Document 12787260
advertisement

/3/.8 ( i
REGULATION OF BUD-BURST TIMING BY TEMPERATURE AND PHOTOREGIMEs;_,_.
DURING DORMANCY
1/
Robert K. Campbell­
. ,r' Hlttra.fiot.tf»l
Abstract,--In Douglas-fir, vegetative buds throughout most
1
or all of the " dormant" period have the potential ability for ex-·
tension growth in response to temperatures above freezing. In
terms of actual growth or development, this ability can be meas­
ured as a rate for each temperature within the normal range.
Thus, potential ability can be conceptualized as a temperature
response curve. Data from several experiments suggest that po­
tential abilit gan be described by an equation of the form
+ x
100/ y
K/ (l+e
); where, 100/ y is developmental rate and
is
temperature. Other parameters in the equation change continu­
ously during dormancy in response to complex, interacting environ­
mental cues provided by chilling temperature, duration, and time
and by photoperiod. Bud-burst timing reflects the growth that
accrues during winter and spring in response to temperature pat­
tern and changing potential developmental rates. The model may
be appropriate, in principle, for other perennial species.
. "{:Ill"-
=
Additional keywords: Douglas-fir, Pseudotsuga menziesii, phenology, chilling, developmental-cycle, growth rhythm. INTRODUCTION
Many temperate region forests include sites with growing seasons that are
severely restricted by cold or moisture deficit or both. Such sites are often
difficult to regenerate naturally or artificially--synchronization of the
plant developmental cycle to the seasonal cycle appears to be a critical fac­
tor (Eiche 1966), Problems arising from poor synchronization usually can be
minimized by the close matching of seed source to plantation site, a compel­
ling reason for the development of seed-transfer rules,
As one basis for rules, I have been investigating the annual development­
al cycle of Douglas-fir (Pseudotsuga menziesii vat. menziesii [Mirb.] Franco),
with the help of A. I. Sugano. We are concentrating on the system by which
Douglas-fir uses temperature and photoperiod as environmental cues for optim­
izing timing of the developmental cycle. This paper presents my concept of
the system for bud-burst timing, up-dated from a previous report (Campbell and
Sugano 1975), but still provisional and incomplete.
Supporting examples are drawn from several experiments, mainly designed
to study the genecology of reaction to environmental cues. Results have been
or are being reported elsewhere. In this paper, documentation of experimental
and analytical procedures is consequently minimal.
!
/
Principal Plant Geneticist, Forestry Sciences Laboratory, Pacific Northwest
Forest and Range Experiment Station, USDA Forest Service, Corvallis, Oreg.
20
Spring timing of bud-burst is an important component of adaptation for
many species in north temperate climates, and especially in western Oregon,
particularly in valleys or at high elevations. Plants in valleys are exposed
regularly to severe summer droughts. At high elevations frost-free periods
are short. In either case, by appropriate timing of bud burst, the risks of
spring frost are optimally balanced with the advantages of an early display of
the new needles--in the valleys, to make best use of spring soil moisture; at
high elevations, to maximize the use of warm days for photosynthesis and
growth.
THE SETTING
The bud-burst timing mechanism works remarkably well. Spring frost is
seldom a problem in more than a small fraction of native populations, in spite
of the highly heterogeneous habitat occupied by Coastal Douglas-fir. In al­
pine areas, the frost-free season may be less than 90 days. In southern Ore­
gon valleys, the total growing-season moisture deficit may reach 45 em and may
start to accumulate as early as May 1. Such sites can be found within a few
kilometers horizontally, or within 1 500 m vertically, of sites with frost­
free seasons of 200 days, or total sumner moisture deficits of only 5-10 em.
The year-to-year variation at a site can be as significant as the varia­
tion between sites. For example, in the Pacific Northwest, the standard
deviation of the last spring frost of -2.2 C (25 F) ranges from 15 days at
high elevations to 27 days in coastal valleys. In a sample of dates of last
spring frosts (-2.2 C) obtained from 36 weather stations in western Washing­
ton and Oregon, year-to-year variation accounted for 43% of total variation.
°
Weather stations spanned coastal and alpine regions of 6 of latitude.
The system for bud-burst timing in Douglas-fir apparently can preadapt
populations to much of the region's heterogeneity. When population samples
from different parts of the Pacific Northwest are grown together at Corvallis,
Oregon, in any given year they will usually burst buds within 2 or 3 weeks of
one another. Usually only the earliest individuals of the earliest samples
will be injured by frost.
Apparently the system is based mainly on a common response to spring
temperatures. Except in years with highly unusual temperature patterns,
photoperiod appears to have a comparatively minor role. For example, some of
the population-samples in the illustration above would have burst buds up to
half a month earlier in their native habitats than in Corvallis and some as
much as 2 months later. Thus, photoperiods at bud-burst in native habitats
would have been from 1/ 2 hour shorter to 2-1/ 2 hours longer than at bud-burst
in Corvallis. Also, for any one of the samples, bud-burst dates in succeeding
years at Corvallis may have been separated by a month; i.e., by 1-1/4 hours in
photoperiod, Therefore, any effect of photoperiod is probably in the nature
of a modification of the basic response.
That such modifications exist is indicated by an interaction of bud-burst
timing with year of observation in nursery tests. The year-to-year differences
among population-samples depend on the population involved. Since modifica­
tions are population-specific, they probably serve to adapt local populntions
to deviations from the average temperature pattern.
21
The modifications are likely to be fairly complex. Populations are adapt­
ed to greatly different temperature distributions within the region. For
example, if temperatures are weighted according to their probability of occur­
rence, near the Pacific Ocean more than 8 0% of temperatures in the period
November 15 to April 15 will fall above 5 C (roughly approximated from proba­
bilities of maximum and minimum temperatures based on 30 years of record; from
Wakefield 1969). For Corvallis, the estimate for similar conditions is 60%;
in the high Cascades, 12%. Temperatures near the Pacific Coast seldom drop
into the severe frost range except in January. Also, they seldom exceed 24 C.
In the high mountains, temperatures in December and January rarely exceed 7 C,
but in summer months they may reach 35 C and can fall to near 0 C almost any
night.
THE SYSTEM
My idea of the system is based on two postulates: 1) buds during dor­
mancy will respond to any temperatures above freezing with continued morpho­
logical development, extension growth, or change in physiological activity
(Romberger 1963, p. 59-61; Perry 1971, p. 32). 2) Environmental cues received
by the bud during dormancy act to change the bud' s response to temperature.
Dormancy in this paper is defined in the general sense, i.e., the temporary
suspension of visible growth; in conifers, the superficially inactive, bud­
scale-covered phase between bud-set and bud-burst.
At any time during dormancy, a bud has a potential ability to respond to
temperatures. In terms of actual development toward bud-bursting, this ability
is expressed as a rate for each temperature within the range of temperatures
normal for the species. Therefore, potential ability at any point in time can
be conceptualized as a temperature response curve; quantitatively it is de­
scribed by a response equation. Each change in potential ability means a
change in potential bud-developmental rates throughout the temperature range-­
hence a change in the temperature response curve. Parameters in the equation
describing the curve change continuously as the season progresses from bud-set
to bud-burst, in response to cues provided by chilling and photoperiod.
Temperature, therefore, influences bud-development in two, partly inde­
pendent, quantitative ways:
1) In the range -2 C to + 12 C, approximately (from Wommack 1964, Sarvas
1974, Campbell and Sugano 1975), temperature acts mainly as information. It
induces incremental changes in potential developmental rates (a)--letters in
parenthesis here and in the following discussion refer to schematic paths in
figure 1.
22
..---.v__,...,
)9
L--
CELL
PHYSIOLOGIC AL
S T ATE
POTENTIAL
DEVELOPMENTAL
RATE
DEVELOPMENT
AND
GROWT H
-----
Figure 1.--Model of the system by which temperature and photoperiod during
dormancy are used to regulate bud extension-growth and bud-burst timing.
2) Temperatures greater than approximately 12 C act mainly to release
energy for growth (c). Temperatures below 12 C also can release energy for
growth, very slowly when potential developmental rates are low, and more
rapidly after high potential rates have been induced by prolonged chilling.
Cool temperatures change the cell physiological state (b) by processes
(collectively termed as "induction ") still imperfectly k nown (Romberger 1963,
Smith and Kefford 1964) and outside the scope of this paper. Photoperiod
during the flushing period also influences the cell physiological state (d).
Photo-effects are also manifested as a change in parameters of the temperature
equation. The degree of change depends on the physiological state already in­
duced by chilling. Thus, photoperiod effects are considered here as a modif­
ication, secondary to induction by chilling, but still influencing potential
developmental rates (e).
Some factors which condition the influence of chilling on potential de­
velopmental rates are:
1) The temperature of chilling, 2) the timing of chilling, i. e., whether
chilling occurs early or late in dormancy--this may reflect the state of bud
development reached before chilling occurs (i) as well as the passage of time
between bud-set and chilling (Dormling, et al. 1968 , Sugano 1971), and 3) the
physiological state already induced by previous chilling (h and j).
The photoperiod immediately preceding or during chilling may also in­
fluence induction (k) (Lavender and Wareing 1972).
MATERIALS AND METHODS
Several inter-relations within the system are described quantitatively
in the next section to illustrate major features of the system and also to
suggest its complexity. Relationships, which are presented graphically, are
based on data primarily from three experiments. As noted earlier, these ex­
periments are being reported in detail elsewhere.
As genetic entries, the three experiments each used samples from the same
°
°
23 populations of Douglas-fir, Origins ranged from 42 to 49 N latitude, from
23
6 to 1 460 m, and from Pacific Coast to mountain passes on the Cascades crest,
all in western Washington and Oregon,
Germinated seed from five open-pollinated, individual tree collections at
each location were planted in pots in March, grown in a greenhouse until May,
then transferred to a lathhouse (SO% shade). On or about Oct 1, pots were put
in growth chambers held at a constant 16 C and 9-h daylength until scheduled
chilling treatments began.
Seedlings were subjected to various chilling and flushing treatments in
growth chambers, starting in November or December. Depending on the experi­
mental objectives, chamber treatments were factorial combinations of chilling
duration and flushing temperatures, or chilling duration and photoregime during
flushing, or flushing temperature and photoregime. A caution is necessary.
Photoregimes are designated in illustrations by a daylength, e g, 8 h. This
represents the initial photoperiod of a regime in which photoperiod was in­
creased periodically and regularly. The rate varied from experiment to
experiment, from 15 to 60 min/wk.
.•
Treatment results were assessed by their effect on length of the flushing
period, measured in days to bud-burst from the time a plant entered the flush­
ing treatment. To m1n1m1ze problems associated with correlated inhibition,
only terminal buds were scored.
Data were analyzed by multiple regression. Then the equation resulting
for each experiment was solved for a factorial set of selected values for
chilling period and flushing temperature (e.g., chilling 11, 44, 77 days;
flushing temperature, 100 14, 18 , 22 C) and for provenance topographic vari­
°
°
ables (43 , 45.5 and 48 N latitude; 100-, 600-, 1 100-m elevation; 60, 128,
180 km from the ocean). Solutions of equations provided several hundred ex­
pected values (e.g. , 3 chilling periods x 4 flushing temperatures x 3 latitudes
x 3 elevations x 3 distances = 324 values). Values for each chilling- and
flushing-period combination were then averaged over all provenance points in
the system (e. g. , 3 latitudes x 3 elevations x 3 distances) to provide average
effects for chilling, flushing, and photoperiod treatments. Most of the rela­
tionships illustrated in this paper are based on calculations using these
average effects. Thus, they describe an "average " response of coastal Douglas­
fir. Responses of individual population-samples deviated significantly from
this average response.
QUANTITATIVE RELATIONSHIPS
With some restrictions that are discussed below, it is proposed that the
energy-release or growth effects of temperature on rates of development toward
bud-burst (path c, figure 1) can be described by an empirical logistic equa­
tion of the form:
a+bx
);
100/ y = K/ (1 + e
where, 100/y is the developmental rate and represents the average per­
centage d evelopment made by the buds per day at a given temperature x,
! represents the distance in units of developmental rate between the upper
and lower asymptotes of the curve,
represents the relative position of the curve's origin on the abscissa,
quantifies the degree of increasing developmental rate with increasing
temperature ' and
24
e is the base of the natural logarithm.
Davidson (1944) has used this equation-form to express the relationship
between temperature and speed of development in insects. Sarvas' data (1972, table 5) on pollen-catkin developmental rates in Betula fit the curve form quite precisely (figure 2). 7
;;:....
.......
-
r--r�����-r�
6
100/ -
Y
0
0
-
Betula pubescens
-
7 40 .
1 + e3.!59-o.
2ox
R2 = 0.996
5
w
a::
4
_J
3
z
w
::::E a..
0
_J 2
w
>
w
0
100/y:
4.42
389-0 19X
l+e'
·
R2= 0.999
0
8
12
16
20
24
28
TEMPERATURE (C)
Figure 2.--Pollen-catkin developmental rates fitted to temperature response
equations (rates derived from data by Sarvas 1972).
I suggest that, over the normal range of autumn, winter, and spring
temperatures, the same equation type describes the growth effects of temper­
ature on bud-development in Douglas-fir. We have not tested this relationship
by sampling temperatures as intensively as did Sarvas for lack of facilities;
but at temperatures above 12 C, it satisfactorily describes our data. Below
12 C, we have not yet experimentally separated chilling-induction effects from
temperature growth-effects--below 12 C the actual response to temperature has
been greater than would be expected from the logistic relationship. Partly
for this reason, and partly because nonlinear equations are excessively expen­
sive to analyze in complicated designs, we have used a simpler type of
equation in published reports. Nevertheless, the logistic equation is probably
the correct form; it is used for illustrations in this paper.
25
Chilling has a general effect on the ability of buds to respond to flush­
ing temperature; the longer the chilling, the steeper the temperature response
curve.
Consequently, parameters in the equations describing response to temp­
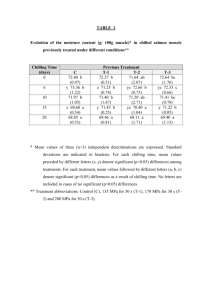
erature are affected by chilling duration (table 1). Induced differences in
developmental rate due to added chilling are more evident at higher tempera­
tures (figure 3).
Table 1.--Coefficients of equations used to prepare curves in figures 3 and 6
a/
Parameters-
Curve
K
a
b
11
44
77
3.78
5.93
6.97
4.11
4.31
4.36
-0.251
-0.245
-0.245
9
11
13
4.76
5.19
5.61
3.17
3.22
3.30
-0.247
-0.257
-0.272
Figure 3
Chilling
Figure 6
Photoregime
a/
K
a
b
distance in developmental units between upper and lower asymptotes. relative position of curve origin on abscissa. degree of change in slope. 240
6
5
>.
'
0
0
4=-
>.
IU)
0::
:::>
CD
0
:::>
CD
II
DAYS
OF
CHILLING
0
IU)
0
0
3
2
w
I_j
::::!
0...
0
_j
w
>
w
0
12
TEMPERATURE
Figure 3.--The influence of chilling duration (4 C) on potential develop­
mental rates and on days to bud-burst in Douglas-fir (derived from data by
Campbell and Sugano, In press).
26
It is, however, timing of bud-burst, rather than developmental rate,
that is the critical factor in natural selection. In this respect, chilling
duration has its most important influence on bud-development at lower flushing
temperatures. For example, consider Douglas-fir seedlings that have been
chilled for 11 days at 4 C. In a constant temperature of 10 C, they are ex­
pected to flush in 156 days (figure 3). If chilled for 12 days rather than 11,
the expected number of days to bud-burst is decreased by 1.38 days (figure 4).
If chilled 11 days and flushed at 20 C, buds are expected to burst in 37 days
(figure 3). And, in this case, an added day of chilling hastens bud-burst by
only 0.52 days (figure 4). Even after 77 days of chilling, the expected date
of flushing is altered more drastically by additional chilling if buds are
flushed in cool temperatures (figure 4). Over short periods, the development
of relatively unchilled buds in low temperatures is almost imperceptible.
From 100 to 28 0 days are required for flushing in temperatures of 10 C and
8 C, respectively, even if buds are flushed in long, 14-h daylengths (figure
3).
FLUSHING
TEMPERATURE
0
CUMULATIVE
DAYS OF CHILLING TO WHICH
.
THE ADDITIONAL DAY IS ADDED
Figure 4.--Decrease in days to bud-burst due to one added day of chilling
(4 C) as influenced by length of previous chilling and by flushing temper­
ature.
The change in the temperature response curve induced by chilling is influ­
enced by chilling te peratures as well as by duration. Sarvas (1974) proposed
that effects of different temperatures could be described quantitatively by an
"optimal" curve, with a high, median peak and steeply sloping sides. For
Betula, the optimum is about 3.5 C. Above and below 3.5 C, effectiveness falls
off sharply so that temperatures of -3 C and +10 C are only about one-tenth as
effective as the optimum.
Our experience indicates that the actual relationship is far more complex
than is implied by the optimal curve. Sarvas' curve form may, however, be ade­
quate for some tissues or under some conditions. For example, it may describe
the chilling-temperature differences induced by initial stages of chilling.
In Douglas-fir, when a high-elevation provenance was chilled for 20 days start­
ing in October (Campbell and Sugano 1975), potential developmental rates were
27
increased more by a median temperature (4.4 C) than by higher temperatures
(7.4 C and 10.0 C). The optimal curve may also be appropriate in circum­
stances where induced rates reflect a summation of the chilling effects of
several weeks (Wommack 1964). A less consistent picture emerges when the
total influence of chilling is broken into its incremental daily contribu­
tions. In our experiment after the initial 20 days of chilling, 10 C was
more effective per added day of chilling than was 4.4 c--an added day of
chilling at 10 C decreased days to bud-burst by 0.74 d3ys compared to 0.52
days if chilled at 4.4 C (figure 5, b). Plants chilled at 4.4 C were at
higher developmental rates after 20 days, and added chilling may have been
less effective for that reason only. Nevertheless, on any given day in fall
or winter, chilling at 10 C can be more effective than chilling at 4 C,
probably depending on previous conditions.
a.
LOW-ELEVATION
b. HIGH- ELEVATION
.... <.!)
(J)Z
O::....J 1.00
=>....J
co:r:
cu
::::>LL
coo
g
.80
(J)c
...... ,
'
....,
..
....,
............
0
oW
0
CHILLING
TEMPERATURE
(C)
.......,
'
.........
w
<ta.
w
0:::(1)
u
,
,'
.......,
...........
zc
-<{
Wo::
(J)
"\,
"\.,
'
4.4
'
......
... ..
....
'
~
'
,4.4
10.0
.....
'10.0
CHILLING STARTED OCTOBER
'
4.4
""' 10.0
10.0
4.4
----CHILLING STARTED DECEMBER 5
we
c_
0
20
30
40
20
30
40
CUMUL ATIVE DAYS OF CHIL LING TO WHICH
ADDI T I O NAL DA Y IS ADDED
Figure 5.--Decrease in days to bud-burst by one added day of chilling at
4.4 C vs. 10.0 C. The amount of decrease is influenced by the date chilling
is started, the duration of previous chilling and the elevation of the popu­
lations being sampled (derived from data by Campbell and Sugano 1975).
There is some other evidence that the changes induced by chilling are
influenced by conditions at the time of chilling, i.e., by the time of season
at which chilling is experienced. In the above experiment, if chilling was
started December 5 rather than October 22, 4 C was more effective than 10 C,
even after the initial 20 days (figure 5, b),
Thus, the relative chilling
effectiveness of 4 C and 10 C depended on time of chilling, perhaps because
the chilling periods started at different cell physiological-states. It
almost certainly started at different stages of bud-development. In Douglas­
fir, bud morphogenesis continues after bud-set, in some population-samples
until mid-December (Allen and Owens 1972). The high-elevation population­
28
sample used in th s illustrat on had completed bud-set by August 10.
In the
same experiment, a low-elevation population-sample had completed bud-set much
later, by Septell\ber 22, Patterns of chilling temperature effectiveness were
quite different for the two samples (compare figures 5, a and 5, b),
The shapes of temperature response curves change with lengthening photo­
periods just as they do with longer chilling periods. This occurs even after
buds have been exposed to 104 continuous days of chilling at 4 C (table 1).
Also, just as with chilling effects, photoperiod-effects are more pronounced,
in respect to bud-burst timing, when buds flush in lower temperatures (figure
6). In a photoregime starting at 9 hours, an increase in flushing temperature
from 10 C to 11 C decreased days to bud-burst by 5.3 days (figure 7). If the
regime started at 13 hours, the same increase in flushing temperature de­
creased days to bud-burst by only 3.8 days.
w 7
a:
w
Q.
5
>.
'-
0
0
w
1(f)
0::
::)
CD
a
::)
CD 120
(f) a
3
0::
_j
z
w
2
Q_
0
_j
w
>
w
�-(l')w
a: I-
i<£
ow
:>a:
6
5
Ill
PHOTOREGIME
4
0
1-0
C/)u
><(W
ow
a:
Z<!>
-w
o
w
(l')lr
<(W
wQ.
a:(/)
U)-,
W<(
9
3
II
13
2
0
a
20
TEMPERATURE TO WHICH AN ADDITIONAL
DEGREE (C) IS ADDED
Figure 6.--The influence of photoregime on developmental rates and on days
to bud-burst in Douglas-fir (derived from data by Campbell and Sugano, In
prep.). Response of seedlings that had been chilled 104 days at 4 C.
Figure 7.--Decrease in day to bud-burst due to an added degree (C) of tempera­
ture during flushing, as influenced by photoregime and previous temperature.
Had the chilling duration been shorter than 104 days, the differences
among photoregimes in figure 6 would have been greater. The longer the
chilling period, the less the spread among photoregimes (figure 8 ).
Moreover, potential developmental rates are augmented more by an added day
of chilling if the preceding chilling period has been short and if buds are
The longer the photoperiods in the photo­
flushed in shorter daylengths,
regime, the less the influence of added chilling (figure 9). Longer
photoperiods tend to compensate for chilling, as has been noted previously for
several species (Jensen and Gatherum 1965, Nienstaedt 1966, Worrall and
Mergen 1967, Farmer 1968 ).
29
1.3
200
P H OTORE GI ME
l-<9
(f)Z
1-
(/)
0:: :::J 1.1
0:::J
CD_
160 :r:
ou
:J (D :J
CDLL
0
0
:J 120 (D
PHOTOREGI ME
0
1(/) >:
<:!
(f)O
0
0
ow
80
9
0
II
13
40 II
44
DAYS
9
.9
zo
-<J:
.7
II
Wa::
<!a_
(/)w
w
0:: (/)
u Wo
0
----13
77
OF CHI L LI NG
0
20
40
C U MULA T I VE DAYS OF CHI L LI NG TO
WHI CH THE ADDI TIONAL DAY I S ADDED
Figure 8 .--The influence of chilling duration (4 C) and photoregime on days
to bud-burst. Seedlings flushed at 15.5 C (derived from data by Campbell and
Sugano, In prep.).
Figure 9.--Decrease in days to bud-burst due to one added day of chilling, as
influenced by length of previous chilling and photoregime in which seedlings
are flushed.
In Douglas-fir, there is question whether photoperiod and chilling ever
completely compensate for one another. After 8 0 days of chilling, an added
day decreased days to bud-burst by about one-half day, if plants were flushed
in a 13-h photoregime at 15 C (figure 9). Even after 104 days (15 weeks) of
continuous chilling at 4 C, plants that were flushed in 13-h photoregimes at
15 C burst their buds 8 days before plants in a 9-h photoregime (figure 6).
DISCUSSION
Our experience indicates that responses to stimuli during dormancy can be
measured quite precisely by their effects on developmental rate. Thus, compli­
cated interrelations become susceptible of study. It is these interrelations
that are emphasized in this paper, in support of a suggested quantitative
model for the bud-burst timing mechanism.
The proposed system appears to suffice as a preliminary model for
Douglas-fir. It also may be appropriate, in principle, for describing respons­
es in other perennial, temperate-region species. Our results agree, qualita­
tively, with results in many previous reports. Chilling effects, first
reported by Coville (1920), and effects of a substitution of photoperiod for
chilling as reported by Gustafson (1938 ) and others cited earlier in this
paper, usually are similar to those we found. As in Douglas-fir, experimental
data in these other species, hardwoods as well as conifers, appear to be
30
quantitatively interpretable as resulting from changes in developmental rates.
Vegis (1963) clearly recognized the quantitative interaction of photo­
period and temperature, and a "widening of the temperature response" with pas­
This latter can also be interpreted quantitatively as
sage through dormancy.
a change in the temperature response curve induced by chilling--in early
dormancy it is only the lower temperatures that can effectively increase
actual developmental rates, by first increasing the potential developmental
rates. Sarvas (1974) added to Vegis' conceptual framework by providing a
semi-quantitative model. His model predicted bud-development as the passage
in time of the developmental cycle through three succeeding categories of dor­
mancy.
Sarvas did not conceptually differentiate between potential and actual
development rates, and he did not account for effects of photoperiod.
For some species, a division of dormancy into phases of rest (Samish 1954,
Vegis 1964) or stages of dormancy, whatever they may be called (Doorenbos 1953,
Sarvas 1974), may be both unnecessary and undesirable. Categories of dormancy
imply steady states and boundaries. Categorical definitions within dormancy
are apparently difficult to apply and seemingly inadequate (Romberger 1963,
Sarvas 1974). Romberger advised more detailed physiological studies to provide
a more satisfactory nomenclature. But, for some species, it may be that it is
the concept of a partitioned dormancy rather than the definition of categories
that is at fault. In these species, e.g. Douglas-fir, we do not appear to be
dealing with steady states, or even with steady states separated by transi­
tional phases as proposed by Smith and Kefford (1964). In Douglas-fir, dor­
mancy is apparently constantly transitional, with potential developmental
rates changing continuously in response to cool-season environmental stimuli.
Also, buds apparently can grow continuously during dormancy, either by cell
production or by cell elongation, the degree depending on potential develop­
mental-rate and temperature. In this case, categories such as predormancy,
true dormancy, and postdormancy have little functional meaning (Sarvas 1973).
They tend to confuse by providing an apparent rationale for delineating bound­
aries, or for seeking qualitative differences between steady states when such
differences do not exist.
The system as proposed here is still incomplete in several ways. In de­
ciduous trees, continuous chilling has been reported to be more effective than
alternating warm and cold periods (Overcash and Campbell 1955). Also, warm
periods can reverse the effects of previous cold periods (Bennett 1950).
These phenomena have not been investigated for Douglas-fir, or, to my know­
ledge, for any other conifer. The model presented here assumes strict addi­
tivity of development toward bud burst--each day's actual development is added
to previous development. Also, the model does not make provision for revers­
als in potential developmental rates. Whether these are serious deficiencies,
or are even applicable to conifers, will require further experimentation.
The structure of the temperature response curve also needs refinement.
In Douglas-fir, diurnally fluctuating temperatures give rise to developmental
rates that are larger than would be predicted from rates in constant tempera­
tures (Campbell and Sugano 1975). In the cited study, night temperatures were
low enough to have contributed to chilling. Thus, increased rates may have
been partly due to higher potential rates induced by chilling. Still, fluctu­
ating temperatures were so much more effective than constant temperatures that
31
some otl1er stimulus may
have
been involved.
fn my experiments I h ave taken the view that details in the dormancy system that are not directly related to developmental-cycle timing are not acted on by natural selection and therefore cannot be treated as meaningful parts of the system. Xn other words, for differences exposed in growth cham­
bers or nurseries to have an accepted place in the system, they must have functional relevance within the genetic structure of the species. The parts of the system we have. so far studied apparently satisfy this
requirement. Not only did diff erent population-samples respond differently
to chilling, photoperiod, and temperature, but many of these differences
could be related clinally to postulated selecting agents at population origin.
I have proposed that these differential responses to stimuli are responses to
environmental cues for adjusting bud-burst to its seasonal cycle. Elsewhere,
we (Campbell and Sugano, In press) suggest that the degree of response to any
particular cue is specified by the conditional probabilities of future select­
ing events such as frost and drought, at the population origin, given that
particular cue.
LITERATURE CITED
Allen, G. S., and Owens, J. N. 1972.
The life history of Douglas-fir.
Can., Can. For. Serv., Ottawa, 139 p.
Inf.
Bennett, J. P. 1950. Temperature and bud rest period. Effect of temperature
and exposure on the rest period of deciduous plant leaf buds investigated.
Calif. Agric. 4 (1): 11, 13, 15, 16.
Campbell, R. K. , and Sugano, A. I. 1975. Phenology of bud burst in Douglas­
fir related to provenance, photoperiod, chilling and flushing temperature.
Bot. Gaz. 136: 290-298 .
(In press). Genecology of bud-burst phenology in Douglas-fir--response
to flushing temperature and chilling. Bot. Gaz.
Coville, F. V.
1920. The influence of cold in stimulating the growth of
plants. J. Agric. Res. 20: 151-160,
Davidson, J. 1944. On the relationship between temperature and rate of de­
velopment of insects at constant temperatures. J. Animal Ecol. 13: 26-38.
Doorenbos, J,
1953. Review of the literature on dormancy in buds of woody
plants. (Wageningen) Landbouw. hoogeseh. Meded. 53: 1-23.
The experimental
Dormling, I. Gustafsson,
., and von Wettstein, D. 1968 .
control of the life cycle in Picea abies (L.) Karst. I. Some basic experi­
ments on the vegetative cycle. Silvae Genet. 17: 44-64.
Eiche, V. 1966. Cold damage and plant mortality in experimental provenance
plantations with Scots pine in northern Sweden. Stud. For. Suec. 36: 1-219.
32
1968 ,
Sweetgum dormancy release: effects of chilling,
Farmer, R, E., Jr.
photoperiod, and genotype. Physiol. Plant. 21: 1241 1248 .
Gustafson, F. G. 1938 . Influence of the length of day on the dormancy of
tree seedlings. Plant Physiol. 13: 655-658 ,
Jensen, K. F., and Gatherum, G, E. 1965. Effects of temperature, photo­
period, and provenance on growth and development of Scotch pine seedlings.
For. Sci, 11: 18 9-199.
Lavender, D. P., and Wareing, P. F. 1972. Effects of daylength and chilling
on the responses of Douglas-fir (Pseudotsuga menziesii (Mirb,) Franco)
seedlings to root damage and storage. New Phytol. 71: 1055-1067.
Nienstaedt, H. 1966.
Sci. 12: 374-38 4.
Dormancy and dormancy release in white spruce.
For.
Overcash, J. P., and Campbell, J. A. 1955. The effects of intermittent warm
and cold periods on breaking the rest period of peach leaf buds. Proc.
Amer. Soc. Hort. Sci. 66: 8 7-92.
Perry, T. 0,
1971.
Dormancy of trees in winter.
Science 171: 29-36.
Romberger, J. A. 1963. Meristems, growth and development of woody plants.
U.S.D.A. For. Serv., Tech. Bull. No, 1293, 214 p.
Samish, R. M.
5: 18 3-204.
1954.
Dormancy in woody plants.
Ann. Rev. Plant Physiol.
Sarvas, R. 1972. Investigations on the annual cycle of development of
forest trees--active period, Communications Instituti Forestalis
Fenniae 76.3, 110 p.
1973,
The annual developmental cycle in forest trees. IUFRO Working
Party S2.01.4 Symposium on Dormancy in Trees. Kornik, Poland, Sept. 5-9,
17 p.
1974,
Investigations on the annual cycle of development of forest
trees.
II. Autumn dormancy and winter dormancy. Communications Instituti
Forestalis fenniae 8 4,1, 101 p.
1964, The chemical regulation of the dormancy
Smith, H., and Keiford, N. p,
phases of bud d,eyelopment. Amer. J. Bot. 51: 1002-1012.
Sugano, A. I. 1971. The effects of low temperatures on dormancy release in
Douglas-fir from western Oregon, Washington and California. MSc. Thesis,
Oreg. State Univ,, Corvallis.
1963. Climatic control of germination, bud break, and dormancy.
Vegis, A.
P. 265-285, In Evans, L. T. Environmental Control of Plan t Growth.
Academic Press, New York, 449 p.
33
Vegis, /\.
1964.
15: 185-224.
Dormancy in higher plants.
Ann , Rev. Plan t PhysioJ.
Wakefield, J. D., ed. 1969, Cl imatological Handbook, Columbia Basin States
temperature. Vol. 1, pt. A. Pacific Northwest River Basins Commission,
Vancouver, Wash.
Wommack, D. E. 1964. Temperature effects on the growth of Douglas-fir.
Ph.D. Thesis, Oregon St. Univ., Corvallis.
Worrall, J. , an d Mergen , F. 1967. Environmental and gen etic control of
dormancy in Picea abies. Physiol. Plant. 20: 733-745.
Reproduced from PROCEEDINGS:
WORKSHOP
1978,
Anthony E. Squillace,
of Agriculture,
FIFTH
NORTH AMERICAN FOREST
compiled and edited by
by the FOREST
for official use
GPO 988-894
C harles A. Hollis
SERVICE,
BIOLOGY
and
U.S . Department