and roseophilin; the deoxybouvardin and RA-I class of antitumor agents; vancomycin, teicoplanin, ristocetin, chloropeptins and related agents; ramoplanin; the luzopeptins, quinoxapeptins, thiocoraline, BE-22179 and sandramycin; bleomycin A2 and functional analogs; HUN-7293; chlorofusin; CI-920 (fostriecin); the combretastatins; storniamide A; phomazarin; ningalins; lamellarin O; lukianol A; piericidins; nothapodytine and mappicine; rubrolone; and vinblastine (Figs. 2 and 3). BIOORGANIC CHEMISTRY The agents listed in the previous paragraph were selected on the basis of their properties; in many instances, they are agents related by a projected property. For example, (+)-CC-1065 and the duocarmycins are antitumor antibiotics and related sequence-selective DNA minor groove alkylating agents. Representa- CHEMISTRY 2005 71 F i g . 3 . Additional recent total syntheses. F i g . 2 . Recent total syntheses. tive of such efforts, studies to determine the structural features of (+)-CC-1065 and the duocarmycins that Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. contribute to the sequence-selective DNA alkylation properties of these agents have resulted in the identification of a unique source of catalysis for the DNA alkylation reaction. Efforts are under way to develop DNA 72 CHEMISTRY 2005 cross-linking agents of a predefined cross-link, to further understand the nature of the noncovalent and covalent interactions between agents and DNA, and to apply this understanding to the de novo design of DNA-binding and DNA-effector agents. Techniques for the evaluation of the agent-DNA binding and alkylation properties, collaborative efforts in securing biological data, nuclear magnetic resonance structures of DNA-agent complexes, molecular modeling, and studies of DNA-agent interactions are integral parts of the program. Additional ongoing studies include efforts to define the fundamental basis of the DNA-binding or cleavage properties of bleomycin A2, sandramycin, and the luzopeptins; to design inhibitors of the folate-dependent enzymes glycinamide ribonucleotide transformylase and aminoimidazole carboxamide ribonucleotide transformylase as potential antineoplastic agents; to establish the chemical and biological characteristics responsible for the sleep-inducing properties of the endogenous lipid oleamide; to inhibit tumor growth through inhibition of angiogenesis; to inhibit aberrant gene transcription associated with cancer; and to control intracellular signal transduction through the discovery of antagonists or agonists that affect protein-protein interactions, including receptor dimerization. PUBLICATIONS Chen, L., Yuan, Y., Helm, J.S., Hu, Y., Rew, Y., Shin, D., Boger, D.L., Walker, S. Dissecting ramoplanin: mechanistic analysis of synthetic ramoplanin analogues as a guide to the design of improved antibiotics. J. Am. Chem. Soc. 126:7462, 2004. Crowley, B.M., Mori, Y., McComas, C.C., Tang, D., Boger, D.L. Total synthesis of the ristocetin aglycon. J. Am. Chem. Soc. 126:4310, 2004. Ham, Y.W., Boger, D.L. A powerful selection assay for mixture libraries of DNA alkylating agents. J. Am. Chem. Soc. 126:9194, 2004. Kastrinsky, D.B., Boger, D.L. Effective asymmetric synthesis of 1,2,9,9a-tetrahydrocyclopropa[c]benzo[e]indol-4-one (CBI). J. Org. Chem. 69:2284, 2004. Lee, P.S., Du, W., Boger, D.L., Jorgensen, W.L. Energetic preferences for α,β versus β,γ unsaturation. J. Org. Chem. 69:5448, 2004. Lichtman, A.H., Leung, D., Shelton, C.C., Saghatelian, A., Hardouin, C., Boger, D.L., Cravatt, B.F. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J. Pharmacol. Exp. Ther. 311:441, 2004. Lillo, A.M., Sun, C., Gao, C., Ditzel, H., Parrish, J., Gauss, C.-M., Moss, J., Felding-Habermann, B., Wirsching, P., Boger, D.L., Janda, K.D. A human single-chain antibody specific for integrin α3β1 capable of cell internalization and delivery of antitumor agents. Chem. Biol. 11:897, 2004. Parrish, J.P, Hughes, T.V., Hwang, I., Boger, D.L. Establishing the parabolic relationship between reactivity and activity for derivatives and analogues of the duocarmycin and CC-1065 alkylation subunits. J. Am. Chem. Soc. 126:80, 2004. Parrish, J.P., Trzupek, J.D., Hughes, T.V., Hwang, I., Boger, D.L. Synthesis and evaluation of N-aryl and N-alkenyl CBI derivatives. Bioorg. Med. Chem. 12:5845, 2004. Rew, Y., Shin, D., Hwang, I., Boger, D.L. Total synthesis and examination of three key analogues of ramoplanin: a lipoglycodepsipeptide with potent antibiotic activity. J. Am. Chem. Soc. 126:1041, 2004. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Shin, D., Rew, Y., Boger, D.L. Total synthesis and structure of the ramoplanin A1 and A3 aglycons: two minor components of the ramoplanin complex. Proc. Natl. Acad. Sci. U. S. A. 101:11977, 2004. Tao, H., Hwang, I., Boger, D.L. Multidrug resistance reversal activity of permethyl ningalin B amide derivatives. Bioorg. Med. Chem. Lett. 14:5979, 2004. Tichenor, M.S., Kastrinsky, D.B., Boger, D.L. Total synthesis, structure revision, and absolute configuration of (+)-yatakemycin. J. Am. Chem. Soc. 126:8396, 2004. Tse, W.C., Boger, D.L. A fluorescent intercalator displacement assay for establishing DNA binding selectivity and affinity. Acc. Chem. Res. 37:61, 2004. Tse, W.C., Boger, D.L. Sequence-selective DNA recognition: natural products and nature’s lessons. Chem. Biol. 11:1607, 2004. Chemical and Functional Genomic Approaches to Regenerative Medicine S. Ding, R. Abu-Jarour, R. Ambasudhan, A. Brunkhorst, P. Descargues, C. Despon, N. Emre, H.S. Hahm, S. Hilcove, J. Hsu, S. Takanashi, W. Xiong, S. Yao, D. Yue, Y. Zhao, X. Zhu ecent advances in stem cell biology may make possible new approaches for the treatment of a number of diseases, including cardiovascular disease, neurodegenerative disease, musculoskeletal disease, diabetes, and cancer. These approaches could involve cell replacement therapy and/or drug treatment to stimulate the body’s own regenerative capabilities by promoting survival, migration/homing, proliferation, and differentiation of endogenous stem/progenitor cells. However, such approaches will require identification of renewable cell sources of engraftable functional cells, an improved ability to manipulate proliferation and differentiation of stem cells, and a better understanding of the signaling pathways that control the fate of stem cells. Equipped with large arrayed molecular libraries— combinatorial chemical libraries (>100,000 discrete and diverse small molecules), cDNA overexpression libraries (>30,000 human and mouse genes), and small interfering RNA libraries (targeting >20,000 human and mouse genes)—and a high-throughput screening platform, we are developing and integrating chemical and functional genomic tools to study stem cell biology and regeneration. We screen these libraries to identify small molecules and genes that can control the fate of stem cells in various systems, including (1) self-renewal, as well as directed neuronal, cardiac and R CHEMISTRY 2005 pancreatic differentiations of pluripotent mouse and human embryonic stem cells; (2) directed neuronal differentiation and subtype neuron specification of human and rodent neural stem cells; (3) directed differentiation of mesenchymal stem cells to osteogenic, adipogenic, chondrogenic, and myogenic lineages; (4) functional proliferation of adult cardiomyocytes and islets/beta cells; (5) cellular plasticity and dedifferentiation of lineage-restricted somatic cells; and (6) developmental signaling pathways. In addition, we are doing systemic biochemical and cellular studies, including detailed investigations of the structure-activity relationship, affinity chromatography for target identification, genome-wide expression analysis, and cDNA and/or RNA interference complementation screens to map signaling pathways, to characterize the molecular mechanism of these identified small molecules and genes. The results may ultimately facilitate the therapeutic application of stem cells and the development of small-molecule drugs to stimulate tissue and organ regeneration in vivo. 73 us to consider the triazines (2,4-diamino-triazines and their oxygen analogs) as alternative nucleobases that may be able to function as informational base pairs through a type of hydrogen-bond arrangement that differs from the canonical Watson-Crick type with its pairing axis parallel to the nucleosidic bond. Because carboxyl groups can easily be converted to suitably functionalized triazine rings, a large variety of oligomer backbones tagged with informational triazines (instead of conventional nucleobases) could be envisioned (Fig. 1). In collaboration with B. Han, Swiss Federal Institute of Technology, Zürich, Switzerland, we developed the triazination of the carboxyl group of a variety of α-amino acids such as glycine, serine, cysteine, aspartic acid, glutamic acid, β-amino-alanine, and α-carboxy-glycine to produce correspondingly triazine-tagged building blocks of potentially informational oligomers. PUBLICATIONS Chen, S., Zhang, Q., Wu, X., Schultz, P.G., Ding, S. Dedifferentiation of lineagecommitted cells by a small molecule. J. Am. Chem. Soc. 126:410, 2004. Ding, S., Schultz, P.G. A role for chemistry in stem cell biology. Nat. Biotechnol. 22:833, 2004. Liu, J., Wu, X., Mitchell, B., Kintner, C., Ding, S., Schultz, P.G. A small-molecule agonist of the Wnt signaling pathway. Angew. Chem. Int. Ed. 44:1987, 2005. Wu, X., Ding, S., Ding, Q., Gray, N.S., Schultz P.G. Small molecules that induce cardiomyogenesis in embryonic stem cells. J. Am. Chem. Soc. 126:1590, 2004. Wu, X., Walker, J., Zhang, J., Ding, S., Schultz, P.G. Purmorphamine induces osteogenesis by activation of the hedgehog signaling pathway. Chem. Biol. 11:1229, 2004. Zheng, L., Liu, J., Batalov, S., Zhou, D., Orth, A., Ding, S., Schultz, P.G. An approach to genomewide screens of expressed small interfering RNAs in mammalian cells, Proc. Natl. Acad. Sci. U. S. A. 101:135, 2004. F i g . 1 . 2,4-Diamino-triazine–tagged oligomeric systems. O L I G O M E R S B A S E D O N T R I A Z I N E - TA G G E D BACKBONES Chemical Etiology of the Structure of Nucleic Acids A. Eschenmoser, R. Krishnamurthy, O. Munoz, H. Xiong, G. Kumar, F. De Riccardis, R. Kondreddi, S. Eppacher, J. Nandy D uring the past year, we worked on the following projects. T R I A Z I N E - TA G G E D A M I N O A C I D D E R I VAT I V E S Our earlier work on the synthesis of C-nucleosides with a family of allopurines (formerly isopurines) led Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Of the 2 planned variants (compounds 1 and 2 in Fig. 1) of ethylenediamine-based oligomer systems containing triazine as recognition elements, we were able to synthesize and study oligomers (up to dodecamer) of 1 of them (2 in Fig. 1). As expected, oligomers of this chemical structure underwent efficient cross-pairing with polyuracil (RNA) and polythymine (DNA) (Table 1). However, to our surprise, the backbones of oligomers of this type were unstable because of a triazine-assisted eliminative fragmentation. A comparative conformational analysis relative to RNA (tagged with the conventional nucleobases) of oligomer backbones tagged with triazines predicted that oligodipeptides of type 2 and 4 (Fig. 1) might be 74 CHEMISTRY 2005 T a b l e 1 . T m values of duplexes formed by the triazine-tagged oligomers 2–5 with RNA and DNA* System Tm (°°C) 2 12-mer 3 6-mer 12-mer 4 6-mer 12-mer 5 8-mer 12-mer DNA poly(T) RNA poly(U) DNA d(T 12) RNA r(T 12) Organic, Materials, and Analytical Chemistry M.G. Finn, W.G. Lewis, D. Díaz, S. Punna, V. Rodionov, S. Sen Gupta 44.1† 29.2† 30.3† 28.5† <6 26.6 <10 33.1 – <11.0 – 28.0 41.7 59.4 50.0 65.0 32.2 53.8 42.8 57.2 26.7 35.2 19.7 29.1 n.m. 22.7 n.m. n.m. n addition to synthetic chemistry research on viruses, our program encompasses organic, organometallic, and materials chemistry. Special emphasis is placed on methods of chemical synthesis, the discovery of functional molecules, and catalysis. I M E C H A N I S M S A N D A P P L I C AT I O N S O F C L I C K CHEMISTRY *Measurements were made at 260 nm, c ≈ 5 µM + 5 µM in 1 M NaCl, 10 mM NaH2PO 4, 0.1 mM Na2EDTA, pH 7.0. T m values are given in degrees Celsius (°C) and are derived from maxima of the first derivative of the heating curve. – indicates no pairing observed, n.m. = no measurement, † = in 0.15 M NaCl, T = thymine, U = uracil, d = DNA, r = RNA. oligomer systems that cross-pair with RNA, whereas oligopeptides of type 3 should not (or less efficiently so). Experimental results obtained so far are in accord with the analysis, except that oligopeptides of type 3 also cross-pair with RNA, yet much more weakly than those of type 4 do (Table 1). The oligopeptides of type 4, composed of a triazine-tagged oligomer consisting of alternating glutamic and aspartic acid residues, cross-pairs with RNA (polyuracil) strongly (Table 1). Studies on the self-pairing and cross-pairing properties of type 4 oligopeptides are under way. A variation of oligodipeptide system 3 is the oligodipeptoid of type 5 (Fig. 1), constructed from the iminodiacetic acid unit, wherein the triazine- and carboxylate-containing side chains are now appended to the nitrogen atoms along the backbone, making this system achiral. We used a solid-support strategy starting from the requisite monomers to synthesize oligomers (up to dodecamer). The resultant oligodipeptoids crosspaired with RNA (polyuracil) and DNA (polythymine) (Table 1). PUBLICATIONS Ferencic, M., Reddy, G., Wu, X., Guntha, S.G., Nandy, J., Krishnamurthy, R., Eschenmoser, A. Base-pairing systems related to TNA containing phosphoramidate linkages: synthesis of building blocks and pairing properties. Chem. Biodivers. 1:939, 2004. Han B., Jaun, B., Krishnamurthy, R., Eschenmoser, A. Mannich type C-nucleosidations in the 5,8-diaza-7,9-dicarba-purine family. Org. Lett. 6:3691, 2004. Han, B., Rajwanshi, V., Nandy, J., Krishnamurthy, R., Eschenmoser, A. Mannichtype C-nucleosidations with 7-carba-purines and 4-aminopyrimidines. Synlett 744, 2005, Issue 4. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. The copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction, discovered in 2002 by V.V. Fokin and K.B. Sharpless, Department of Chemistry, has been adopted by chemists all over the world for organic synthesis, drug development, and materials science. In the past year, we reported the first experimental study of the mechanism of the reaction (Fig. 1). Two copper centers are required, and the rate-limiting step of the catalytic cycle changes under different conditions of concentration and reactant ratios. Copper acetylide intermediates are important, and we obtained kinetic evidence that the existence of a discrete azide-copper interaction is also important. We observed unusually large rates for the reactions of 1,3-diazides such as compound 1 (Fig. 1). F i g . 1 . Mechanistic outline of the CuAAC reaction as determined by kinetics measurements. New ligands have been discovered for copper, which provide large accelerations in rate in the CuAAC reaction. We used a fluorescence-quenching catalysis screen in aqueous solutions with low concentrations of reagents to find systems that operate under conditions optimized for bioconjugation. We found that a commercially available sulfonated bathophenanthroline ligand imparts superior performance to the copper catalyst under nitrogen atmosphere. This catalyst has had an extraordinary CHEMISTRY 2005 impact on our bioconjugation efforts, enabling us to attach a wide variety of structures to virus particles with far less material than required by any other method. We also pioneered the use of the CuAAC reaction in the synthesis and derivatization of polymeric materials. The deposition of molecules bearing 2, 3, or 4 azide and alkyne groups between metallic copper surfaces makes especially strong adhesive mixtures that glue the copper pieces to each other. The catalytic copper ions are provided by the surface, and much of the adhesion power to the metal is provided by the 1,2,3triazole moieties formed in the azide-alkyne cycloaddition process. We can also use the CuAAC process to connect polymers to each other and to other materials. Last, the CuAAC reaction mediates an unprecedented cyclic dimerization of peptides containing azide and alkyne groups on solid supports (Fig. 2). Rings as large as 36 amino acids and more than 120 atoms have been made selectively and in high yield. The bimetallic nature of the reaction appears to play a crucial role, and we are exploring its scope with both peptide and nonpeptide structures. 75 Díaz, D.D., Lewis, W.G., Finn, M.G. Acid-mediated amine exchange of N,Ndimethylformamidines: preparation of electron-rich formamidines. Synlett, in press. Díaz, D.D., Lewis, W.G., Finn, M.G. Activation of urea as a leaving group in substitution reactions of formamidine ureas. Chem. Lett., in press. Díaz, D.D., Punna, S., Holzer, P., McPherson, A.K., Sharpless, K.B., Fokin, V.V., Finn, M.G. Click chemistry in materials synthesis, I: adhesive polymers from coppercatalyzed azide-alkyne cycloaddition. J. Polym. Sci. A Polym. Chem. 42:4392, 2004. Gissibl, A., Finn, M.G., Reiser, O. Cu(II)-aza(bisoxazoline)-catalyzed asymmetric benzoylations. Org. Lett. 7:2325, 2005. Keller, K.A., Guo, J., Punna, S., Finn, M.G. A thermally-cleavable linker for solidphase synthesis. Tetrahedron Lett. 46:1181, 2005. Lewis, W.G., Magallon, F.G., Fokin, V.V., Finn, M.G. Discovery and characterization of catalysts for azide-alkyne cycloaddition by fluorescence quenching. J. Am. Chem. Soc. 126:9152, 2004. Meng, J.-C., Siuzdak, G., Finn, M.G. Affinity mass spectrometry from a tailored porous silicon surface. Chem. Commun. (Camb.) 2108, 2004, Issue 18. Narayan, S., Muldoon, J., Finn, M.G., Fokin, V.V., Kolb, H.C., Sharpless, K.B. “On water”: unique reactivity of organic compounds in aqueous suspension. Angew. Chem. Int. Ed. 44:3275, 2005. Punna, S., Díaz, D.D., Finn, M.G. Palladium-catalyzed homocoupling of arylboronic acids and esters using fluoride in aqueous solvents. Synlett 2351, 2004, Issue 13. Punna, S., Kuzelka, J., Wang, Q., Finn, M.G. Head-to-tail peptide cyclodimerization by copper-catalyzed azide-alkyne cycloaddition. Angew. Chem. Int. Ed. 44:2215, 2005. Punna, S., Meunier, S., Finn, M.G. A hierarchy of aryloxide deprotection by boron tribromide. Org. Lett. 6:2777, 2004. Rodionov, V.O., Fokin, V.V., Finn, M.G. Mechanism of the ligand-free Cu(I)-catalyzed azide-alkyne cycloaddition reaction. Angew. Chem. Int. Ed. 44:2210, 2005. F i g . 2 . An example of the cyclodimerization of peptides by the CuAAC reaction. SYNTHESIS AND USE OF FORMAMIDINE COMPOUNDS We have continued to explore the synthesis and properties of formamidines and formamidine ureas. We optimized efficient and modular routes to their preparation and found that these compounds are active binders and inhibitors of several families of enzymes. The properties of greatest importance are the basicity and electrophilicity of the compounds, an unusual combination that provides multiple ways for them to interact and react with target proteins. We found that fluorescently labeled formamidines are strong ligands for acetylcholinebinding proteins and that formamidine urea derivatives have promise as unique “soft dry” (degradable) binders. We are exploring these modes of action in collaboration with P. Taylor, University of California, San Diego. PUBLICATIONS Converso, A., Saaidi, P.-L., Sharpless, K.B., Finn, M.G. Nucleophilic substitution by Grignard reagents on sulfur mustards. J. Org. Chem. 69:7336, 2004. Díaz, D.D., Finn, M.G. Modular synthesis of formamidines and their formation of stable organogels. Chem. Commun. (Camb.) 2514, 2004, Issue 21. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Shen, Z., Go, E.P., Gamez, A., Apon, J.V., Fokin, V., Greig, M., Ventura, M., Crowell, J.E., Blixt, O., Paulson, J.C., Stevens, R.C., Finn, M.G., Siuzdak, G. A mass spectrometry plate reader: monitoring enzyme activity and inhibition with a desorption/ionization on silicon (DIOS) platform. Chembiochem 5:921, 2004. Stray, S.J., Bourne, C.R., Punna, S., Lewis, W.G., Finn, M.G., Zlotnick, A. A heteroaryldihydropyrimidine activates and can misdirect hepatitis B virus capsid assembly. Proc. Natl. Acad. Sci. U. S. A. 102:8138, 2005. Design of Functional Synthetic Systems M.R. Ghadiri, G. Ashkenasy, N. Ashkenasy, J. Beierle, A. Chavochi, N. Gianneschi, W.S. Horne, Z.-Z. Huang, P. Imming, L. Leman, A. Loutchnikov, A. Montero, L. Motiei, D. Nicoletti, Y. Norikane, J. Picuri, N. Rahe, D. Radu, S. Rahimipour, J. Shin, R. Yamasaki, Y.S. Yoo e are engaged in a multidisciplinary research effort to uncover new chemical and biochemical approaches for the design of functional molecular, supramolecular, and complex self-organized systems. Our endeavors span disciplines ranging from synthetic organic, bioorganic, and physical organic chemistry to nanotechnology, biophysics, enzymology, and W 76 CHEMISTRY 2005 molecular biology. Current research includes the design of synthetic peptide catalysts, antimicrobial self-assembling peptide nanotubes, semisynthetic allosteric enzymes, self-replicating molecular systems and emergent networks, single-molecule stochastic DNA sensing, molecular computation, and prebiotic chemistry. ANTIMICROBIAL PEPTIDE NANOTUBES F i g . 2 . Schematic representation of an intrasterically inactivated We showed that appropriately designed cyclic peptide subunits can self-assemble through hydrogen bond–directed ring stacking into open-ended hollow tubular structures that have marked antibacterial and antiviral activities in vitro. The effectiveness of this novel supramolecular class of bioactive species as selective antibacterial agents was highlighted by the high efficacy of one of these antimicrobials against lethal methicillinresistant Staphylococcus aureus infections in mice. Currently, we are exploring rational design of cyclic glycopeptides and selections from combinatorial libraries to discover novel antiviral and anticancer supramolecular compounds (Fig. 1). inhibitor-DNA-enzyme construct (left) and the DNA hybridization– triggered enzyme activation (right). The construct can be used to sense low concentrations of cDNA because of its built-in capacity for signal amplification via rapid turnover of substrate. F i g . 1 . Antiviral agents based on self-assembling cyclic peptide nanotubes. Cyclic D ,L-α-peptides act on endosomal membranes to prevent the development of low pH in endocytic vesicles, arrest the escape of virions from the endosome, and abrogate adenovirus infection. S T O C H A S T I C A N A LY S I S O F S I N G L E - M O L E C U L E D N A R O TA X A N E S We are interested in the study of matter at the level of single molecules. For these studies we use the transmembrane protein α-hemolysin as a rapid and highly sensitive sensor element for stochastic analysis of the molecules lodged or trapped inside the protein pore; the analysis relies on detecting the perturbations in the conductance levels produced in the ion channel in the native protein. Using this technique, we developed an approach by which a single-stranded DNA molecule can be trapped in a specific configuration inside an α-hemolysin channel (Fig. 3), manipulated, and studied with high sensitivity at the single-molecule level. Moreover, a single adenine nucleotide at a specific location on a strand of polydeoxycytidine can be detected by its characteristic effect in reducing the ion conductance in α-hemolysin. We are extending this approach to the design of rapid single-molecule DNA sensing and sequencing. DESIGN OF SIGNAL SELF-AMPLIFYING DNA SENSORS We constructed a novel sequence-specific DNA detection system based on rationally designed semisynthetic enzymes. The system is composed of covalently associated inhibitor-DNA-enzyme modules that function via DNA hybridization–triggered allosteric enzyme activation and signal amplification through substrate turnover (Fig. 2). The functional capacity of the system is highlighted by the sequence-specific detection of approximately 10 fmol of DNA in less than 3 minutes under physiologic conditions. Our studies suggest that rationally designed intrasterically regulated enzymes may be a promising new class of reagents for highly sensitive, rapid, 1-step detection of label-free DNA sequences that does not depend on polymerase chain reactions. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. F i g . 3 . Functional supramolecular chemistry at the single-molecule level. Single strands of DNA can be captured inside an α-hemolysin transmembrane pore protein to form single-species pseudorotaxanes composed of α-hemolysin and DNA. This process can be used to identify a single adenine nucleotide at a specific location on a strand of DNA on the basis of the characteristic reductions in the α-hemolysin ion conductance. SYNTHETIC NETWORKS Living cells use complex networks of evolutionary selected biomolecular interactions and chemical trans- CHEMISTRY 2005 formations to process multiple extracellular input signals rapidly and simultaneously. We are interested in understanding and experimentally modeling the organizational and functional properties of biological networks. We have developed a general strategy for the design and construction of self-organized synthetic peptide networks based on the sequence-selective autocatalytic and cross-catalytic template-directed coiled coil peptide fragment condensation reactions in aqueous solutions. The synthetic networks have some of the basic architectural and dynamic features of the living networks, reorganize in response to changes in environmental conditions and inputs (Fig. 4), and perform basic Boolean logic functions such as OR, NOR and NOTIF logic. We suggest that the ability to rationally construct predictable chemical circuitry might be useful in advancing the modeling and in better understanding some of the basic dynamic information-processing characteristics of the more complex cellular networks. 77 day volcanoes, is a condensing agent that brings about the formation of peptides from amino acids under mild conditions in aqueous solution (Fig. 5). We have studied the carbonyl sulfide–mediated condensations of α-amino acids under aerobic and anaerobic conditions in the absence of any added reagents and in the presence of metal ions, oxidizing agents, or alkylating agents. Depending on the reaction conditions and additives used, exposure of α-amino acids to carbonyl sulfide generates peptides in yields of up to 80% in minutes to hours at room temperature. F i g . 5 . Peptide formation under plausibly prebiotic reaction con- ditions. Carbonyl sulfide, a volcanic gas, is the most simple and effective amino acid–condensing agent for the formation of peptides in aqueous solutions. F i g . 4 . Adaptive reorganization in a synthetic peptide network. The graph structure or wiring of a synthetic peptide network responds PUBLICATIONS Ashkenasy, G., Ghadiri, M.R. Boolean logic functions of a synthetic peptide network. J. Am. Chem. Soc. 126:11140, 2004. dramatically to changes in the environmental stimuli (pH or salt content). Ashkenasy, G., Jagasia, R., Yadav, M., Ghadiri, M.R. Design of a directed molecular network. Proc. Natl. Acad. Sci. U. S. A. 101:10872, 2004. PREBIOTIC CHEMISTRY Leman, L., Orgel, L., Ghadiri, M.R. Carbonyl sulfide-mediated prebiotic formation of peptides. Science 306:283, 2004. In almost all discussions of prebiotic chemistry, it is assumed that amino acids, nucleotides, and possibly other monomers were first formed on Earth or brought to it in comets and meteorites and that the monomers subsequently condensed nonenzymatically to form oligomeric products. Unfortunately, attempts to create plausibly prebiotic polymerization reactions have met with limited success. Direct heating of solid mixtures leads to nonspecific products, and the condensing agents that have been studied, with the possible exception of inorganic polyphosphates, are relatively inefficient and/or marginally prebiotic. We showed that carbonyl sulfide, a simple gas present in the emissions from presentPublished by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Sánchez-Quesada, J., Saghatelian, A., Cheley, S., Bayley, H., Ghadiri, M.R. Single DNA rotaxanes of a transmembrane pore protein. Angew. Chem. Int. Ed. 43:3063, 2004. 78 CHEMISTRY 2005 A Merging of Chemistry and Biology K.D. Janda, J. Ashley, M. Atsumi, C. Berndt, G. Boldt, A. Brogan, R. Carrera, B. Clapham, T. Dickerson, L. Eubanks, G. Kaufmann, J. Kennedy, S.-J. Kim, Y.-S. Kim, B.-S. Lee, M. Lillo, Y. Liu, C. Lowery, H. Ma, S. Mahajan, H. Matsushita, M. Matsushita, L. McAllister, G. McElhaney, K. McKenzie, J. Mee, M. Meijler, J. Moss, L. Qi, C. Rogers, A. Shigenaga, P. Wirsching, Y. Xu, M. Yamashita, B. Zhou uring the past year, we explored various applications of organic chemistry at the interface of chemistry and biology. Representative examples of our results were obtained in 3 research programs: the immunologic consequences of methamphetaminebased protein glycation, the treatment via viruses of the effects of exposure to cocaine in the CNS, and the nonenzymatic formation of a bactericidal product from a compound known to act as a quorum-sensing agent. D IMMUNOLOGIC CONSEQUENCES OF M E T H A M P H E TA M I N E - B A S E D P R O T E I N G LY C AT I O N We extended our studies on the aberrant glycation of proteins by nornicotine to other drugs of abuse that contain reactive secondary amine groups. In this context, we found that methamphetamine can produce the corresponding Amadori product in vitro, and we examined the potential roles of this process in vivo. For example, protein glycation can increase the immunogenicity of the modified protein. The initial stage of protein glycation is accepted to proceed via the Amadori rearrangement, although the specific mechanism varies widely, depending on factors such as pH, ionic strength, and temperature. We analyzed the reaction of methamphetamine with glucose in buffer to detect the corresponding Amadori rearrangement product under physiologic conditions. Using commercially available sera containing polyclonal antibodies to a methamphetamine-protein conjugate, we found specific covalent modification of bovine serum albumin after extended incubation periods as indicated by enzyme-linked immunosorbent assays and dot blots (Fig. 1). In essence, the biochemical formation of a methamphetamine-derived advanced glycation end product (AGE) has numerous similarities to traditional hapten preparation, in which a nonimmunogenic molecule is covalently conjugated to a carrier protein via a linker by Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. F i g . 1 . Generation of an aberrant methamphetamine vaccine ini- tiated by the reaction of methamphetamine and glucose. using a chemical coupling reagent. Because of this parallel, we postulated that proteins glycated by methamphetamine could evoke an abnormal immune response. To examine the validity of this hypothesis, we prepared methamphetamine-glycated mouse serum albumin (MSA) and immunized mice with this modified protein. Serum samples from the mice were analyzed to determine if an antibody response to methamphetamine-AGE or to MSA had occurred. After a preliminary series of injections, the mice had appreciable amounts of antibodies to methamphetamine-AGE but no significant amounts of antibodies to the MSA carrier protein or to control injections of MSA alone. Of note, no adjuvant was required to achieve significant titers against methamphetamine-modified MSA, suggesting that the immune system needs little priming to recognize foreign glycation motifs. The discovery that these antibodies bind to methamphetamine is of potential significance in the context of addiction. Our observations suggest that once a methamphetamine-AGE is administered, an immune response could develop to the modified protein, and the antibodies produced could bind some proportion of the serum methamphetamine, thereby reducing the available concentration of the drug and ensuing high. Furthermore, autoantibodies against methamphetamine-modified proteins could have undesirable consequences, such as the misregulated activation of inflammatory pathways, leading to extensive tissue damage. In total, our results provide an intriguing possibility for an unrecognized mechanism underlying methamphetamine addiction and the associated health consequences. CHEMISTRY 2005 U S I N G V I R U S E S T O T R E AT C O C A I N E A D D I C T I O N Cocaine addiction continues to be a major health and social problem in the United States and other countries. The pharmacologic agents currently used to treat cocaine abuse are inadequate, and few treatment options are available. An alternative is to use protein-based therapeutic agents, such as antibodies, that can eliminate the load of cocaine and thereby attenuate its effects. This approach is especially attractive because the therapeutic agents exert no pharmacodynamic action of their own and therefore have little potential for side effects. The effectiveness of these agents, however, is limited by their inability to act directly within the CNS. Bacteriophages are viruses that infect bacteria, and unlike animal and plant viruses, they lack intrinsic tropism for eukaryotic cells. The filamentous bacteriophage fd can be produced at high levels in bacterial culture, making production simple and economical. However, perhaps the most important characteristic of this virus is its genetic flexibility. With phage display technology, a wide variety of proteins, antibodies, and peptides can be presented on the phage coat. The recent discovery that intranasally administered phage can penetrate the CNS caused us to speculate that by using phage as a vehicle for CNS entry, a method for treating the effects of cocaine addiction directly within the brain could be developed. To prove this hypothesis, we prepared phage molecules that display the cocaine-binding antibody GNC92H2. We then administered this modified phage intranasally to rats over a period of 3 days and assayed the animals’ cocaine-induced locomotor behavior. Gratifyingly, psychomotor responses to cocaine differed significantly between rats given phage GNC92H2 and rats given control phage. In rats given phage GNC92H2, movement was almost 50% less than at baseline (before administration of phage), whereas in the control group, locomotor activity actually increased compared with baseline values. To understand the role of the nasal vaccine, we investigated potential limitations. The CNS is considered an immune privileged site; however, the possibility that phage enters the bloodstream cannot be discounted. Filamentous phage in itself, and with displayed proteins on its surface, is a foreign entity to the immune system. However, analysis of serum from vaccinated animals showed only marginal concentrations of antibodies to phage and thus provides further evidence that potential toxic side effects are not being manifested in animals administered filamentous phage. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 79 Together these results indicate a novel approach for treating cocaine addiction directly within the CNS. We are examining the combination of this phage-based approach with either passive or active immunization protocols to determine whether any synergistic benefits can be obtained. We envision that this new proteinbased treatment for cocaine abuse can be modified to provide therapeutic agents for treatment of other drug abuse syndromes in which areas of the CNS are targeted. N O N E N Z Y M AT I C F O R M AT I O N O F T E T R A M I C A C I D S FROM QUORUM-SENSING MOLECULES The term quorum sensing has been coined to describe the ability of a population of unicellular bacteria to act as a multicellular organism in a cell density–dependent manner, that is, a way to sense “how many are out there.” Bacteria use small diffusible molecules to exchange information among themselves. An important class of “quormones,” or autoinducers, is the family of N-acylhomoserine lactones used by gram-negative bacteria. Upon reaching a critical threshold concentration, these compounds bind to their cognate receptor proteins, triggering the expression of target genes. Recently, we showed that N-(3-oxo-dodecanoyl) homoserine lactone (compound 1 in Fig. 2) performs a previously unrecognized role: the autoinducer itself and a corresponding degradation product derived from an unusual Claisen-like condensation reaction function as innate bactericidal agents. Incubation of compound 1 in water produced an undocumented compound in addition to the expected hydrolysis product (compound 3 in Fig. 2). Structural characterization of this anomalous molecule revealed that it was 3-(1-hydroxydecylidene)-5-(2-hydroxyethyl)pyrrolidine-2,4-dione (compound 2 in Fig. 2), a molecule that belongs to a F i g . 2 . Reaction of N-(3-oxo-dodecanoyl) homoserine lactone (1) to generate lactone hydrolysis product 3 (path a) and 3-(1hydroxydecylidene)-5-(2-hydroxyethyl)pyrrolidine-2,4-dione (compound 2; path b). 80 CHEMISTRY 2005 class of antibacterial and antifungal compounds known as tetramic acids. Because Pseudomonas aeruginosa uses compound 1 as the principal autoinducer and because of the known bactericidal activity of tetramic acids, we hypothesized that compound 2 could have biological function in the context of bacterial viability. Indeed, it had significant antibacterial activity against all tested gram-positive bacterial strains and no activity against P aeruginosa or other tested gram-negative bacteria. The effective concentration of compound 2 is biologically relevant, because high concentrations of compound 1 have been detected in P aeruginosa biofilms. Notably, compound 1 was also cytotoxic, suggesting a dual role for N-acylhomoserine lactones in P aeruginosa communities as both quorum-sensing molecules and as an interference mechanism against bacterial competitors. Additionally, compound 2 tightly binds essential metals such as iron, possibly providing a previously unrecognized primordial siderophore. Iron plays an essential role in physiologic processes and in the pathogenesis of bacterial infections. Many bacteria produce siderophores to sequester iron, an element that although essential for their growth has poor solubility under physiologic conditions. The 3-acetyl-pyrrolidine-2,4dione heterocycle found in compound 2 and in many other naturally occurring tetramic acids can efficiently chelate a variety of metal cations, including iron. Our studies have revealed the ability of compound 2 to compete for available iron in solution and potentially provide an additional method for iron solubilization. We propose that P aeruginosa uses compound 2 as an interference strategy to preclude encroachment by competing bacteria. Although the complexation of critical metals such as iron may play a role in this process, further study is required into the mechanism and scope of the observed bactericidal activity and the potential of compound 2 to act as a primordial siderophore. Dickerson, T.J., Lovell, T., Meijler, M.M., Noodleman, L., Janda, K.D. Nornicotine aqueous aldol reactions: synthetic and theoretical investigations into the origins of catalysis. J. Org. Chem. 69:6603, 2004. Dickerson, T.J., Reed, N.N., La Clair, J.J., Janda, K.D. A precipitator for the detection of thiophilic metals in aqua. J. Am. Chem. Soc. 126:16582, 2004. Dickerson, T.J., Yamamoto, N., Janda, K.D. Antibody-catalyzed oxidative degradation of nicotine using riboflavin. Bioorg. Med. Chem. 12:4981, 2004. Dickerson, T.J., Yamamoto, N., Ruiz, D.I., Janda, K.D. Immunological consequences of methamphetamine protein glycation. J. Am. Chem. Soc. 126:11446, 2004. Felding-Habermann, B., Lerner, R.A., Lillo, A., Zhuang, S., Weber, M.R., Arrues, S., Gao, C., Mao, S., Saven, A., Janda, K.D. Combinatorial antibody libraries from cancer patients yield ligand-mimetic Arg-Gly-Asp-containing immunoglobulins that inhibit breast cancer metastasis. Proc. Natl. Acad. Sci. U. S. A. 101:17210, 2004. Kaufmann, G.F., Meijler, M.M., Sun, C., Chen, D.W., Kujawa, D.P., Mee, J.M., Hoffman, T.Z., Wirsching, P., Lerner, R.A., Janda, K.D. Enzymatic incorporation of an antibody-activated blue fluorophore into DNA. Angew. Chem. Int. Ed. 44:2144, 2005. Kaufmann, G.F., Sartorio, R., Lee, S.H., Rogers, C.J., Meijler, M.M., Moss, J.A., Clapham, B., Brogan, A.P., Dickerson, T.J., Janda, K.D. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc. Natl. Acad. Sci. U. S. A. 102:309, 2005. Kim, Y.S., Moss, J.A., Janda, K.D. Biological tuning of synthetic tactics in solidphase synthesis: application to Aβ(1-42). J. Org. Chem. 69:7776, 2004. Lee, B.S., Mahajan, S., Janda, K.D. Cross-linked poly(4-vinylpyridine/styrene) copolymers as a support for immobilization of ytterbium triflate. Tetrahedron 61:3081, 2005. Lee, B.S., Mahajan, S., Janda, K.D. Molecular iodine-catalyzed imine activation for three-component nucleophilic addition reactions. Synlett, in press. Lee, B.S., Mahajan, S., Janda, K.D. Novel method for catalyst immobilization using an ionic polymer: a case study using recyclable ytterbium triflate. Tetrahedron Lett. 46:807, 2005. Lee, S.H., Matsushita, H., Koch, G., Zimmermann, J., Clapham, B., Janda, K.D. Smart cleavage reactions: the synthesis of an array of ureas from polymer-bound carbamates. J. Comb. Chem. 6:822, 2004. Lee, S.H., Yoshida, K., Matsushita, H., Clapham, B., Koch, G., Zimmermann, J., Janda, K.D. N-H insertion reactions of primary ureas: the synthesis of highly substituted imidazolones and imidazoles from diazocarbonyls. J. Org. Chem. 69:8829, 2004. Lillo, A.M., Sun, C., Gao, C., Ditzel, H., Parrish, J., Gauss, C.-M., Moss, J., Felding-Habermann, B., Wirsching, P., Boger, D.L., Janda, K.D. A human single-chain antibody specific for integrin α3β1 capable of cell internalization and delivery of antitumor agents. Chem. Biol. 11:897, 2004. Lowery, C.A., McKenzie, K.M., Qi, L., Meijler, M.M., Janda, K.D. Quorum sensing in Vibrio harveyi: probing the specificity of the LuxP binding site. Bioorg. Med. Chem. Lett. 15:2395, 2005. Matsushita, H., Lee, S.H., Yoshida, K., Clapham, B., Koch, G., Zimmermann, J., Janda, K.D. N-H insertion reactions of Boc-amino acid amides: solution- and solidphase synthesis of pyrazinones and pyrazines. Org. Lett. 6:4627, 2004. PUBLICATIONS Carrera, M.R.A., Kaufmann, G.F., Mee, J.M., Meijler, M.M., Koob, G.F., Janda, K.D. Treating cocaine addiction with viruses. Proc. Natl. Acad. Sci. U. S. A. 101:10416, 2004. McDunn, J.E., Dickerson, T.J., Janda, K.D. Antibody catalysis of disfavored chemical reactions. In: Catalytic Antibodies. Keinan, E. (Ed.). Wiley & Sons, New York, 2005, p. 184. Carrera, M.R.A., Meijler, M.M., Janda, K.D. Cocaine pharmacology and current pharmacotherapies for its abuse. Bioorg. Med. Chem. 12:5019, 2004. Meijler, M.M, Kaufmann, G.F., Qi, L., Mee, J.M., Coyle, A.R., Moss, J.A., Wirsching, P., Matsushita, M., Janda, K.D. Fluorescent cocaine probes: a tool for the selection and engineering of therapeutic antibodies. J. Am. Chem. Soc. 127:2477, 2005. Carrera, M.R.A., Trigo, J.M., Roberts, A.J., Janda, K.D. Evaluation of the anticocaine monoclonal antibody GNC92H2 as an immunotherapy for cocaine overdose. Pharmacol. Biochem. Behav., in press. Moss, J.A., Lillo, A., Kim, Y.S., Gao, C., Ditzel, H., Janda, K.D. A dimerization “switch” in the internalization mechanism of a cell-penetrating peptide. J. Am. Chem. Soc. 127:538, 2005. Dickerson, T.J., Kaufmann, G.F., Janda, K.D. Bacteriophage-mediated protein delivery into the central nervous system and its application in immunopharmacotherapy. Expert Opin. Biol. Ther. 5:773, 2005. Reed, N.N., Dickerson, T.J., Boldt, G.E., Janda, K.D. Enantioreversal in the Sharpless asymmetric epoxidation reaction controlled by the molecular weight of a covalently appended achiral polymer. J. Org. Chem. 70:1728, 2005. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. CHEMISTRY 2005 Rogers, C.J., Dickerson, T.J., Brogan, A.P., Janda, K.D. Hammett correlation of nornicotine analogues in the aqueous aldol reaction: implications for green organocatalysis. J. Org. Chem. 70:3705, 2005. Xu, Y., Yamamoto, N., Janda, K.D. Catalytic antibodies: hapten design strategies and screening methods. Bioorg. Med. Chem. 12:5247, 2004. Protein Misfolding Diseases: Cell Biology and Bioorganic and Biophysical Chemistry J.W. Kelly, L. Bazhenova, J. Bieschke, D. Bosco, P. Braun, M.T.A. Dendle, W. D’Haeze, T. Foss, D. Fowler, K. Frankenfield, Y. Fu, J. Gao, M.-Y. Gao, A. Hurshman, S. Johnson, E.T. Powers, A. Sawkar, S. Siegel, J.-Y. Suk, L. Wiseman, I. Yonemoto, Z. Yu, Q. Zhang ur goal is to contribute to the understanding of the molecular mechanisms of protein folding and misfolding; protein misfolding ultimately leads to neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease. We use cell biological approaches, in collaboration with W.E. Balch, Department of Cell Biology, and spectroscopic and biophysical approaches in combination with chemical synthesis and recombinant DNA technology. O INHIBITION OF TRANSTHYRETIN AMYLOIDOGENESIS As a consequence of a mutation or denaturation stress associated with aging and/or oxidative stress, the transthyretin tetramer dissociates, and subsequent changes in the tertiary structure of the monomer make it competent to misassemble into aggregates, including fibrils. An attractive strategy to slow or prevent the formation of aggregates is to inhibit the rate-limiting dissociation of the tetramer and stabilize the native state. In this respect, we synthesized various structurally distinct small molecules that inhibit transthyretin amyloidogenesis. For example, bisarylaldoxime ethers substituted with a carboxylic acid on one aromatic ring and halogens or a trifluoromethyl group on the other aryl ring substantially inhibit the formation of transthyretin fibrils, as do oxazoles carrying a carboxyl group at C-4, a 3,5dichlorophenyl group at C-2, and an ethyl, a propyl, or a trifluoromethyl group at C-5. We showed that hydroxylated polychlorinated biphenyls bind transthyretin in blood and plasma with a high affinity and specificity and inhibit the formation of transthyretin fibrils in vitro. Such small molecules might be suitable drug candiPublished by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 81 dates for treatment of transthyretin amyloidoses, as illustrated by the drug diflunisal, which is being tested in humans in a multicenter placebo-controlled clinical trial because of previous discoveries in our laboratory. These small-molecule inhibitors typically bind with negative cooperativity, and thus investigating the ability of a transthyretin tetramer to undergo amyloidogenesis when bound to only a single inhibitor is important. We showed that occupancy of only 1 of the 2 possible sites was sufficient to stabilize the entire tetramer and prevent amyloidogenesis. In these studies, we tethered a small-molecule inhibitor to 1 of the 4 transthyretin subunits, allowing it to bind to 1 of the ligand-binding sites within the transthyretin tetramer. We also found that we could stabilize the same interface by linking the N terminus of one subunit to the C terminus of a second subunit, a sequence modification that conferred kinetic stability on the quaternary structure. We developed a new method to demonstrate kinetic stabilization of the tetramer mediated by binding of 1 ligand under physiologic conditions. In this method, we used the correlation between the tetramer dissociation rate and the rate at which unlabeled wild-type transthyretin homotetramers and wild-type transthyretin homotetramers labeled at the N terminus exchanged subunits. The latter exchange was dramatically slowed in the presence of small-molecule inhibitors. In other research, we found that only the most destabilized transthyretin variants are degraded by endoplasmic reticulum–associated degradation, and then only in certain tissues. We discovered that endoplasmic reticulum–assisted folding determines protein secretion in a tissue-specific manner, and we propose that its competition with endoplasmic reticulum–associated degradation may explain the appearance of tissue-selective amyloid diseases, especially for highly destabilized transthyretin variants that lead to CNSselective disease, because the CNS is the only tissue that can secrete these highly unstable transthyretin variants. Surprisingly, we found the brain is a more permissive transthyretin secretor than is the liver. O X I D AT I V E M E TA B O L I T E S A N D P R O T E I N A G G R E G AT I O N Oxidative stress leads to the generation of oxidative metabolites that can modify proteins. We discovered that oxidative metabolites generated upon cholesterol ozonolysis or lipid peroxidation can covalently modify amyloid β-peptides (Aβ), dramatically accelerating the amyloidogenesis of these peptides, which are associ- 82 CHEMISTRY 2005 ated with Alzheimer’s disease. Metabolite modification of Aβ amyloidogenesis occurs via a 2-step mechanism involving the nucleation-independent formation of spherical aggregates by Aβ-metabolite adducts before the generation of fibrillar aggregates. This mechanism may explain the formation of Aβ aggregates in the brain at nanomolar concentrations. α-Synucleinopathies, including Parkinson’s disease and dementia with Lewy bodies, are characterized by cytoplasmic α-synuclein–rich aggregates within degenerating dopaminergic neurons in the substantia nigra. Observations suggest a correlation between oxidative stress or inflammation and Parkinson’s disease. Feasibly, reactive oxygen species react with metabolites to generate oxidized metabolites that can interact with α-synuclein, triggering misfolding and subsequent aggregation. We aim to determine whether such metabolites accelerate the aggregation of α-synuclein in the same way they accelerate the aggregation of Aβ. Those studies will provide insight into the correlation between oxidative stress and α-synucleinopathies. β-SHEET FOLDING The native state of a protein is stabilized by hydrophobic interactions between side chains (the hydrophobic effect) and by hydrogen bonding. We studied the role of backbone hydrogen bonds in the folding kinetics and stability of the PIN WW domain, a 34-residue β-sheet protein composed of 3 β-strands and 2 intervening loops. For these studies, we used amide-to-ester backbone mutations, alterations that do not affect backbone conformational preferences or the structure of side chains. We synthesized 19 variants, allowing the perturbation of 11 backbone hydrogen bonds. Thermodynamic analysis indicated that the location of a backbone hydrogen bond determines the extent of protein destabilization caused by the elimination of a hydrogen bond by amide-to-ester mutation. Elimination of buried hydrogen bonds in the hydrophobic core substantially destabilizes the PIN WW domain; in contrast, elimination of hydrogen bonds present at or near loops exposed to solvent is only slightly destabilizing. In addition, we found that the destabilization of the PIN WW domain was greater when a backbone hydrogenbond donor was eliminated than when a hydrogen-bond acceptor was weakened. These findings are important because they suggest that only a subset of hydrogen bonds is energetically important in protein folding. Previously, we synthesized a peptidomimetic composed of a dibenzofuran template substituted at C-2 Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. and C-8 with propanoic acid linkers to which valinethreonine-valine-threonine peptides were attached via N-terminal amide bonds. This peptidomimetic forms protofilaments and filaments via a side-chain hydrophobic collapse model. In this model, the inward association of the amino acid side chains is such that intramolecular interactions are mediated by the side chains and not by hydrogen-bonded intermolecular formation of β-sheets. Investigation of the means by which this peptidomimetic self-assembles revealed that it is strictly analogous to the structure of Aβ fibrils recently characterized by using solid-state nuclear magnetic resonance. FAMILIAL AMYLOIDOSIS OF FINNISH TYPE Familial amyloidosis of Finnish type is caused by the D187N/Y mutation in plasma gelsolin. This disease is characterized by amyloid deposits composed of 5- and 8-kD internal fragments of plasma gelsolin. The disease is triggered by a loss of a calcium-binding site in domain 2 that allows aberrant furin cleavage in the Golgi complex, yielding a 68-kD fragment. This fragment is then cleaved by a transmembrane matrix metalloprotease, resulting in 5- and 8-kD fragments that are deposited as amyloid fibrils in the extracellular matrix. Currently, we are testing the effect of a variety of structurally distinct glycosaminoglycans on the rate of gelsolin amyloidosis. We expect that effective treatment of gelsolin amyloid disease will involve decreasing the amount of the amyloidogenic gelsolin fragments. This decrease can be achieved, for instance, by inhibiting proteases that cleave mutant gelsolin. Inhibitors of either or both of these proteases could serve as drugs for familial amyloidosis of Finnish type. CHEMICAL CHAPERONES The N370S mutation in glucocerebrosidase, a lysosomal hydrolase, leads to an accumulation of glucocerebrosidase substrate in lysosomes and consequently to Gaucher disease, the most common lysosomal storage disorder. We showed that N-(n-nonyl)deoxynojirimycin increases the activity of N370S glucocerebrosidase in a cell line derived from a patient with Gaucher disease. Most likely, the small molecule acts as a chemical chaperone for glucocerebrosidase. In other words, binding of the small molecule stabilizes glucocerebrosidase, allowing the enzyme to be trafficked successfully from the endoplasmic reticulum to the lysosome. Recently, we examined additional disease-associated glucocerebrosidase mutants and new classes of small CHEMISTRY 2005 molecules. We found that some other glucocerebrosidase mutants are also amenable to chemical chaperoning and that several of the compounds tested increased the activity of multiple mutants. PUBLICATIONS Bieschke, J., Zhang, Q., Powers, E.T., Lerner, R.A., Kelly, J.W. Oxidative metabolites accelerate Alzheimer’s amyloidogenesis by a two-step mechanism, eliminating the requirement for nucleation. Biochemistry 44:4977, 2005. Deechongkit, S., Dawson, P.E., Kelly, J.W. Toward assessing the position-dependent contributions of backbone hydrogen bonding to β-sheet folding thermodynamics employing amide-to-ester perturbations. J. Am. Chem. Soc. 126:16762, 2004. 83 Total Synthesis, New Synthetic Technologies, and Chemical Biology K.C. Nicolaou, I. Andrews, S. Arseniyadis, W. Brenzovich, P. Bulgar, G. Carenzi, J. Chen, A. Converso, A. Corbu, J. Crawford, P. Dagneau, K. Dellios, R. Denton, A. Estrada, D. Edmonds, R. Faraoni, T. Francis, M. Frederick, M. Freestone, R. Harbach, D. Harris, S. Harrison, V. Jeso, Deechongkit, S., Nguyen, H., Powers, E.T., Dawson, P.E., Gruebele, M., Kelly, J.W. Context-dependent contributions of backbone hydrogen bonding to β-sheet folding energetics. Nature 430:101, 2004. Deechongkit, S., Powers, E.T., You, S.-L., Kelly, J.W. Controlling the morphology of cross β-sheet assemblies by rational design. J. Am. Chem. Soc. 127:8562, 2005. Foss, T.R., Kelker, M.S., Wiseman, R.L., Wilson, I.A., Kelly, J.W. Kinetic stabilization of the native state by protein engineering: implications for inhibition of transthyretin amyloidogenesis. J. Mol. Biol. 347:841, 2005. Johnson, S.M., Petrassi, H.M., Palaninathan, S.K., Mohamedmohaideen, N.N., Purkey, H.E., Nichols, C., Chiang, K.P., Walkup, T., Sacchettini, J.C., Sharpless, K.B., Kelly, J.W. Bisaryloxime ethers as potent inhibitors of transthyretin amyloid fibril formation. J. Med. Chem. 48:1576, 2005. Petrassi, H.M., Johnson, S.M., Purkey, H.E., Chiang, K.P., Walkup, T., Jiang, X., Powers, E.T., Kelly, J.W. Potent and selective structure-based dibenzofuran inhibitors of transthyretin amyloidogenesis: kinetic stabilization of the native state. J. Am. Chem. Soc. 127:6662, 2005. Purkey, H.E., Palaninathan, S.K., Kent, K.C., Smith, C., Safe, S.H., Sacchettini, J.C., Kelly, J.W. Hydroxylated polychlorinated biphenyls selectively bind transthyretin in blood and inhibit amyloidogenesis: rationalizing rodent PCB toxicity. Chem. Biol. 11:1719, 2004. Razavi, H., Powers, E.T., Purkey, H.E., Adamski-Werner, S.L., Chiang, K.P., Dendle, M.T.A., Kelly, J.W. Design, synthesis, and evaluation of oxazole transthyretin amyloidogenesis inhibitors. Bioorg. Med. Chem. Lett. 15:1075, 2005. Sekijima, Y., Wiseman, R.L., Matteson, J., Hammarström, P., Miller, S.R., Sawkar, A.R., Balch, W.E., Kelly, J.W. The biological and chemical basis for tissue-selective amyloid disease. Cell 121:73, 2005. Wiseman, R.L., Green, N.S., Kelly, J.W. Kinetic stabilization of an oligomeric protein under physiological conditions demonstrated by a lack of subunit exchange: implications for transthyretin amyloidosis. Biochemistry 44:9265, 2005. Wiseman, R.L., Johnson, S.M., Kelker, M.S., Foss, T., Wilson, I.A., Kelly, J.W. Kinetic stabilization of an oligomeric protein by a single ligand binding event. J. Am. Chem. Soc. 127:5540, 2005. You, S.-L., Kelly, J.W. The total synthesis of bistratamides F-I. Tetrahedron 61:241, 2005. You, S.-L., Kelly, J.W. Total synthesis of didmolamides A and B. Tetrahedron Lett. 46:2567, 2005. Zhang, Q., Kelly, J.W. Cys-10 mixed disulfide modifications exacerbate transthyretin familial variant amyloidogenicity: a likely explanation for variable clinical expression of amyloidosis and the lack of pathology in C10S/V30M transgenic mice? Biochemistry 44:9079, 2005. F. Kaiser, D. Kim, T. Koftis, S. Lee, T. Ling, D. Lizos, E. Loizidou, N. Mainolfi, S. Mandal, C. Mathison, R. Milburn, R. Mogul, A. Nold, R. de Noronha, A. Ortiz, C. Papageorgiou, L. Pasunoori, G. Petrovic, J. Piper, B. Pratt, A. Roecker, B. Safina, D. Sarlah, P. Sasmal, C. Schindler, D. Schlawe, S. Snyder, C. Stathakis, C. Solorio-Alvarado, X. Sun, W. Tang, C. Turner, H. Xu, M. Zak e focus on the total synthesis of natural products, the discovery and development of new synthetic technologies, and chemical biology. Naturally occurring substances are selected as synthetic targets because of their novel molecular architectures, important biological properties, and interesting mechanisms of action. The projects are designed to optimize the opportunities for discovery and invention in the areas of chemistry, biology, and medicine. The anticancer drug paclitaxel (Taxol), the antitumor epothilones, the neurotoxins brevetoxins A and B, the antibiotic vancomycin, the cholesterol-lowering CP-molecules, the antibiotic everninomicin, the TNF α–associated trichodimerol, the tetrahydropyran class of natural products, apoptolidin, diazonamide A, thiostrepton, and azaspiracid-1 exemplify this philosophy. Current projects include total synthesis of the antibiotic nocathiacin, the antifeedant azadirachtin, various other azaspiracids, and the antitumor agents lomaiviticins A and B (Fig. 1). In addition, we are developing synthetic technologies and strategies for chemical synthesis and chemical biology studies. Our overall aims are to advance the art and science of chemical synthesis and to develop enabling technologies for biology and medicine while maximizing educational opportunities and training of young men and women in chemistry. W PUBLICATIONS Nicolaou, K.C., Bulger, P.G., Sarlah, D. Metathesis reactions in total synthesis. Angew. Chem. Int. Ed. 44:4490, 2005. Nicolaou, K.C., Bulger, P.G., Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 44:4442, 2005. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 84 CHEMISTRY 2005 Nicolaou, K.C., Sasmal, P.K., Koftis, T.V., Converso, A., Loizidou, E., Kaiser, F., Roecker, A.J., Dellios, K., Sun, X.-W., Petrovic, G. Studies toward the synthesis of azadirachtin, 2: construction of fully functionalized ABCD ring frameworks and unusual intramolecular reactions induced by close-proximity effects. Angew. Chem. Int. Ed. 44:3447, 2005. Nicolaou, K.C., Sasmal, P.K., Roecker, A.J., Sun, X.-W., Mandal, S., Converso, A. Studies toward the synthesis of azadirachtin, 1: total synthesis of a fully functionalized ABC ring framework and coupling with a norbornene domain. Angew. Chem. Int. Ed. 44:3443, 2005. Nicolaou, K.C., Snyder, S.A. Chasing molecules that were never there: misassigned natural products and the role of chemical synthesis in modern structure elucidation. Angew. Chem. Int. Ed. 44:1012, 2005. Nicolaou, K.C., Snyder, S.A. The essence of total synthesis. Proc. Natl. Acad. Sci. U. S. A. 101:11929, 2004. Nicolaou, K.C., Snyder, S.A., Giuseppone, N., Huang, X., Bella, M., Reddy, M.V., Rao, P.B., Koumbis, A.E., O’Brate, A., Giannakakou, P. Studies toward diazonamide A: development of a hetero-pinacol macrocyclization cascade for the construction of the bis-macrocyclic framework of the originally proposed structure [published correction appears in J. Am. Chem. Soc. 126:15316, 2004]. J. Am. Chem. Soc. 126:10174, 2004. Nicolaou, K.C., Snyder, S.A., Huang, X., Simonsen, K.B., Koumbis, A.E., Bigot, A. Studies toward diazonamide A: initial synthetic forays directed toward the originally proposed structure. J. Am. Chem. Soc. 126:10162, 2004. Nicolaou, K.C., Snyder, S.A., Longbottom, D.A., Nalbandian, A.Z., Huang, X. New uses for the Burgess reagent in chemical synthesis: methods for the facile and stereoselective formation of sulfamidates, glycosylamines, and sulfamides. Chemistry 10:5581, 2004. Nicolaou, K.C., Tang, W., Dagneau, P., Faraoni, R. A catalytic asymmetric threecomponent 1,4-addition/aldol reaction: enantioselective synthesis of the spirocyclic system of vannusal A. Angew. Chem. Int. Ed. 44:3874, 2005. Nicolaou, K.C., Vyskocil, S., Koftis, T.V., Yamada, Y.M.A., Ling, T., Chen, D.Y.-K., Tang, W., Petrovic, G., Frederick, M.O., Li, Y., Satake, M. Structural revision and total synthesis of azaspiracid-1, part 1: intelligence gathering and tentative proposal. Angew. Chem. Int. Ed. 43:4312, 2004. F i g . 1 . Selected target molecules. Nicolaou, K.C., Carenzi, G.E.A., Jeso, V. Construction of highly functionalized medium-sized rings: synthesis of hyperforin and perforatumone model systems. Angew. Chem. Int. Ed. 44:3895, 2005. Nicolaou, K.C., Chen, D.Y.-K., Huang, X., Ling, T., Bella, M., Snyder, S.A. Chemistry and biology of diazonamide A: first total synthesis and confirmation of the true structure [published correction appears in J. Am. Chem. Soc. 126:15316, 2004]. J. Am. Chem. Soc. 126:12888, 2004. Nicolaou, K.C., Xu, H., Wartmann, M. Biomimetic total synthesis of gambogin and rate acceleration of pericyclic reactions in aqueous media. Angew. Chem. Int. Ed. 44:756, 2005. Nicolaou, K.C., Zak, M., Safina, B.S., Lee, S.H., Estrada, A.A. Total synthesis of thiostrepton, 2: construction of the quinaldic acid macrocycle and final stages of the synthesis. Angew. Chem. Int. Ed. 43:5092, 2004. Tan, C., de Noronha, R.G., Roecker, A.J., Pyrzynska, B., Khwaja, F., Zhang, Z., Zhang, H., Teng, O., Nicholson, A.C., Giannakakou, P., Zhou, W., Olson, J.J., Pereira, M.M., Nicolaou, K.C., Van Meir, E.G. Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway. Cancer Res. 65:605, 2005. Nicolaou, K.C., Estrada, A.A., Zak, M., Lee, S.H., Safina, B.S. A mild and selective method for the hydrolysis of esters employing trimethyltin hydroxide, Angew. Chem. Int. Ed. 44:1378, 2005. Nicolaou, K.C., Hao, J., Reddy, M.V., Rao, P.B., Rassias, G., Snyder, S.A., Huang, X., Chen, D.Y.-K., Brenzovich, W.E., Giuseppone, N., O’Brate, A., Giannakakou, P. Chemistry and biology of diazonamide A: second total synthesis and biological investigations [published correction appears in J. Am. Chem. soc. 126:15316]. J. Am. Chem. Soc. 126:12897, 2004. Nicolaou, K.C., Koftis, T.V., Vyskocil, S., Petrovic, G., Ling, T., Yamada, Y.M.A., Tang, W., Frederick, M.O. Structural revision and total synthesis of azaspiracid-1, part 2: definition of the ABCD domain and total synthesis. Angew. Chem. Int. Ed. 43:4318, 2004. Nicolaou, K.C., Lee, S.H., Estrada, A.A., Zak, M. Construction of substituted N-hydroxyindoles: synthesis of a nocathiacin I model system. Angew. Chem. Int. Ed. 44:3736, 2005. Nicolaou, K.C., Montagnon, T., Vassilikogiannakis, G., Mathison, C.J.N. The total synthesis of coleophomones B, C, and D. J. Am. Chem. Soc. 127:8872, 2005. Nicolaou, K.C., Safina, B.S., Zak, M., Estrada, A.A., Lee, S.H. Total synthesis of thiostrepton, 1: construction of the dihydropiperidine/thiazoline-containing macrocycle. Angew. Chem. Int. Ed. 43:5087, 2004. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Chemical, Biological, and Biophysical Approaches to Understanding Evolution F.E. Romesberg, J. Chin, R. Cirz, M. Cremeens, D. Harris, A. Henry, R. Holmberg, G. Hwang, Y. Kim, A. Leconte, E. Lis, S. Matsuda, E. Oakman, B. O’Neill, T. Roberts, L. Sagle, P. Smith, M. Thielges, P. Weinkam, W. Yu, J. Zimmermann he molecules of biology are unique because they have been evolved for function. We take a unique multidisciplinary approach to understanding these processes. T CHEMISTRY 2005 INCREASING THE CHEMICAL AND GENETIC POTENTIAL OF DNA Biological information storage is based on the natural genetic alphabet, composed of the 2 base pairs guaninecytosine and adenine-thymine. We are interested in increasing the information potential of DNA by expanding the genetic alphabet with a third base pair composed of unnatural nucleobases. Using hydrophobicity, polarity, shape complementarity, and hydrogen bonding, we developed several promising unnatural base pairs, including some that are replicable in vitro. Currently, we are refining these base pairs and synthesizing and characterizing additional novel unnatural base pairs. Nature developed the natural genetic code, not only by optimizing DNA and RNA but also by evolving the polymerases that synthesize these nucleic acids. We developed a selection system that can be used to evolve polymerases for any desired function. The selection system is based on the codisplay of DNA polymerases and their DNA substrates on phage particles (Fig. 1). Polymerases that can efficiently synthesize DNA containing the unnatural substrates covalently attach a biotin tag to the corresponding phage particle, allowing selective recovery on a strepavidin solid. Using this activity-based selection system, we evolved polymerases with a variety of novel functions, including the synthesis of DNA containing an unnatural base 85 pair. We are optimizing these polymerases and evolving new ones. DNA DAMAGE RESPONSE Evolution requires mutation, but mutations also make cells susceptible to aging and cancer. It is now understood that at times of sufficient stress, cells induce error-prone replication to facilitate their own evolution. We used high-throughput methods to screen the entire yeast genome for genes involved in both error-free and error-prone responses to DNA stress. We identified and characterized a variety of proteins with functions ranging from cell-cycle control to recombinational repair of stalled replication forks to ubiquitination. Characterization of the proteins required for mutation in eukaryotic cells will not only revolutionize our understanding of cancer and aging but also result in identification of targets whose inhibition might actually inhibit these processes. Pathogenic bacteria have plagued humanity since its beginnings. With the advent of antibiotics, many scientists suggested that this problem had been solved. However, today, because of their evolution, bacteria exist that are resistant to all available antibiotics. Consequently, we are also characterizing how prokaryotes induce mutation, which is required for the evolution of drug resistance. We have fully characterized the mechanisms in Escherichia coli and are characterizing the pathways associated with drug resistance in Pseudomonas aeruginosa and Staphylococcus aureus. Perhaps most exciting, we have initiated efforts to design a drug that inhibits bacterial mutation and thus evolution. EVOLUTION OF PROTEIN DYNAMICS F i g . 1 . Activity-based selection system in which a DNA poly- merase and its unnatural substrate are codisplayed on the same phage particle so that activity results in biotinylation and recovery of active polymerase mutants. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. The products of evolution are molecules with unique vibrational dynamics. The study of vibrational dynamics in proteins and nucleic acids has been limited by spectral complexity, but selective deuteration of a protein or a nucleic acid results in a carbon-deuterium oscillator that absorbs light in an otherwise transparent region of the infrared spectrum. The synthesis of selectively deuterated proteins has provided us with a residue-specific probe of flexibility, function, and folding. We are also using multidimensional femtosecond spectroscopy to characterize how protein motion is evolved during the somatic evolution of antibodies (Fig. 2). We quantitatively showed that somatic evolution systematically evolves an antibody from a flexible receptor into a more rigid receptor and that the immune system can manipulate protein dynamics, findings that suggest a role for these dynamics in molecular recognition. 86 CHEMISTRY 2005 F i g . 2 . Structural and dynamic data are used to understand how antibodies are evolved for molecular recognition. PUBLICATIONS Cirz, R.T., Chin, J.K., Andes, D.R., de Crecy-Lagard, V., Craig, W.A., Romesberg, F.E. Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol. 3:e176, 2005. Henry, A.A., Jimenez, R., Hanway, D., Romesberg, F.E. Preliminary characterization of light harvesting in E. coli DNA photolyase. Chembiochem 5:1088, 2004. Holmberg, R.C., Henry, A.A., Romesberg, F.E. Directed evolution of novel polymerases. Biomol. Eng. 22:39, 2005. Matsuda, S., Romesberg, F.E. Optimization of interstrand hydrophobic packing interactions within unnatural DNA base pairs. J. Am. Chem. Soc. 126:14419, 2004. O’Neill, B.M., Hanway D., Winzeler, E.A., Romesberg, F.E. Coordinated functions of WSS1, PSY2, and TOF1 in the DNA damage response. Nucleic Acids Res. 32:6519, 2004. Romesberg, F.E., Schowen, R.L. Isotope effects and quantum tunneling in enzyme-catalyzed hydrogen transfer, I: the experimental basis. Adv. Phys. Org. Chem. 39:27, 2004. Biological Chemistry P.G. Schultz, L. Alfonta, E. Brustad, S. Chen, J. Chitturulu, C. Cho, J. Graziano, J. Grbic, D. Groff, J. Hong, W.Y. Hur, M. Jahnz, J. Lee, K.-B. Lee, J. Liao, J. Liu, H. Luesch, F. Marr, S. Matsuda, J. Melnick, J. Mills, K.H. Min, M. Mukherji, B. Okram, Y. Ryu, S. Schiller, D. Summerer, L. Supekova, E. Tippmann, M.-L. Tsao, J. Turner, J. Wang, A. Willingham, J. Xie, H. Zeng, Q. Zhang A lthough chemists are remarkably adept at synthesizing molecular structures, they are far less sophisticated in designing and synthesizing mol- Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. ecules with defined biological or chemical functions. Nature, on the other hand, has produced an array of molecules with remarkably complex functions, ranging from photosynthesis and signal transduction to molecular recognition and catalysis. Our aim is to combine the synthetic strategies and biological processes of Nature with the tools and principles of chemistry to create new molecules with novel chemical and biological functions. By studying the properties of the resulting molecules, we hope to gain new insights into the molecular mechanisms of complex biological and chemical systems. For example, we have shown that the tremendous combinatorial diversity of the immune response can be chemically reprogrammed to generate selective enzymelike catalysts. We have developed antibodies that catalyze a wide array of chemical and biological reactions, from acyl transfer to redox reactions. Characterization of the structure and mechanisms of these catalytic antibodies has led to important new insights into the mechanisms of biological catalysis. In addition, the detailed characterization of the properties and structures of germ-line and affinity-matured antibodies is revealing fundamental new aspects of the evolution of binding and catalytic function, in particular, the role of structural plasticity in the immune response. Most recently, we have focused on in vitro evolution methods that involve the development of novel chemical screens and selections for identifying mutants with enhanced function and the rational design of proteolytic antibodies. Our work on catalytic antibodies redirects natural combinatorial diversity to produce new function. We are extending this combinatorial approach to many other problems, including the generation of sequencespecific recombinases, small-molecule regulators of DNA transcription, and the ab initio evolution of novel protein domains. We are also generating structure-based combinatorial libraries of small molecules, including purine, pyrimidine, and fatty acid derivatives. These libraries are being used in conjunction with novel cellular and organismal screens to identify important proteins involved in such cellular processes as differentiation, proliferation, and signaling. Indeed, we have identified molecules that control stem cell differentiation and self-renewal and that dedifferentiate lineage-committed cells. We are using x-ray crystallography and biochemical studies, together with genomics experiments with gene chip array technology and genetic complementation, to characterize the mode of action of these CHEMISTRY 2005 compounds and to study their effects on cellular processes. We are also developing modern genomics tools (cell-based phenotypic screens of arrayed genomic cDNA and small interfering RNA libraries) and proteomics tools (mass spectrometric phosphoprotein profiling) and are applying them to a variety of significant biomedical problems in cancer biology, neurodegenerative disease, aging, and virology. We have also developed a general biosynthetic method that can be used to site specifically incorporate unnatural amino acids into proteins in vitro and in vivo. Using this method, we have effectively expanded the genetic codes of bacteria and yeast by adding new components to the biosynthetic machinery of living cells. We have added amino acids with novel spectroscopic and chemical properties (e.g., keto- and heavy atom– containing amino acids, photocross-linking and photoisomerizable amino acids) to the genetic codes of Escherichia coli, yeast, and mammalian cells. Our results have removed a billion-year constraint imposed by the genetic code on the ability to chemically manipulate the structures of proteins. PUBLICATIONS Ding, S., Schultz, P.G. A role for chemistry in stem cell biology. Nat. Biotechnol. 22:833, 2004. Liu, J., Bang, A., Kintner, C., Orth, A.P., Chanda, S.K., Ding, S., Schultz, P.G. Identification of the Wnt signaling activator leucine-rich repeat in Flightless interaction protein 2 by a genome-wide functional analysis. Proc. Natl. Acad. Sci. U. S. A. 102:1927, 2005. Wang, L., Schultz, P.G. Expanding the genetic code. Angew. Chem. Int. Ed. 44:34, 2004. Wu, X., Walker, J., Zhang, J., Ding, S., Schultz, P.G. Purmorphamine induces osteogenesis by activation of the hedgehog signaling pathway. Chem. Biol. 11:1229, 2004. Xie, J., Wang, L., Wu, N., Brock, A., Spraggon, G., Schultz, P.G. The site-specific incorporation of p-iodo-L-phenylalanine into proteins for structure determination. Nat. Biotechnol. 22:1297, 2004. Catalysis and Click Chemistry K.B. Sharpless, V.V. Fokin, B. Boren, M. Cassidy, B. Colasson, T. Chan, A. Feldman, R. Fraser, T.V. Hansen, B. Hatano, T. Hirose, A. Krasinski, Y. Liu, J. Loren, R. Manetsch, A. McPherson, S. Narayan, S. Pitram, L.K. Rasmussen, J. Raushel, S. Röper, W. Sharpless, S. Silverman, A. Sugawara, X. Wang, J. Wassenaar, M. Whiting, P. Wu T he aim of our research program is the development of reliable chemical transformations that allow rapid exploration of chemical space. Our Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. 87 goal is to find new molecules with desired functions, whether in medicinal chemistry, materials science, or organic synthesis. CLICK CHEMISTRY The success of any search for new molecules with desired properties often depends on the degree of diversity of the blocks that are used in synthesis: the greater the variety of structures and functional groups that can be used in the construction of candidate compounds, the more likely it is that useful function will be discovered. However, the number and the sophistication of methods that allow synthesis of truly diverse collections of compounds still leave much to be desired. The problems are often due to at least one, and usually a combination, of the following: limited scope; hard-toobtain starting materials; requirements for inert atmospheres, anhydrous solvents, and protecting groups; and difficult purifications. Because organic and medicinal chemistry primarily evolved from the desire to explore and learn from the chemistry of life, organic and medicinal chemists naturally favored reactions that are similar to the prototypical biosynthetic pathways. These pathways center on the transformations of the carbonyl group; Nature’s primary carbon starting material is carbon dioxide, so exquisite enzymatic pathways evolved to bring about these essentially thermoneutral transformations. Because we lack these stratagems, we rely on a more useful and available set of starting materials: a plethora of unsaturated hydrocarbons provided by the petrochemical industry. In the past several years, we sought to develop and use only the best connecting reactions for the synthesis of functional molecules. We coined the term click chemistry to describe the reactions that fulfill the most stringent criteria of usefulness and convenience. Most click reactions form carbon-heteroatom bonds, are tolerant of water, and are often accelerated when water is used as the sole medium (even if the reagents are not soluble in water). C O P P E R - C ATA LY Z E D C Y C L O A D D I T I O N S Among the best click processes are 1,3-dipolar cycloadditions, especially formation of 1,2,3-triazoles from organic azides and alkynes. These modular “fusion” reactions unite 2 unsaturated reactants and result in an enormous variety of useful heterocycles. The recently discovered copper-catalyzed cycloaddition of azides and terminal alkynes is, arguably, the most convenient and reliable way to irreversibly fuse a broad variety of 88 CHEMISTRY 2005 blocks by means of the 1,2,3-triazole connection, a link that is notably stable and inert to severe hydrolytic, reducing, and oxidizing conditions. Although both alkynes and azides are highly reactive, their chemoselectivity profiles are quite narrow, that is, “orthogonal” to an unusually broad range of reagents, solvents, and other functional groups. These features allow reliable and clean sequential transformations of broad scope without the need for any protecting groups. The uncatalyzed, thermal reaction of azides and alkynes is often slow, requires elevated temperatures, and results in mixtures of 2 regioisomeric products. Therefore, we were pleased to find that copper(I) catalyzes this process, accelerating it by a factor of up to 107 and resulting in regiospecific union of azides and terminal acetylenes to give only 1,4-disubstituted-[1,2,3]triazoles. The process is experimentally simple and has enormous scope (Fig. 1). F i g . 2 . Synthesis of the bis-triazole catalyzed by metallic copper. The reactants were dissolved in a 1:1 mixture of tert-butanol and water (left) and were stirred for 24 hours. The reaction was complete then, and the product triazoles were isolated in quantitative yield by filtration. F i g . 1 . Copper(I)-catalyzed synthesis of 1,4-disubstituted1,2,3-triazoles. Although a number of copper(I) sources can be used directly, we found that the catalyst is often better prepared in situ by reduction of copper(II) salts, which are less costly and often purer than copper(I) salts. As the reductant, ascorbic acid (vitamin C) or sodium ascorbate is excellent. Remarkably, even copper metal can be used as a source of the catalytic species, making the experimental procedure even simpler; pure triazoles can be obtained in almost quantitative yield after simply stirring the corresponding azide and alkyne components in water with a small amount of copper turnings (Fig. 2). In collaboration with M.G. Finn, Department of Chemistry, we developed a practical method based on this high-fidelity transformation to selectively label large protein structures without affecting their integrity. The procedure has been used by many researchers around the world for activity-based profiling of whole proteomes, selective labeling of bacterial cell walls, and preparation of various bioconjugates. We also discovered that the ligand tris-(benzyltriazolylmethyl) amine (Fig. 3) enables efficient coupling of azides and alkynes even Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. F i g . 3 . Tris-(benzyltriazolylmethyl) amine. in extremely dilute solutions contaminated with myriad other molecules. The ligand is readily synthesized by the very same copper-catalyzed transformation and represents a new class of 1,2,3-triazole–derived tripodal ligands. To date, it has been used in more than 50 laboratories worldwide. Together with a team of researchers from IBM, San Jose, California, and the University of California, Santa Barbara, led by C. Hawker, we devised a high-efficiency approach to synthesis of dendrimers that is based on the copper(I)-catalyzed synthesis of triazoles. The unique properties of these macromolecules, which are a direct consequence of their regular structure, have attracted much attention in recent years. However, their syntheses are often plagued by low yields and tedious purification. Our approach results in almost quantitative yields, and, in many instances, simple filtration or solvent extraction is the only method required for purification (Fig. 4). These features represent a significant CHEMISTRY 2005 89 PUBLICATIONS Chan, T.R., Hilgraf, R., Sharpless, K.B., Fokin, V.V. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 6:2853, 2004. Colasson, B., Feldman, A.K., Sharpless, K.B., Fokin, V.V. The allylic azide rearrangement: a dynamic [3,3] process. J. Am. Chem. Soc., in press. Converso, A., Saaidi, P.-L., Sharpless, K.B., Finn, M.G. Nucleophilic substitution by Grignard reagents on sulfur mustards. J. Org. Chem. 69:7336, 2004. Díaz, D.D., Punna, S., Holzer, P., McPherson, A.K., Sharpless, K.B., Fokin, V.V., Finn, M.G. Click chemistry in materials synthesis, I: adhesive polymers from coppercatalyzed azide-alkyne cycloaddition. J. Polym. Sci. A Polym. Chem. 42:4392, 2004. Feldman, A.K., Colasson, B., Fokin, V.V. One-pot synthesis of 1,4-disubstituted 1,2,3-triazoles from in situ generated azides. Org. Lett. 6:3897, 2004. Himo, F., Lovell, T., Hilgraf, R., Rostovtsev, V.V., Noodleman, L., Sharpless, K.B., Fokin, V.V. Copper(I)-catalyzed synthesis of azoles: DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 127:210, 2005. F i g . 4 . Synthesis of 1,2,3-triazole-based dendrimers. advancement in dendrimer chemistry and illustrate an evolving synergy between organic chemistry and functional materials. In collaboration with scientists at Scripps Research who are involved in finding novel inhibitors of the HIV type 1 protease, we used the copper-catalyzed cycloaddition to rapidly synthesize a library of potential inhibitors of this crucial enzyme. The absence of byproducts and almost quantitative yields allowed the direct screening of compounds in a microtiter plate–based assay. Several compounds with Ki values ranging from 5 to 50 nM, both against the native protease and its mutants, were identified (Fig. 5). Johnson, S.M., Petrassi, H.M., Palaninathan, S.K., Mohamedmohaideen, N.N., Purkey, H.E., Nichols, C., Chiang, K.P., Walkup, T., Sacchettini, J.C., Sharpless, K.B., Kelly, J.W. Bisaryloxime ethers as potent inhibitors of transthyretin amyloid fibril formation. J. Med. Chem. 48:1576, 2005. Krasinski, A., Radic, Z., Manetsch, R., Raushel, J., Taylor, P., Sharpless, K.B., Kolb, H.C. In situ selection of lead compounds by click chemistry: target-guided optimization of aceylcholinesterase inhibitors. J. Am. Chem. Soc. 127:6686, 2005. Lewis, W.G., Magallon, F., Fokin, V.V., Finn, M.G. Discovery and characterization of catalysts for azide-alkyne cycloaddition by fluorescence quenching. J. Am. Chem. Soc. 126:9152, 2004. Loren, J.C., Sharpless, K.B. The Banert cascade: a synthetic sequence to polyfunctional NH-1,2,3-triazoles. Synthesis, in press. Manetsch, R., Krasinski, A., Radic, Z., Raushel, J., Taylor, P., Sharpless, K.B., Kolb, H.C. In situ click chemistry: enzyme inhibitors made to their own specifications. J. Am. Chem. Soc. 126:12809, 2004. Mocharla, V.P., Colasson, B., Lee, L.V., Röper, S., Sharpless, K.B., Wong, C.-H., Kolb, H.C. In situ click chemistry: enzyme-generated inhibitors of carbonic anhydrase II. Angew. Chem. Int. Ed. 44:116, 2004. Narayan, S., Muldoon, J., Finn, M.G., Fokin, V.V., Kolb, H.C., Sharpless, K.B. “On water”: unique reactivity of organic compounds in aqueous suspensions. Angew. Chem. Int. Ed. 44:3275, 2005. Wu, P., Feldman, A.K., Nugent, A.K., Hawker, C.J., Scheel, A., Voit, B., Pyun, J., Fréchet, J.M.J., Sharpless, K.B., Fokin, V.V. Efficiency and fidelity in a click chemistry route to triazole dendrimers via the copper(I)-catalyzed ligation of azides and alkynes. Angew. Chem. Int. Ed. 43:3928, 2004. Chemistry, Biology, and Disease P. Wentworth, Jr., Y. Chen, L. Eltepu, R. Galvé, R.K. Grover, F i g . 5 . Synthesis of inhibitors of HIV type 1 protease and its J. Nieva, M. Puga, A. Shafton, B.D. Song, M.M.R. Peram, mutants. C. Takeuchi, S. Tripurenani, R. Troseth, K. Trygvasson, Studies of other applications, ranging from biology to materials science, are under way in our laboratories and in collaboration with M.G. Finn, P.K. Vogt, and C.-H. Wong, Department of Chemistry; J.H. Elder, Department of Molecular Biology; and others. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. H. Wang, A.D. Wentworth ur research is multidisciplinary and involves bioorganic, biophysical, physical organic, synthetic, and analytical chemistry coupled with biochemical techniques, cell-based assays, and animal models. These diverse approaches are combined to facilitate a better understanding of and generate new thera- O 90 CHEMISTRY 2005 peutic approaches to complex disease states. Ongoing projects include studies on atherosclerosis, neurodegeneration, ischemia-reperfusion injury, macular degeneration, cancer, inflammation, and infectious diseases. T H E A N T I B O D Y - C ATA LY Z E D WAT E R O X I D AT I O N PAT H WAY Antibodies are the classical adapter molecules of the immune system, linking recognition and killing of foreign pathogens. However, we recently discovered that all antibody molecules, regardless of source or antigenic specificity, can catalyze the reaction between singlet oxygen and water to give hydrogen peroxide. This reaction is being studied as a possibly new effector function of the immune system. Both the chemical and the biological aspects of this pathway are being explored intensively, and intriguing new insights into how it may play a role in immune defense and inflammatory damage are emerging. In collaboration with I.A. Wilson, Department of Molecular Biology, we studied, at atomic resolution, the structural modifications of an antibody Fab fragment that was used as the antibody in the antibodycatalyzed water oxidation pathway. X-ray analysis revealed surprisingly few but conserved oxidative modifications to certain residues within the Fab structure (Fig. 1). The most consistent modification is a regioselective hydroxylation of the amino acid tryptophan at position L163. Such a conserved modification suggests that the active site of the antibody-catalyzed water oxidation pathway may be close to position L163 and thus offers an area for site-directed mutagenesis to investigate this observation. One of the most controversial aspects of this pathway is our assertion that trioxygen species may be generated as intermediates and/or byproducts. To increase our experimental evidence for such intermediates, we are generating libraries of molecular probes that are being tested for both their specificity for ozone in a biological setting and for their usefulness in cell-based assays. C H E M I S T R Y O F T H E P O LY O X I D E D I H Y D R O G E N TRIOXIDE Chemical processes by which molecules containing polyoxides are generated are of considerable interest. Although the reaction between hydrogen peroxide and ozone is a time-honored chemical process, because of the complexity of the overall mechanism, intermediates formed when these 2 molecules react are still incompletely characterized. These intermediates recently became central to biological thinking because of our discovery that antibodies catalyze the oxidation of water by singlet oxygen, leading to the formation of hydrogen peroxide, and it has been proposed that dihydrogen trioxide may be formed by antibodies during the formation of hydrogen peroxide. In support of this hypothesis, we recently showed that dihydrogen trioxide is formed in measurable amounts during the thermal reaction between ozone and hydrogen peroxide (Fig. 2). Overlaid 1H nuclear magnetic resonance spectra of the peroxone reaction (A) and the peroxone reaction and authentic dihydrogen trioxide (B). Inset shows the structure of the triplet biradical intermediate theorized as being generated during the reaction between ozone and hydrogen peroxide. Fig. 2. F i g . 1 . Crystal structure of 4C6 Fab prepared after photoirradi- ation with ultraviolet light shows the Cα trace of the light (L, light gray) and heavy (H, dark gray) chains. Inset shows Fourier electron density map for tryptophan at position L163 in the 4C6 Fab. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. CHEMISTRY 2005 91 This first experimental report of a link between these 3 oxidants suggests that dihydrogen trioxide may be involved in oxidation reactions that span biological, atmospheric, and environmental systems. We are investigating new methods for the formation of dihydrogen trioxide and the chemical properties of this novel oxidant. inflammatory artery disease. Thus, the atheronal molecules may be a new thread in the already entangled relationship between cholesterol oxidation, macrophages, and atherosclerosis. CHOLESTEROL SECO-STEROLS AND The ultimate aim of the search for genetic and environmental factors that increase the propensity of a specific protein to misfold is the understanding and treatment of disease states as diverse as atherosclerosis, light-chain deposition disease, systemic amyloidosis, Alzheimer’s disease, and Parkinson’s disease. We recently discovered that the inflammation-derived atheronal-A and atheronal-B can trigger a deformation in the secondary structure of the normally folded protein apolipoprotein B-100 to a proamyloidogenic form. This protein is a component of low-density lipoprotein particles and is misfolded within atherosclerotic arteries, although the cause had never been known. Therefore, this misfolding of apolipoprotien B-100 induced by cholesterol seco-sterols is a new link between inflammation, cholesterol oxidation, protein misfolding, and atherosclerosis. Our goal is to expand this observation and explore its relevance in many disease states and to generate molecules that prevent it. In collaboration with J.W. Kelly, Department of Chemistry, we showed that these cholesterol seco-sterols also trigger the misfolding of amyloid β-peptide (1–40), leading to formation of fibrils similar to those observed in patients with Alzheimer’s disease. Interestingly, analysis of the structure-activity relationship revealed that among a panel of aldehydes, only atheronal-A, atheronal-B, and 4-hydroxynonenal triggered this misfolding of amyloid β-peptide, suggesting that both electrophilicity and hydrophobicity of the adducting aldehyde are critical structural aspects. AT H E R O S C L E R O S I S We recently discovered that the 5,6-seco-sterols atheronal-A and atheronal-B of a class of cholesterol ozonolysis products are present in human atherosclerotic plaques and plasma (Fig. 3). In addition, we found that the atheronals are present in murine models of atherosclerosis and have a range of biological properties that, in combination, would increase the local density of macrophages at sites of vascular inflammation. Atheronal-A and atheronal-B are both chemotactic for cultured macrophages. When in complex with low-density lipoproteins, atheronal-A induces upregulation of the cell-surface adhesion molecule E-selectin on vascular endothelial cells. F i g . 3 . Cholesterol seco-sterols atheronal-A and atheronal-B are present within the walls of inflamed arteries and in plasma. Bottom left, Immunohistochemistry of aortic valve atherosclerosis in an aged mouse that lacked the gene for apolipoprotein E. The primary antibody is a murine monoclonal antibody to atheronal-B, revealing high localization of atheronal-B in the artery wall. Bottom right, Lipid accumulation within primary macrophages is triggered by low-density lipoprotein in complex with atheronal-B. Taken together with our previously shown effects of atheronals on the formation of foam cells and the cytotoxic effects of macrophages, these data indicate that the atheronals have biological effects that would lead to the recruitment, entrapment, dysfunction, and ultimate destruction of the major leukocyte player in Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. I N F L A M M AT O R Y A L D E H Y D E - M E D I AT E D P R O T E I N MISFOLDING PUBLICATIONS Ahn, J.-M., Wentworth, P., Jr., Janda, K.D. Probing lipase/esterase libraries for lipid A hydrolases: discovery of biocatalysts for the detoxification of bacteriallyexpressed recombinant protein. Chem. Commun. (Camb.) 364, 2004, Issue 4. Chen, Y.P., Eltepu, L., Wentworth, P., Jr. Diastereo- and enantio-selective crotylation of α-ketoesters using crotyl boronic acid ester complexes. Tetrahedron Lett. 45:8285, 2004. Granville, D.J., Tashakkor, B., Takeuchi, C., Gustafsson, Å.B., Huang, C., Sayen, M.R., Wentworth, P., Jr., Yaeger, M., Gottlieb, R.A. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc. Natl. Acad. Sci. U. S. A. 101:1321, 2004. Nieva, J., Wentworth, P., Jr. The antibody-catalyzed water oxidation pathway: a new chemical arm to immune defense? Trends Biochem. Sci. 29:274, 2004. Nyffeler, P., Boyle, N.A., Eltepu, L., Wong, C.-H., Eschenmoser, A., Lerner, R.A., Wentworth, P., Jr. Dihydrogen trioxide (HOOOH) is generated during the thermal reaction between hydrogen peroxide and ozone. Angew. Chem. Int. Ed. 43:4656, 2004. 92 CHEMISTRY 2005 Takeuchi, C.T., Wentworth, P., Jr. The antibody-catalyzed water oxidation pathway. In: Catalytic Antibodies. Keinan, E. (Ed.). Wiley & Sons, New York, 2004, p. 336. Toker, J.D., Tremblay, M., Wentworth, P., Jr., Janda, K.D. Investigating the scope of the 29G12 antibody-catalyzed 1,3-dipolar cycloaddition reaction. J. Org. Chem., in press. outside the active site. We are investigating how the remote changes affect the enantioselectivity in the active site. DEVELOPMENT OF INHIBITORS OF ENZYMES AND Wentworth, A.D., Wentworth, P., Jr., Blackburn, G.M. Transition state analogs archetype antigens for catalytic antibody generation. In: Catalytic Antibodies. Keinan, E. (Ed.). Wiley & Sons, New York, 2004, p. 454. Zhang, Q., Powers, E.T., Nieva, J., Huff, M.E., Dendle, M.A., Bieschke, J., Glabe, C.G., Eschenmoser, A., Wentworth, P., Jr., Lerner, R.A., Kelly, J.W. Metabolite-initiated protein misfolding may trigger Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 101:4752, 2004. Zhu, X., Wentworth, P., Jr., Wentworth, A.D., Lerner, R.A., Wilson, I.A. Probing the antibody-catalyzed water-oxidation pathway at atomic resolution. Proc. Natl. Acad. Sci. U. S. A. 101:2247, 2004. Bioorganic and Synthetic Chemistry C.-H. Wong, C. Behrens, M. Best, A. Brik, M. Fujio, S. Hanson, Z.-Y. Hong, J. Hsu, C.-Y. Huang, D.-R. Hwang, A. Krebs, J.-C. Lee, F.-S. Liang, H. Liu, L. Liu, M. Numa, T. Polat, M. Sawa, P. Schanen, M. Sugiyama, D. Thayer, S.-K. Wang, L. Whalen, C.-Y. Wu, D. Wu, M. Wuchrer, Y.-Y. Yang ur research programs involve development of new chemical and enzymatic strategies and methods for the synthesis of biologically active compounds. We use the synthesized materials as molecular probes to explore carbohydrate-mediated biological recognitions, sequence-specific RNA recognition, and enzymatic reactions. O ORGANIC AND BIOORGANIC SYNTHESIS Our work in organic and bioorganic synthesis includes the development of new chemical reactions and the exploitation of native and engineered enzymes for organic synthesis. In the past year, we developed several new synthetic methods. These include development of covalent glycoarrays for high-throughput analysis of protein-carbohydrate interactions, the use of aldolases in synthesis of glycosyltransfer enzyme inhibitors, and enzymatic synthesis of glycoproteins. Using directed evolution, we developed new aldolase variants capable of making both enantiomers of sugars. In collaboration with P.G. Schultz, Department of Chemistry, we evolved a tyrocyl-tRNA synthase to accept N-acetylgalactosamine α-linked to the side chain of threonine for incorporation into proteins in vivo in Escherichia coli. All the mutations found in the new aldolases and the aminoacyl-tRNA synthase occur Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. RECEPTORS Our goals in the area of enzyme and receptor inhibitors are to develop new strategies and discover potential new therapeutic agents with high selectivity. Current strategies involve the design and synthesis of structureand mechanism-based inhibitors of enzymes associated with diseases. Targets for investigation include bacterial transglycosidase, sulfotransferases, retroviral proteases, the lethal factor of Bacillus anthracis, and the enzymes involved in the biosynthesis of carbohydrates essential for biological functions. We developed new iminocyclitols and derivatives as inhibitors of glycosidases and glycosyltransferases for potential treatment of inflammatory diseases. In addition, we used a new strategy based on a rapid microscale synthesis coupled with in situ high-throughput screening to develop new tight-binding inhibitors of anthrax lethal factor, a sulfotransferase, and drug-resistant HIV proteases. We also developed new reactions based on tetrabutylammonium fluoride–mediated N- and O-alkylation in aqueous solution and used the reactions to identify potent enzyme inhibitors. Finally, we designed and synthesized novel aminoglycoside mimetics that target unique bacterial and oncogenic RNA sequences as potential new antibiotics and anticancer agents. C A R B O H Y D R AT E C H E M I S T R Y A N D M O L E C U L A R G LY C O B I O L O G Y We continue to improve the programmable 1-pot oligosaccharide synthesis method for convenient and rapid preparation of oligosaccharides. So far, we have designed approximately 600 building blocks and measured the anomeric reactivity of each building block. Using the computer program OptiMer, developed in our laboratory, we rapidly assembled a number of oligosaccharides. We are using this method to define the specificity of interactions between carbohydrates and their receptors, with particular focus on optimization of the cancer antigen Globo H and gp120 oligomannose as vaccine candidates and development of aminoglycosides to target specific RNA sequences. In collaboration with D.R. Burton, Department of Immunology, and I.A. Wilson, Department of Molecular Biology, we are evaluating a designed oligomannoseprotein conjugate as an antigen to elicit antibodies for neutralizing HIV gp120 and variants. We prepared CHEMISTRY 2005 some bacterial glycolipids and analogs and found that they are active ligands for CD cell markers involved in activation of human natural killer T cells. We also prepared several heparin derivatives and glycoproteins for investigation of their structures and function. In collaboration with J.C. Paulson, Department of Molecular Biology, we developed new methods for microfabrication of saccharides on microtiter plates and glass slides for use in the high-throughput analysis of sugar-protein interactions. We also developed new methods for the discovery of enzyme inhibitors. PUBLICATIONS Blixt, O., Head, S., Mondala, T., Scanlan, C., Huflejt, M.E., Alvarez, R., Bryan, M.C., Fazio, F., Calarese, D., Stevens, J., Razi, N., Stevens, D.J., Shekel, J.J., van Die, I., Burton, D.R., Wilson, I.A., Cummings, R., Bovin, N., Wong, C.-H., Paulson, J.C. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. U. S. A. 101:17033, 2004. Brik, A., Alexandratos, J., Lin, Y.-C., Elder, J.H., Olson, A.J., Wlodawer, A., Goodsell, D.S., Wong, C.-H. 1,2,3-Triazole as a peptide surrogate in the rapid synthesis of HIV-1 protease inhibitors. Chembiochem 6:1167, 2005. 93 Hsu, C.-C., Hong, Z., Wada, M., Franke, D., Wong, C.-H. Directed evolution of D-sialic acid aldolase to L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase. Proc. Natl. Acad. Sci. U. S. A. 102:9122, 2005. Hsu, H.-Y., Hua, K.-F., Lin, C.-C., Lin, C.-H., Wong, C.-H. Extract of Reishi polysaccharides induces cytokine expression on TLR4-modulated protein kinase signaling pathways. J. Immunol. 173:5989, 2004. Kinjo, Y., Wu, D., Kim, G., Xing, G.-W., Poles, M.A., Ho, D.D., Tsuji, M., Kawahara, K., Wong, C.-H., Kronenberg, M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434:520, 2005. Klostermeier, D., Sears, P., Wong, C.-H., Millar, D.P., Williamson, J.R. A threefluorophore FRET assay for high-throughput screening of small-molecule inhibitors of ribosome assembly. Nucleic Acids Res. 32:2707, 2004. Liang, F.-S., Wang, S.-K., Nakatani, T., Wong, C.-H. Targeting RNAs with tobramycin analogues. Angew. Chem. Int. Ed. 43:6496, 2004. Liang, F.-S., Wong C.-H. Surface plasmon resonance study of HCV IRES RNAaminoglycoside interactions. Methods Mol. Biol., in press. Lin, H., Thayer, D.A., Wong, C.-H., Walsh, C.T. Macrolactamization of glycosylated peptide thioesters by the thioesterase domain of tyrocidine synthetase. Chem. Biol. 11:1635, 2004. Liu, J., Numa, M.M.D., Liu, H., Huang, S.-J., Sears, P., Shikhman, A.R., Wong, C.-H. Novel synthesis and high-throughput screening of N-acetyl-β-hexosaminidase inhibitor libraries targeting osteoarthritis. J. Org. Chem. 69:6273, 2004. Brik, A., Wu, C.-Y., Best, M.D., Wong, C.-H. Tetrabutylammonium fluorideassisted rapid N9-alkylation on purine ring: application to combinatorial reactions in microtiter plates for the discovery of potent sulfotransferase inhibitors in situ. Bioorg. Med. Chem. 13:4622, 2005. Mocharla, V.P., Colasson, B., Lee, L.V., Romper, S., Sharpless, K.B., Wong, C.-H., Kolb, H.C. In situ click chemistry: enzyme-generated inhibitors of carbonic anhydrase II. Angew. Chem. Int. Ed. 44:116, 2004. Bryan, M.C., Fazio, F., Lee, H.-K., Huang, C.-Y., Chang, A., Best, M.D., Calarese, D.A., Blixt, O., Paulson, J.C., Burton, D., Wilson, I.A., Wong, C.-H. Covalent display of oligosaccharide arrays in microtiter plates. J. Am. Chem. Soc. 126:8640, 2004. Numa, M.M.D., Lee, L.V., Hsu, C.-C., Bower, K.E., Wong, C.-H. Identification of novel anthrax lethal factor inhibitors generated by combinatorial Pictet-Spengler reaction followed by screening in situ. Chembiochem 6:1002, 2005. Bryan, M.C., Lee, L.V., Wong, C.-H. High-throughput identification of fucosyltransferase inhibitors using carbohydrate microarrays. Bioorg. Med. Chem. Lett. 14:3185, 2004. Nyffeler, P.T., Duron, S.G., Burkart, M.D., Vincent, S.P., Wong, C.-H. Selectflour: mechanistic insight and applications. Angew. Chem. Int. Ed. 44:192, 2005. Chang, C.-F., Ho, C.-W., Wu, C.-Y., Chao, T.A., Wong, C.-H., Lin, C.-H. Discovery of picomolar slow tight-binding inhibitors of α-fucosidase [published correction appears in Chem. Biol. 11:1595, 2004]. Chem. Biol. 11:1301, 2004. Nyffeler, P.T., Eltepu, L., Boyle, N.A., Wong, C.-H., Eschenmoser, A., Lerner, R.A., Wentworth, P., Jr. Dihydrogen trioxide (HOOOH) is generated during the thermochemical reaction between hydrogen peroxide and ozone. Angew. Chem. Int. Ed. 43:4656, 2004. Chen, H.-S., Tsai, Y.-F., Lin, S., Lin, C.-C., Khoo, K.-H., Lin, C.-H., Wong, C.-H. Studies on the immuno-modulating and anti-tumor activities of Ganoderma lucidum (Reishi) polysaccharides. Bioorg. Med. Chem. 12:5595, 2004. Chen, J.H., Chang, Y.-W., Yao, C.-W., Chiueh, T.-S., Huang, S.-C., Chien, K.-Y., Chen, A., Chang, F.-Y., Wong, C.-H., Chen, Y.-J. Plasma proteome of severe acute respiratory syndrome analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 101:17039, 2004. Chien, C.M., Cheng, J.-L., Chang, W.-T., Tien, M.-H., Tsao, C.M., Chang, Y.-H., Chang, H.-Y., Hsieh, J.-F., Wong, C.-H., Chen, S.-T. Polysaccharides of Ganoderma lucidum alter cell immunophenotypic expression and enhance CD56+ NK-cell cytotoxicity in cord blood. Bioorg. Med. Chem. 12:5603, 2004. Fan, G.-T., Pan. Y.-S., Lu, K.-C., Cheng, Y.-P., Lin, W.-C., Lin, S., Lin, C.-H., Wong, C.-H., Fang, J.-M., Lin, C.-C. Synthesis of α-galactosyl ceramide and the related glycolipids for evaluation of their activities on mouse splenocytes. Tetrahedron 61:1855, 2005. Franke, D., Hsu, C.-C., Wong, C.-H. Directed evolution of aldolases. Methods Enzymol. 388:224, 2004. Fridman, M., Belakhov, V., Lee, L.V., Liang, F.-S., Wong, C.-H., Baasov, T. Dual effect of synthetic aminoglycosides: antibacterial activity against Bacillus anthracis and inhibition of anthrax lethal factor. Angew. Chem. Int. Ed. 44:447, 2005. Hanson, S.R., Best, M., Bryan, M.C., Wong, C.-H. Chemoenzymatic synthesis of oligosaccharides and glycoproteins. Trends Biochem. Sci. 29:656, 2004. Hanson, S.R., Best, M.D., Wong, C.-H. Sulfatases: structure, mechanism, biological activity, inhibition, and synthetic utility. Angew. Chem. Int. Ed. 43:5736, 2004. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved. Shie, J.-J., Fang, J.-M., Kuo, C.-J., Kuo, T.H., Liang, P.-H., Huang, H.-J., Yang, W.-B., Lin, C.-H., Chen, J.-L., Wu, Y.T., Wong, C.-H. Discovery of potent anilide inhibitors against the severe acute respiratory syndrome 3CL protease. J. Med. Chem. 48:4469, 2005. Thayer, D.A., Yu, H.N., Galan, M.C., Wong, C.-H. A general strategy toward S-linked glycopeptides. Angew. Chem. Int. Ed. 44:4596, 2005. Tolbert, T.J., Franke, D., Wong, C.-H. A new strategy for glycoprotein synthesis: ligation of synthetic glycopeptides with truncated proteins expressed in E. coli as TEV protease cleavable fusion protein. Bioorg. Med. Chem. 13:909, 2005. Tolbert, T.J., Wong, C.-H. Carbohydrate chains: enzymatic and chemical synthesis. In: Encyclopedia of Biological Chemistry. Lennarz, W.J., Lane, M.D. (Eds.). Academic Press, San Diego, 2004, Vol. 1, p. 307. Tolbert, T.J., Wong, C.-H. Conjugation of glycopeptide thioesters to expressed protein fragments: semisynthesis of glycosylated interleukin-2. Methods Mol. Biol. 283:255, 2004. Tolbert, T.J., Wong, C.-H. Subtilisin-catalyzed glycopetide condensation. Methods Mol. Biol. 283:267, 2004. Wong, C.-H. Protein glycosylation: new challenges and opportunities. J. Org. Chem. 70:4219, 2005. Wu, C.-Y., Jan, J.-T., Ma, S.-H., Kuo, C.-J., Juan, H.-F., Cheng, Y.-S.E., Hsu, H.-H., Huang, H.-C., Wu, D., Brik, A., Liang, F.-S., Liu, R.-S., Fang, J.-M., Chen, S.-T., Liang, P.-H., Wong, C.-H. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. U. S. A. 101:10012, 2004. 94 CHEMISTRY 2005 Wu, D., Xing, G.-W., Poles, M.A., Horowitz, A., Kinjo, Y., Sullivan, B., BodmerNarkevitch, V., Plettenburg, O., Kronenberg, M., Tsuji, M., Ho, D.D., Wong, C.-H. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc. Natl. Acad. Sci. U. S. A. 102:1351, 2005. Xing, G.-W., Wu, D., Poles, M.A., Horowitz, A., Tsuji, M., Ho, D.D., Wong, C.-H. Synthesis and human NKT cell stimulating properties of 3-O-sulfo-α/β-galactosylceramides. Bioorg. Med. Chem. 13:2907, 2005. Xu, R., Hanson, S.R., Zhang, Z., Yang, Y.-Y., Schultz, P.G., Wong, C.-H. Site-specific incorporation of the mucin-type N-acetylgalactosamine-α-O-threonine into protein in Escherichia coli. J. Am. Chem. Soc. 126:15654, 2004. Published by TSRI Press®. © Copyright 2005, The Scripps Research Institute. All rights reserved.