Document 12586574
advertisement

Yosef Cohen
Evolutionary Distributions
November 22, 2007
c 2007, Yosef Cohen
Copyright Consistency is not a virtue, Coherency is.
I have known extremely incoherent men
who were extremely consistent.
Preface
Evolutionary distributions (ED) are a special kind of partial differential equations (PDE).1 They incorporate the processes that lead to population growth,
such as immigration and birth, and decline, such ad emigration and death.
Declines are subject to natural selection; the latter is influenced by the environment and interactions with coevolving organisms. Growth is subject to
mutations (random or otherwise). An open system of ED represents a collection of functionally distinct ED (primary producers, primary consumers, prey,
predators and so on) along with environmental input such as solar radiation,
temperature (as in global warming) and so on.
Within an ED, ecological interactions may involve the usual interactions with
“your own kind”; e.g., collaboration, competition, cannibalism and parasitism.
Between ED, interactions may involve predator-prey, parasitism and so on.
The quintessential premise of ED is that evolution by natural selection acts
directly on phenotypes and indirectly on genotypes—a hemophiliac dies because he bleeds to death, not because he owns (or owned by) some genetic
make up. Turning common belief on its face and contrary to what Richard
Dawkins professed in his ground-breaking The Selfish Gene (Dawkins, 1990),
from our perspective, genes serve phenotypes, not the other way around. ED
present us with the opportunity to supplement and in some cases do away
with evolutionary games as means to study evolutionary ecology
To relate to a comprehensive theory of ED (in the context of evolutionary
theory) requires solid understanding of the potential behavior of solutions of
ED, where the latter may be hyperbolic, elliptic or parabolic systems of PDE.
In most cases, the PDE models of the evolutionary process result in semi- or
at worst quasi-linear systems of PDE. We shall not encounter fully nonlinear
ED.
1
All acronyms are used in both singular and plural.
VIII
We begin with a motivating example (Chapter 1). We then provide an almost self contained introduction to PDE (Chapters 2, 3 and 4). To make the
subject matter accessible, we introduce PDE at an intuitive level as much as
possible. This means that some of the material in the introductory chapters
may seem at first irrelevant. Yet, if you are not familiar with PDE, the introductory chapters will hopefully motivate you to delve further into the subject.
Solutions of PDE represent a rich range of behaviors. Once you go over the
introductory chapters, you should be able to judge for yourself how useful (or
not) are ED to understanding evolution by natural selection.
After the introduction to PDE—which of course you may skip if you are familiar with the subject—we move on to the analysis of the consequences of
ED (single and systems) with respect to ecological interactions such as competition, predation, parasitism and so on. The guiding principles are biological
“reality” (or otherwise). Consequently, we will not be able to solve most of the
ED we discuss. So, we are going to sacrifice generality for biological reality and
thus rely on numerical solutions. We shall assume that for the most part, if a
real (co-)evolutionary system is inadequately encapsulated in a mathematical
framework—as is the case, for example, for ill-posed Cauchy problems—then
attempting numerical solutions will fail. Therefore, we shall not be infatuated with existence problems. If a solution to an ED does not exist, then the
mathematical model is nonsense.
A quick look at the Table of Contents reveals the organization of this work.
All of the results with respect to PDE are well known. Most of the results with
respect to ED are new in the sense that they have not been published before.
In all honesty, it is not possible to pin-point the prerequisite background for
complete comprehension of the material in this book. Much of it depends on
your abilities, background and most of all, level of commitment. To pursue the
subject matter without exposure to advanced calculus and ordinary differential equations (ODE) will probably require more than one reading. You may
find consolation in the fact that other than high school (but then during my
time high schools were different), I have no formal training in Mathematics.
The idea of ED resulted from long discussions about evolutionary games and
my dissatisfaction with some of their aspects. These spanned a period of about
10 years, during which I collaborated with Tom Vincent and Joel Brown,
working on evolutionary games. It was in the summer of 2003, when we met
at Itaska State Park in Minnesota (where the Mississippi river begins) that I
finally decided to abandon my work in the evolutionary games area and follow
the ED path. Were it not for the two of them, I do not know if my work on
ED would have happened.
IX
Yosef Cohen
November 22, 2007
St. Paul, Minnesota
Contents
1
Motivation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
1
1.1 A motivating example . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
1
1.1.1 The population model . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
2
1.1.2 Mutations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
3
1.1.3 Selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
4
1.1.4 The evolutionary distribution . . . . . . . . . . . . . . . . . . . . . . .
5
1.1.5 The evolution of efficiency . . . . . . . . . . . . . . . . . . . . . . . . . .
7
1.1.6 The evolution of efficiency with competition . . . . . . . . . .
9
1.1.7 Evolution through two phenotypic traits . . . . . . . . . . . . . . 11
1.1.8 Cooperation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
1.1.9 Directional mutations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
1.2 Evolutionary distributions in context . . . . . . . . . . . . . . . . . . . . . . . 15
1.2.1 Phenotypes, genotypes and natural selection . . . . . . . . . . 15
1.2.2 Evolutionary ecology and evolutionary games . . . . . . . . . 16
1.2.3 Stability . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
1.2.4 The origins of the theory of ED . . . . . . . . . . . . . . . . . . . . . 18
1.2.5 The ordinary differential equations approach . . . . . . . . . . 18
XII
2
Contents
Preliminaries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
2.1 Vectors and matrices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
2.1.1 Vectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
2.1.2 Matrices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
2.1.3 Eigenvalues and eigenvectors . . . . . . . . . . . . . . . . . . . . . . . . 26
2.2 Derivatives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
2.2.1 Partial derivatives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
2.2.2 The tangent plane and the approximation formula . . . . . 29
2.2.3 Critical points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
2.3 Vector fields . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
2.3.1 Functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
2.3.2 Tangent and normal vectors . . . . . . . . . . . . . . . . . . . . . . . . 36
2.3.3 Gradients . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
2.3.4 Vector fields . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
2.3.5 Lagrange multipliers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
2.4 Integrals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
2.4.1 Multiple integrals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
2.4.2 Line integrals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
2.4.3 Surface integrals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
3
Introduction to first order PDE . . . . . . . . . . . . . . . . . . . . . . . . . . . 69
3.1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 69
3.2 First-order PDE with two independent variables . . . . . . . . . . . . . 70
3.2.1 Integral curves . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72
3.2.2 Semilinear equations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 74
3.2.3 Quazilinear equations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 80
3.2.4 Solutions with discontinuous first derivatives . . . . . . . . . . 89
Contents
XIII
3.2.5 Weak solutions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 92
3.2.6 Shocks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 94
3.2.7 Nonuniqueness of weak solutions . . . . . . . . . . . . . . . . . . . . 98
3.3 First order PDE with n independent variables . . . . . . . . . . . . . . . 101
3.3.1 Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 101
3.3.2 Conservation laws . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 105
3.3.3 Variable transformations . . . . . . . . . . . . . . . . . . . . . . . . . . . 108
3.4 First order systems with two independent variables . . . . . . . . . . 111
3.4.1 Cauchy data and characteristics . . . . . . . . . . . . . . . . . . . . . 111
3.4.2 The Cauchy-Kowalevski theorem . . . . . . . . . . . . . . . . . . . . 113
3.4.3 The system ODE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 117
3.4.4 Weak solutions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 118
3.4.5 Shocks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 118
3.4.6 Causality . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 120
3.4.7 Canonical form . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 121
3.5 First order systems with n independent variables . . . . . . . . . . . . 123
4
Introduction to second order PDE . . . . . . . . . . . . . . . . . . . . . . . . . 127
4.1 Classes of scalar PDE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 127
4.1.1 Linear . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 128
4.1.2 Semilinear . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 128
4.1.3 Quazilinear . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 129
4.2 Variable transformations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 129
4.3 Distributions and Riemann functions . . . . . . . . . . . . . . . . . . . . . . . 130
4.4 Semilinear PDE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 130
4.4.1 Cauchy data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 130
4.4.2 Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 131
XIV
Contents
4.4.3 Types . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 132
4.4.4 Canonical forms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 134
4.5 Semilinear hyperbolic equations . . . . . . . . . . . . . . . . . . . . . . . . . . . 140
4.6 Semilinear elliptic equations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 142
4.6.1 The Poisson’s equation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 142
4.6.2 Maximum principle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 144
4.6.3 Green’s functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 144
4.7 Another approach to elliptic PDE . . . . . . . . . . . . . . . . . . . . . . . . . 146
4.7.1 Self adjoint operators and Green’s identities . . . . . . . . . . 146
4.7.2 Well-posedness . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 148
4.7.3 Green’s functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 151
4.7.4 Laplace’s equation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 157
4.7.5 Eigenvalues and eigenfunctions . . . . . . . . . . . . . . . . . . . . . . 160
4.8 Semilinear parabolic equations . . . . . . . . . . . . . . . . . . . . . . . . . . . . 164
4.8.1 Properties . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 164
4.8.2 Well posed boundary data . . . . . . . . . . . . . . . . . . . . . . . . . . 165
4.8.3 Uniqueness . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 165
4.8.4 Maximum value theorem . . . . . . . . . . . . . . . . . . . . . . . . . . . 166
4.8.5 Green’s functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 167
4.8.6 Transforms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 168
4.8.7 Similarity solutions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 169
4.8.8 Another approach . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 170
5
Single evolutionary distributions . . . . . . . . . . . . . . . . . . . . . . . . . . . 175
5.1 Construction of ED . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 175
5.1.1 Evolution . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 175
5.1.2 Single evolutionary distributions . . . . . . . . . . . . . . . . . . . . 176
Contents
XV
5.1.3 Conservation law derivation . . . . . . . . . . . . . . . . . . . . . . . . 179
5.2 Steady state and stability . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 180
5.2.1 Steady state . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 180
5.3 ED - environment interactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . 185
5.3.1 Evolution of exploited populations . . . . . . . . . . . . . . . . . . . 186
5.3.2 Global climate change . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 189
5.3.3 Carrying capacity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 189
5.3.4 Driven ED . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 190
5.4 Competition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 190
5.5 Cooperation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 190
5.6 Cannibalism . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 190
5.7 Parasitism . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 190
5.8 Directional mutations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 190
5.9 Linked mutations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 190
5.10 Discontinuities and shocks in ED . . . . . . . . . . . . . . . . . . . . . . . . . . 190
5.11 Assortative mating . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 190
6
Multiple-evolutionary-distributions . . . . . . . . . . . . . . . . . . . . . . . . 191
6.1 Conservation law derivation of multiple ED . . . . . . . . . . . . . . . . . 191
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 193
Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 197
Notations
1
Motivation
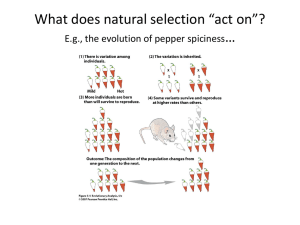
We start with a motivating example and then discuss evolutionary distributions (ED). Section 1.1 is more than just an example. We will use the results of
the example to advance some general ideas about evolution by natural selection and the role that ED might play in “rediscovering” Darwin’s work. If you
are not familiar with partial differential equations (PDE), the presentation in
this chapter might seem obscure on first reading. We shall explain things in
detail in the following chapters.
1.1 A motivating example
Currently, there is much hoopla about the production of ethanol from agricultural crops. It supposedly reduces the energy-dependence of oil-poor countries
on oil-rich countries. For example, sugar beet pulp—otherwise of little economic value to farmers—can be ingested by microorganisms who then effuse
methanol which in turn may be transformed to ethanol. The latter presents
an alternative to energy consumption from fossil fuel.
Imagine a population of microorganisms in a well-mixed liquid mixture of
sugar beet pulp. Suppose that survival and reproduction of each microorganism depends mostly on a single phenotypic trait which effects the organism’s
efficiency in converting sugar beet pulp to methanol and consequently the
ability of the phenotype to multiply.1 Denote this phenotypic trait by x ∈
(0, 1), and assume that x is inherited. The notation implies that x can have
values in the interval (0, 1). In the process of reproduction, the progeny inherit their progenitor’s value of x with small variation. The variation happens
1
Efficiency may be defined as units of energy produced per units of energy consumed.
2
1 Motivation
because of small random mutations. The mutations are manifested in changes
in the microorganism’s efficiency to produce methanol. These mutations are
a fact of life and they arise because, for example, the presence of ultraviolet
radiation that cause “errors” in DNA replication. We also assume that the efficiency of converting pulp to methanol affects the survival of the phenotypic
x value. For us, the survival of values of x in the population is important.
The survival of a an individual organism (phenotype) who carries that trait
is of no consequence unless it is related to the survival of the value of x in
the population. The survival of specific values of x in the population (carriers
of these values are called phenotypes) comes about perhaps because efficient
microorganisms reproduce faster than inefficient ones.
Because the phenotypic trait (the value of a particular x): (i ) affects survival
of the trait value (not necessarily of the organism itself), (ii ) is inherited and
(iii ) is subject to random mutations, we call x an adaptive trait. Next, we cast
this story in a a mathematical framework that mimics the process of evolution
by natural selection.
1.1.1 The population model
There are two approaches to modeling the process as described thus far. One
is discrete and the other continuous. In the discrete approach, we follow the
fate of each individual microorganism at each small time increment, sum these
fates and obtain population dynamics—a tedious and hardly useful approach
in this case. The other is through a continuous approximation. Let u (x, t)
denote the density of phenotypes x at time t of the microorganism population
and write
u (x, t + ∆t) = u (x, t) + ∆t (β (·) − µ (·))
where ∆t is a short time interval, β (·) and µ (·) are rate functions of some
arguments that result in growth and decline of the population density. When
the decline function µ (·) depends at least on x and u (written as µ (u, x)), we
call it the selection function. Rearranging, we obtain
u (x, t + ∆t) − u (x, t)
= β (·) − µ (·) .
∆t
We assume that the limit on the left hand side when ∆t → 0 exists and equals
the right hand side. Therefore,
∂
u (x, t) = β (·) − µ (·)
∂t
(∂u/∂t denotes the partial derivative of u with respect to t). It is well known
that when small (i.e., when resources are plenty), populations grow exponentially. So to a first approximation, we define
1.1 A motivating example
3
β (u (x, t)) := ru (x, t)
(:= denotes equal by definition) where r—the growth coefficient when the
population is small—is a constant. Assume that as the population grows,
the microorganisms exhaust their supply of raw material to convert and also
produce autotoxins. Again, to a first approximation, we may write
µ (u (x, t)) = µ
e (u (x, t)) u (x, t)
r
u (x, t) u (x, t)
=
k
r
2
= [u (x, t)] .
k
Letting u ≡ u (x, t), where ≡ denotes equal for all x and t), we write
∂t u = ru −
r 2
u
k
(1.1)
where ∂t u := ∂u/∂t is the partial derivative of u with respect to t. This is the
most ubiquitous single population model, the so-called logistic growth.
1.1.2 Mutations
Next, we assume that mutations: (i ) occur at birth; i.e., they are imposed on
the growth term of (1.1), (ii ) are small and (iii ) are random. Let η be the
mutation rate. Then after each instant ∆t, we have the following contributions
to u (x, t):
no mutations = (1 − η) ru (x, t) ,
1
mutations from u (x + ∆x, t) = ηru (x + ∆x, t) ,
2
1
mutations from u (x − ∆x, t) = ηru (x − ∆x, t)
2
where ∆x is small compared to x. So
1
ru (x, t) = (1 − η) ru (x, t) + ηr (u (x + ∆x, t) + u (x − ∆x, t)) .
2
(1.2)
Using Taylor series expansion around v, we obtain
u (x + ∆x, t) = u (v, t) + ∆x∂v u (v, t) +
x ≤ v ≤ x + ∆x
and around w
1
2
(∆x) ∂vv u (v, t) + o (u (v, t)) ,
2
4
1 Motivation
u (x − ∆x, t) = u (w, t) − ∆x∂w u (w, t) +
1
2
(−∆x) ∂ww u (w, t) + o (u (w, t)) ,
2
x − ∆x ≤ w ≤ x
where o (u(x)) means that lim∆y→0 o (u (y)) /∆y = 0 and ∂xx denotes the
second partial derivative. Taking ∆x → 0, substituting into (1.2) and dropping
o (·), we obtain
ru (x, t) = (1 − η) ru (x, t) +
1
1
2
ηr u (x, t) + ∆x∂x u (x, t) + (∆x) ∂xx u (x, t) +
2
2
1
2
u (x, t) − ∆x∂x u (x, t) + (−∆x) ∂xx u (x, t)
2
which gives
1
2
ru (x, t) = r u (x, t) + (∆x) η∂xx u (x, t) .
2
With mutations added, (1.1) becomes
2
(∆x)
∂t u = r u +
η∂xx u
2
!
−
r 2
u .
k
2
Absorbing (∆x) /2 into η (in which case η becomes very small), we write
∂t u = r (u + η∂xx u) −
r 2
u .
k
(1.3)
Equation (1.3) is known as the Fisher reaction diffusion equation It has been
analyzed extensively (see for example Murray, 2003) from a different perspective than ours. Rearranging and abstracting, we write (1.3) in a more general
form
∂t u + b∂xx u = f (x, t, u) .
(1.4)
In the mathematical vernacular, if f is nonlinear in u, and b is positive (certainly the case here), then (1.4) is referred to as a second order semi-linear
elliptic partial differential equation. If f is linear in u, then (1.4) is said to be
linear. Classifying PDE; e.g., as elliptic and semi-linear, is useful for then we
can say something about certain attributes of their solutions (e.g., existence)
without further ado.
1.1.3 Selection
So far, (1.3) incorporates mutations. To add selection, we must admit that
there is some advantage–in terms of transferring certain values of x to progeny
1.1 A motivating example
5
more than other values—to being a phenotype x as opposed to y for x 6= y at
some time, t. This advantage depends not only on x and t, but also on u(x, t).
The latter reflects the fact that selection is density dependent. One way to
effect the advantage of being x as opposed to y is to assume that there is some
value of x, say x
b, at which mortality of the x phenotypes is at its minimum.
This is where the environment results in selection of some phenotypes over
others. We use the term selection to emphasize the differences in mortality
rate among phenotypes because of differing values of their trait. If we suppose
that selection is reflected in differing carrying capacity for different values of
x, then one choice for k in (1.3) is
" 2 #
x−x
b
k (x) = k1 exp −
(1.5)
σx
which we may refer to as a phenotypic specific carrying capacity. Equation
(1.5) says: “If you are a phenotype with value x
b, then your carrying capacity
is at its maximum (and your mortality is at its minimum) compared to other
phenotypes with values different from x
b. Any deviation from x
b results in
lower carrying capacity. The amount by which the carrying capacity declines
depends on the magnitude of σx ; the smaller it is, the larger the decline per
unit of deviation from x
b.” For this reason, we call σx the phenotypic plasticity.
So in our example, we admit that increased efficiency in converting pulp to
methanol comes with a price (say vulnerability to autotoxins) and that there
is some optimal value, x
b. In other words, there is no “free lunch”. If there was,
we would have seen an evolution toward 100% efficiency, which contradicts
(at least) the laws of thermodynamics. An alternative to (1.5) is using |x −
2
x
b| instead of (x − x
b) , which is then known as the double exponential. We
can, if we so please, let x := σ, in which case phenotypic plasticity itself is an
adaptive trait.
1.1.4 The evolutionary distribution
With mutations and selection influencing the density of phenotypes via the
adaptive trait values, we have captured the essence of evolution by natural
selection in
r
∂t u = r (u + η∂xx u) −
u2 .
(1.6)
k (x)
To obtain a solution, we must specify the data which consist of initial and
boundary conditions. For initial data, we specify
u (x, 0) = g (x)
where g (x) is a known function. Now boundary conditions arise because x
manifests a physical quantity, and as such, it is usually constrained. For example, body temperature cannot exceed about 42◦ C because proteins denaturate (like in a hard boiled egg). It cannot go below 0◦ C because then proteins
6
1 Motivation
crystallize and harm cell membranes. For our methanol producing microorganisms, x, in representing efficiency, must be between 0 and 1. We write it
as x ∈ (0, 1) (for x and set {y}, x ∈ {y} says that x is a member of (in) {y}).
Once we add the boundary conditions along 0 and 1 for any t ≥ 0, we have
x ∈ [0, 1] for all t ≥ 0. The notation (a, b) for a < b says that the interval
between a and b includes neither a nor b. The interval [a, b] includes both a
and b. These are called open and closed intervals. There are two corresponding
half open intervals.
Both initial and boundary conditions must be compatible in the sense that at
t = 0, the boundary conditions must equal to the initial condition u (x, 0) at
the boundaries; i.e.,
g (x) = u (x, 0) , g (x) = u (x, 0)
(1.7)
where x and x are the lower and upper boundaries of x. In choosing the
boundary conditions, we have a few alternatives. The most common are the
so-called Dirichlet, Neumann and periodic boundary conditions. We write
these boundary conditions as
Dirichlet: u (x, t) = c1 , u (x, t) = c2 ,
Neumann: ∂x u (x, t) = ∂x u (x, t) = 0,
Periodic: u (x, t) = u (x, t) .
This is not the most general way to write boundary conditions, but for our
needs it suffices. In the case of the Neumann conditions, the derivatives are
taken with respect to the outward normal (a directional derivative perpendicular to the tangent to the boundary at x and at x). For our purpose,
the Neumann boundary conditions are the most natural. They simply say
that there is no flow across boundaries. The Dirichlet boundary conditions
are somewhat unnatural—there is no particular reason to believe that u =
some value on the boundaries. The periodic boundary conditions are useful
in cases where we have reason to believe that the u on both boundaries are
equal. We shall use mostly the Neumann boundary conditions. Occasionally,
and for (admittedly) numerical convenience, we shall use periodic boundary
conditions. We invite those who prefer the Dirichlet or any other boundary
conditions (e.g., Robbins) to follow the developments along a parallel track. It
will be extremely interesting to show that different boundary conditions lead
to substantially different conclusions about the the outcome of the evolutionary processes involved.
Our fully specified model of evolution under natural selection, which we call
the evolutionary distribution (ED), is
1.1 A motivating example
∂t u = r (u + η∂xx u) −
r
u2 ,
k (x)
7
x ∈ (x, x) ,
(1.8)
u (x, 0) = g (x) ,
∂x u (x, t) = ∂x u (x, t) = 0
where k (x) is given in (1.5). Because we use the Neumann boundary conditions, it will often be convenient to rescale x to x ∈ (0, 2π) and set u (x, 0)
= A + A sin (x) where A and B are constants. This results in compatible
Neumann boundary and initial conditions in (1.7). Figure 1.1 illustrates com-
u (x ,0 )
∂ x u (x ,0 ) at x = x
∂ x u (x ,0 ) at x = x
x
x
Fig. 1.1: The Neumann boundary conditions must be compatible with the
initial conditions.
patible boundary and initial conditions conditions: at t = 0, we have ∂x u(x, 0)
= 0 at x and at x. For t > 0, the solution of our PDE must continue to satisfy
∂x u(x, t) = 0 on both ends.
We will follow three ED: with selection operating through phenotypes efficiency with respect to carrying capacity, with selection operating on u (x, t)
through the carrying capacity and competition and with two adaptive traits.
1.1.5 The evolution of efficiency
With the following parameter values
8
1 Motivation
r = 0.25, k1 = 1 000, η = 5 × 10−7 , σk = 2,
x = 0, x = 2π, x
b = π, T = 40, g (x) = 100
(1.9)
and data
u (x, 0) = g (x) ,
∂x u (x, t) = ∂x u (x, t) = 0
we obtain the numerical solution illustrated in Figure 1.2.2 The left panel
Fig. 1.2: Left - trajectory of the ED (1.8). Right - initial (horizontal line) and
the stable fixed ED at T = 40.
shows the trajectory of the evolving distribution and the right the initial and
the fixed stable distribution of phenotypes at T = 40. The density of the most
fit phenotypes (with processing efficiency of 50%) is 1 998 (say individuals per
mm3 ).
For us, fitness of a phenotype is a function of its density when the ED is
stable. If the stability is fixed, then fitness is simply the phenotype’s density.
When we say phenotype’s density, we mean the density of individuals who
carry a particular value of the phenotypic trait, x. If the ED is stable, but
periodic, with period τ , then the fitness of a phenotype is given by
Z t+τ
φ(x) =
u
e(x, ζ)dζ
(1.10)
t
where φ denotes fitness and u
e denotes the stable ED. If the ED is chaotic,
then once it enters its attractor, fitness is given by
2
We shall obtain an explicit solution to this ED later.
1.1 A motivating example
Z
φ(x) =
t
9
∞
u
e(x, ζ)dζ.
If the ED is unstable, then fitness is undefined, but instantaneous fitness is
by u(x, t). From this quick introduction of fitness and the solution of (1.8) we
conclude that at any time, fitness, or instantaneous fitness, is a function of x.
Contrary to the traditional mathematical literature in evolutionary ecology
(where ODE are used), we do not require that at fixed stable equilibrium φ(x)
= 0. In fact, phenotypes of different fitness can (and must) coexist.
Thus, we already see that ED give us a way to answer the following: How
come we see so many phenotypes in natural systems that may be stable? For
example, mature trees in old growth forests (wherever they still are) are of
a variety of heights, trunk diameters, shades of leaves (or needles) and so
on. Some of these differences are produced by local random conditions and
events, such as soil nutrients. Others are maintained by genes at the service
of phenotypes.
1.1.6 The evolution of efficiency with competition
Now consider competition among the microorganisms. We assume that similar
phenotypes compete most for resources. Operationally, one may envision the
microorganisms living in a mixture where there are small differences in the
quality of the sugar beet pulp. Similar phenotypes exhaust the supply of pulp
that suit them most. Competition is mediated through mortality. So in (1.6),
we modify u2 and write F (u, x) u (x, t) instead. Here, F (·) accounts for the
competition between phenotypes. We now write
" 2 #
Z x
x−ξ
F (u, x) := k2
exp −
u (ξ, t) dξ, k2 ≥ 1.
(1.11)
σF
x
The exponent takes its maximum when x = ξ. At t, we integrate the competition between x and all phenotypes, weighed by the densities u (ξ, t) and
u (x, t). Thus competition and thereby selection become density dependent.
Let
Z x
2
exp −ξ 2 dξ.
erf (x) := √
π 0
Then
√ σF π
x−x
x−x
F (u, x) = k2
erf
− erf
u (x, t) .
2
σF
σF
Now the ED (1.8) becomes
10
1 Motivation
∂t u = r (u + η∂xx u) −
u (x, 0) = g (x) ,
∂x u (x, t) = ∂x u (x, t) = 0
rF (x)
2
(u (x, t)) ,
k (x)
x ∈ (0, 2π) ,
(1.12)
where F (x) does not depend on u (x, t). We keep the same parameter values
as in (1.9) and add
k2 = 100, σF = 1
(σF is the phenotypic plasticity with respect to competitive ability) and thus
obtain Figure 1.3. To view the impact of competition on the ED, compare
Fig. 1.3: Trajectory of the ED (left) and initial (horizontal line) and fixed
stable distribution at T = 40.
the left panels of Figures 1.2 and 1.3. Without competition, the most fit
phenotypes reach a density of 1 998; with competition, they reach 11.28. In
addition, the total density without competition is 5 × 107 , with competition,
it is 596 938 (all numerical results are approximate).
Figure 1.3 reveals an interesting feature: the density of the phenotypes at
the extremes (the most and least efficient microorganisms with regard to producing methanol) is higher than that of nearby phenotypes. Why? because
they are exposed to less competition than the phenotypes in the interior—
recall that we are integrating from x to x so the phenotypes at the extreme
left do not compete with phenotypes on their left and those on the extreme
right do not compete with phenotypes on their right. Hence, the density of
phenotypes on the boundaries is less depressed than that of phenotypes in
the interior. In fact, it is a matter of the choice of parameter values to obtain
complete isolation of the extreme phenotypes from the rest of the phenotypes.
1.1 A motivating example
11
Given small mutations, they then become reproductively isolated (a kind of
sympatric speciation if you will).
1.1.7 Evolution through two phenotypic traits
Consider two evolutionary traits, x := [x1 , x2 ], where x1 is associated with the
efficiency of processing sugar beet pulp to methanol and x2 is associated with
the competitive ability (say resistance to autotoxins). Assume that mutations
on x1 , denoted by η1 are independent of those on x2 , denoted by η2 . We
now reconsider (1.1). The mutations into ru (x1 , x2 , t) come from (with the
assigned proportions):
1
1 1
η1 η2 u (x1 − ∆, x2 − ∆, t) , η1 (1 − η2 ) u (x1 − ∆, x2 , t) ,
2 2
2
1
1 1
η1 (1 − η2 ) u (x1 − ∆, x2 + ∆, t) , . . . , η1 η2 u (x1 + ∆, x2 + ∆, t) .
2
2 2
Expanding all of these terms in u around (x1 , x2 ) and collecting terms we
obtain
!
2
X
rF (x2 ) 2
ηi ∂xi xi u −
u .
(1.13)
∂t u = r u +
k (x1 )
i=1
(with x in the appropriate set). To examine the ED, we use (1.5) with x
replaced by x1 and (1.11) with x replaced by x2 . Equation (1.13) with the
data
x : = [x1 , x2 ] , x := [x1 , x2 ] , u (x, 0) = g (x) ,
∂xi u (xi , t) = ∂xi u (xi , t) = 0, i = 1, 2
establish our ED. We adopt the same parameter values as before with the
appropriate substitutions for x1 and x2 . Figure 1.4 illustrates the fixed stable
ED. The density of the (π, π) phenotypes is now depressed (to 7.09) and
the total population is 2.593 1 × 106 . The release from extra competition
at the boundaries has now more marked effect than when selection for both
competition and carrying capacity are mediated by a single adaptive trait. The
remarkable fact is that when we link selection for efficiency and for competitive
ability to two different adaptive traits, the density of all phenotypes is larger
compared to when both selective pressures are linked to a single trait. In other
words, evolution by natural selection favors ED with multiple adaptive traits!
Let n be the cardinality (number of elements) of the set of adaptive traits.
This set of traits, along with their boundaries, define what we call the adaptive
space. So each ED is living in a space of n + 2 dimensions (n for x, one for
t and and one for u). In the case of a system of say m ED, each living in
an ni (i = 1, . . ., m) adaptive spaces, we define the evolutionary space as the
12
1 Motivation
Fig. 1.4: Fixed stable ED (left) and cross cuts at x1 = 0 and 2π (lower curve)
and at x1 = π (upper curve).
collection of m adaptive spaces along with m densities, ui (x, t). Therefore, the
evolutionary space has n1 + . . . + nm + m + 1 dimensions. The evolutionary
space is comprised of m manifolds, all intersecting along t.
If one is willing to interpret multiple traits as a measure of diversity, then
little wonder that evolution by natural selection results in ever more diverse
organisms with respect to their number of adaptive traits. Often we refer to
such organisms as “higher” or more complex compared to those with just a few
adaptive traits. Of course, what leads to jumps in the dimensionality of ED
(i.e., increase in the number of adaptive traits) remains an open question that
must be considered in the context of evolution by natural selection. A jump
in the dimensionality of the adaptive space may be likened to macroevolution.
The latter—according to our framework—occurs when a mutation, however
small, is “novel” in the sense that it allows phenotypes who carry them to
adapt to selection which previously had not been part of their repertoire of
adaptive traits. Note that we do not require large mutations to effect changes
in the dimensionality of the adaptive space (and thereby the dimensionality
of the evolutionary space), which we call macroevolution.
Here is another tantalizing possibility. Recall the definition of fitness; e.g.,
as in (1.10). To keep the discussion simple, assume that the ED is stable
and fixed. Does fitness increase monotonically with the dimensionality of the
adaptive space? Or perhaps dimensionality in the adaptive (and evolutionary)
space has some value at which fitness is at a global maximum. In other words,
you adapt, but not to everything for at some point, adapting to too many
things might hurt your fitness. All of these possibilities raise the specter of an
evolutionary space with dimensionality that is not fixed. This is in addition
to the idea of ever changing landscape of the adaptive space itself, as defined,
1.1 A motivating example
13
for example, by Vincent and Brown (2005). We shall have more to say about
these possibilities later.
1.1.8 Cooperation
Let us modify (1.11) to
Z
"
x
F (u, x) := k2
exp
x
x−ξ
σF
2 #
u (ξ, t) dξ, k2 ≥ 1.
(1.14)
Here, like phenotypes cooperate more than dislike phenotypes. Keep all parameters as before; just increase T to 1 000 and observe what happens (Figure
1.5): As opposed to the case of competition (Figure 1.4), now all phenotypes
Fig. 1.5: Fixed stable ED with selection on carrying capacity and cooperation.
bunch up in the middle. Remarkably, the density is depressed compared to
evolution with competition. Because the qualitative behavior of the numerical
solution (e.g., bunching here or there), is not specific to the choice of parameter values, we have a general result: Phenotypes achieve higher fitness under
competition than under cooperation. If the results of this example reflect a
ubiquitous phenomenon, then no wonder that in Nature, competition is more
frequently observed than cooperation. This conclusion is model-specific. It will
be interesting to see if such conclusion is not model-specific.
14
1 Motivation
1.1.9 Directional mutations
Let us revisit the case of a single trait with selection for carrying capacity and
competition (Section 1.1.6). Assign η1 to mutations to the left (to x − ∆x)
and η2 to mutations to the right. Then Taylor series expansion results in
1
u − ∆ (η1 − ∆η2 ) ∂x u + ∆2 (η1 + η2 ) ∂xx u.
2
1 2
Redefine ζ1 := ∆ (η1 − η2 ), ζ2 = ∆ (η1 + η2 ) and now
2
r
F (u, x) u.
∂t u = r (u − ζ1 ∂x u + ζ2 ∂xx u) −
k (x)
motivating-example.nb
To observe the effect of unequal mutation rates, we adopt the ED from Section
1.1.6 with η2 vastly larger than η1 (Figure 1.6). The selection for efficiency
1
t
18
z
16
z
14
12
10
0
1
2
3
T
4
x
Fig. 1.6: Unequal mutation rates.
now plays a minor role and competition a major role. As expected, the faster
mutation rate pushes phenotypes to the right edge of the ED with slight
increase in the total density of phenotypes (666 292) compared to the same
ED with equal mutation rates. These effects become recognizable only when
the mutation rate to the faster side is ridiculously large—as in the case of
selective breeding in animal husbandry.
By how much does the solution change when we drop the second term in the
Taylor series expansion? With η1 = 0.05 and η2 = 0.015, we obtain ζ1 = 10−4
5
6
1.2 Evolutionary distributions in context
15
and ζ2 = 10−6 . The results with the model parameters as in Section 1.1.6
were virtually identical. With this in mind, it makes sense to pursue analysis
of the ED of the first order partial differential equation (PDE):
∂t u = r (u − ζ1 ∂x u) −
r
F (u, x) u, x ∈ (x, x)
k (x)
(1.15)
and data
u (x, 0) = g (x) (x) , u (x, t) = ux , u (x, t) = ux
where x, x, g (x), ux and ux are given. As usual, we must make sure that
u (x, 0) = g (x) , u (x, 0) = g (x) .
Later, we shall obtain an explicit solution to a flavor of this ED.
1.2 Evolutionary distributions in context
Here we put the theory of evolutionary distributions in context (Figure 1.7).
Evolutionary theory
Ecology
Evolutionary
Ecology
ED
Molecular
Genetics
Population Ecology
Population
Genetics
Genetics
Fig. 1.7: Evolutionary distributions in the context of Evolution, Ecology and
genetics.
1.2.1 Phenotypes, genotypes and natural selection
The theory of ED reflects a quintessential recognition: Natural selection works
on phenotypes, never directly on genotypes. An organism does not die directly
16
1 Motivation
because a certain genetic makeup does not agree with a certain cause of mortality; it dies directly because of a certain phenotypic trait does not agree with
a certain cause of mortality. Take for example hemophilia. Between the death
from bleeding and the hemophiliac genotype there stands a chain of events.
In broad brush terms, blood does not reach vital organs. It does not because
of bleeding. The bleeding occurs because of the inability of blood to coagulate. Blood coagulation is influenced by certain proteins whose production is
engineered by genes (one can identify many more chains, but these suffice to
make our point). So we may choose some time-scaling phenotypic trait that
reflects how fast (if at all) blood coagulates.
If we admit that death is an instantaneous event, then it occurs for one, and
only one reason. Therefore, if (i ) we have a framework to follow the dynamics
of phenotypes in a Darwinian evolutionary context and (ii ) if we can map
genotypes to phenotypes and then back to genotypes, then (iii ) the problem of
the genetic population dynamics is reduced to algebraic relationship between
it and the dynamics of ED. The mapping need not be one-to-one for we can
always use probability to map phenotypes to genotypes.
1.2.2 Evolutionary ecology and evolutionary games
Evolutionary ecology models—that is, models that integrate evolutionary processes with population ecology—usually start with
u̇ = H (x, u) u
(1.16)
where u is a vector, H is a matrix of “instantaneous fitness” functions and x is
a vector (possibly of vectors) of adaptive traits—all of appropriate dimensions.
From here on, dots denote derivatives with respect to time. To (1.16) one then
adds dynamics of the adaptive traits (called strategies)
ẋ = f (x, u) .
(1.17)
With these two equations, ecological and evolutionary dynamics become intertwined (Brown and Vincent, 1987; Abrams et al., 1993; Vincent et al.,
1993, 1996; Taylor and Day, 1997; Cohen et al., 2000b). This starting point,
sparked by the work of Maynard-Smith (1982), turns the system into an evolutionary game. The approach above has variations such as matrix games and
differential games. One important variation is the inclusion of space. This
turns a model of a coevolutionary system from ordinary differential equations
to a system of partial differential equations (see Dieckmann et al., 2000, in
particular Chapter 22).
The approach in (1.16) and (1.17)—and its relatives—triggered a profound
change in the perception of coevolution—one no longer looks to maximize
1.2 Evolutionary distributions in context
17
adaptations under constraints (essentially an optimization criterion), but
rather maximize adaptations in the context of other interacting organisms
whose adaptations are maximized by coevolution (essentially a game theoretic criterion). These developments in evolutionary ecology parallel those in
economics, where starting with Nash (1954), emphasis has shifted from optimization to game theoretic solutions of capitalistic market problems.
As an aside, there are some fundamental differences between evolutionary
games in ecology and most games in economics. In evolutionary games, the
players are not individuals, they are strategies. The players are not required
to be rational, they are not required to know the rules and they might not
even know they are playing.
The game theoretic criterion for solution (a solution concept if you like) in
evolutionary ecology is the so-called evolutionarily stable strategies (ESS),
introduced by Maynard-Smith (1982). According to this criterion, coevolving
organisms exhibit a set of genetically-based adaptations that taken together
prevent mutants from coexisting with the ESS set of phenotypes. But alas, in
the course of its development, evolutionary game theory “lost” interest in the
input of the environment to the outcome of the game (but see for example
Cohen et al., 2000a). For example, the solution to the prisoner’s dilemma game
should be quite obvious if a lynching mob is lurking outside the jail house.
When stable, ED meet the criteria of ESS and do away with the various
stability concepts that are associated with ESS. In the limited case of smooth
(dynamical) evolutionary games, ED may serve as an alternative approach
to the study of evolution by natural selection. The addition of interactions
among phenotypes, as articulated with F in (1.11) and (1.14) incorporate the
elements of games in the usual sense.
1.2.3 Stability
The justification for using the ESS concept is that without stability we are not
likely to observe the consequences of evolution in Nature. In general, stability
has been the holy grail of mathematical ecology. This is one place where we
part from traditional approaches to evolutionary ecology problems through
mathematical modeling. There are at least two reasons to object to the concept of stability in mathematical ecology. First, all species are doomed to
extinction. One needs go no further than the famous gambler’s ruin theorem
in probability theory (e.g. Chiang, 1968) to justify this statement. Heuristically, the theorem states that if one gambles against a house with unlimited
resources, then regardless of one’s winning odds, destruction is guaranteed. So
if we view evolution by natural selection as a very large number of gambling
contests between organisms (the players) and Nature, and if we admit that
18
1 Motivation
Nature has unlimited resources (in the sense that it never “dies”), then all
individuals of a species will sooner or later perish.
The second reason we object to the edification of the stability concept has to
do with the argument that “if it is not stable, you are not likely to observe
it”. Stability is an absolute concept. For the sake of argument, suppose that a
mathematical model indicates a slow decay of a population to zero. Then who
is to say that we are not likely to observe the population if it goes extinct at t
= 1020 (whatever t’s units are)? How short should time to extinction be before
we can claim that we are not likely to observe the population? Consequently,
we are not going to be overly concerned with the concept of stability. We will
content ourselves by asserting that a mathematical model of the evolutionary
process does not explode arbitrarily close to its initial conditions. With all
this in mind, stability is a useful concept. Particularly in exploring where a
system is going, even if it never gets there.
1.2.4 The origins of the theory of ED
The theory here builds on the foregoing by using the concept of ED. In fact,
stable ED correspond to ESS. In the case of ED, reaction diffusion models are
derived from first principles concerning ecological and evolutionary processes.
Reaction diffusion is a large topic both in mathematics and ecology. Smoller
(1982) and Murray (2003) are good references on the subject. The former
is more mathematically oriented than the latter. An expository account is
given in Britton (1986). Reaction-diffusion models are of considerable interest
in ecology (Segel and Jackson, 1972; Levin and Segel, 1976; Rosen, 1977;
Mimura and Murray, 1978; Okubo, 1980; Conway, 1984; Pease et al., 1989;
Dieckmann et al., 2000; Alonso et al., 2002). A somewhat related approach to
the one taken here was introduced by Slatkin (1981).
In developing the theory of ED, the influential work by Kimura (1994) must
be kept in mind. The approach Kimura and his coworkers took, however, concentrated on population genetics and they were mostly interested in deriving
moments of the distribution of genotypes.
1.2.5 The ordinary differential equations approach
Equations (1.16) and (1.17) represent a large class of models in ecology and
epidemiology. We shall refer to these as the ordinary differential equations
approach, or ODE models. There are several assumptions implicit in models
that belong to this class: (i ) reproduction is by cloning; (ii ) u is large; (iii )
when strategy is included (as in 1.17), u represents some moment of the
1.2 Evolutionary distributions in context
19
dynamics (usually the mean with respect to x) of u (x, t); (iv ) stochastic
effects can be faithfully represented by u; and (v ) u are smooth functions of
x and t. Some of these assumptions were discussed by Dieckmann et al. (1995),
Doebeli and Ruxton (1997), Law et al. (1997) and Geritz and Kisdi (2000). In
practice, conclusions from ODE models routinely violate the assumptions or
are stated without regard to the assumptions. For example, some applications
of (1.16) produce negative population densities; this is particularly true when
oscillations are involved. To circumvent such problems, authors—explicitly or
implicitly—enlarge this class of models to
f (u (t)) if u (t) ≥ ε
u̇ (t) =
, u (0) = f0
(1.18)
0
otherwise
where ε > 0. This is a class of non-smooth (or even discontinuous) functions
and care must be taken in solving (1.18).
To proceed from (1.16) and (1.17), one usually identifies a set of adaptive
traits and writes (1.17) as
u̇ (xi , x, t) = f (u (xi , x, t)) ,
u (0) = f0
(1.19)
where now xi is the set of adaptive traits of species i. The dimension of xi
is mi . Here x is a setPof real numbers of all adaptive traits of all species
n
with dimension m := i=1 mi . Next, the dynamics of x (called the strategy dynamics) may be isolated by several methods, each involving additional
assumptions (see Murray, 2003; Weibull, 1995; Fudenburg and Levine, 1998;
Hofbauer and Sigmund, 1998; Samuelson, 1998; Gintis, 2000; Cressman, 2003;
Hofbauer and Sigmund, 2003; Vincent and Brown, 2005). For example, in the
case of adaptive dynamics (Dieckmann and Law, 1996; Champagnat et al.,
2001), the additional assumptions are: (i ) mutations are rare and at small
time intervals the probability that more than one mutation occurs is of order
zero; (ii ) x is a moment (usually mean) of some distribution of phenotypes.
Without implicitly assuming some distribution of phenotypes and encapsulating the distribution in x, one cannot observe strategy dynamics. Therein lies
the rub (see below). The nice thing about the adaptive dynamics approach is
that with considerations from measure theory (Doob, 1994), the derivation of
the strategy dynamics justifies the fact that no matter how large the population, it still can be viewed as a collection of individuals (Dieckmann and Law,
1996). Furthermore, in analyzing predator-prey coevolution, Dieckmann et al.
(1995) showed that with some level of stochasticity, deterministic models (i.e.,
where u is the mean path) do represent the mean of the stochastic path.
Other approaches to deriving the strategy dynamics (e.g. Abrams et al., 1993)
involve the assumption that population densities change much faster than
strategy values. Therefore, one assumes that the populations are at equilibrium and only strategy dynamics need to be considered. This assumption is
circumvented by the approaches that Abrams (1992) and Vincent and Brown
20
1 Motivation
(2005) take (see also Cohen et al., 2000b), where population dynamics need
not be ignored. Vincent and Brown (2005) refer to the strategy dynamics with
the related population dynamics as “Darwinian dynamics”.
Regardless of the approach one takes, strategy dynamics usually take on the
form
ẋi (t) = gi (v, x, t) , x (0) = x0 , i = 1, . . . , n.
(1.20)
Here gi is a vector valued function (usually a gradient of some other function)
of dimension mi and v is a vector (of dimension mi ) of the so-called virtual
strategies. To derive (1.20) from (1.19), one needs to add the assumptions
that f and g are at least twice differentiable. These assumptions are required
because one needs to examine special points (e.g., maxima and minima) on
the strategy dynamics.
The basic achievement in deriving gi is that it gives invasion-ability criteria.
e, that result in negative
Specifically, from gi we derive values of x, call them x
e (with respect to u). It should be
gradient on gi for all i and for all x 6= x
noted that in the case of Darwinian dynamics, (1.20) becomes
ẋi (t) = gi (v, x, u, t) , x (0) = x0 ,
i = 1, . . . , n
(1.21)
and the strategy dynamics can no longer be divorced from the population
e on the dynamics of all other values of
dynamics. Because of the effect of x
e the ESS of the system (1.20) or the system (1.19) and (1.21). So
x we call x
far, the strategies are assumed unbounded. Bounded strategy spaces introduce
e is in the interior of
complications that can be avoided if one assumes that x
the strategy space.
At any rate, the same violations or misuse of the assumptions behind (1.16)
apply to evolutionary models, with one crucial addition: Behind each x hide
distributions of the density of phenotypes of u. In fact, the dynamics of x
represent the dynamics of moments of these distributions (usually the mean).
This raises the possibility that we may be following dynamics of phenotypes
whose distribution can hardly be represented by their mean. Worse yet, we
may be following the dynamics of phenotypes that do not exist (Figure 1.8).
In fact, we can change the parameter values in the example with competition
(Section 1.1.6) such that all surviving phenotypes are on the boundaries. The
mean of the surviving phenotypes, as evolutionary games would have it, is in
a place that no phenotypes exist! Furthermore, because ESS is an outcome
e cannot be determined by traditional stability
of a game, the stability of x
analysis (except for the case of Darwinian dynamics). Hence, the proliferation
of stability criteria for ESS (Eshel, 1996; Apaloo, 1997; Taylor and Day, 1997).
By examining the stability of ED, some of the ESS related issues can be
simplified and enriched: (i ) stability of ED satisfies the ESS criterion; (ii ) ED
bypass the potential problems that arise when strategy dynamics are followed
by some distribution moments; (iii ) for fixed point stability, ESS requires that
21
u ( x, t )
1.2 Evolutionary distributions in context
x
^x
x
Fig. 1.8: A bounded distribution (between x and x) of phenotypes at time
t. Note that there are no phenotypes at the mean adaptive trait, x
b.
The figure illustrates a snapshot of some ED at t.
the fitness of all species participating in the ESS be zero whereas ED admit
any finite value of fitness at stability; (iv ) the ODE approach either assumes
a number of species or obtains them from the solution of the game whereas
the ED approach is infinite dimensional; (v ) using the machinery of PDE,
ED can deal with solutions that are more general—in a sense to be clarified
later—than the ODE approach (in particular, the idea of weak solutions); (vi )
a related issue is that ED (through PDE) can sometimes deal with solutions
in which continuity of solutions breaks down or the initial conditions are not
continuous. Yet, ED do suffer from limitations which will be pointed out as
we proceed. Some of these limitation are common to the ODE approach.
For example, we do require large populations so that it remains valid to use
derivatives. Other limitations relate to the complexity and richness of the
behavior of (even linear) PDE and to the fact that a comprehensive theory of
PDE is not going to be available any time (if ever) soon.
Finally, we emphasize that for us, the “stuff” of evolution is not species. It is
a distribution of phenotypes in the adaptive space. For example, one may be
tempted to identify two species in Figure 1.8. Yet, here we are, over 150 years
since Darwin’s time, and we are yet to come up with a satisfactory definition
of species, which is one of the most fundamental concepts in all of biology.
So rather talk about species, we talk about types. The latter are identified
as intervals around special points on the ED. Such intervals may indicate
reproductive isolation of phenotypes.