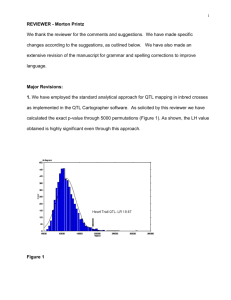
AN ABSTRACT OF THE THESIS OF
advertisement

AN ABSTRACT OF THE THESIS OF Theerayut Toojinda for the degree of Doctor of Philosophy in Crop Science presented on December 9, 1998. Title: Mapping and Introgression of Disease Resistance Genes in Barley (Hordeum vulgare L.). Redacted for privacy Abstract approved: Patrick M. Hayes Molecular tools, coupled with unique germplasm stocks and rigorous phenotyping, are useful for developing a better understanding of qualitative and quantitative disease resistance genes in plants. The identification of molecular markers linked to all types of resistance genes provides opportunities for implementing a range of resistance breeding strategies, ranging from gene pyramiding to gene deployment. This thesis consists of two chapters. The first describes a disease resistance gene mapping effort and the second describes a disease resistance gene introgression effort. The number, location, and effects of genes determining resistance to stripe rust, leaf rust and Barley Yellow Dwarf Virus were determined using a population of doubled haploid (DH) lines from the cross of Shyri x Galena. Resistance to leaf rust was qualitatively inherited, and the locus was mapped to the long arm of chromosome 1. Resistance to stripe rust and BYDV was quantitatively inherited. Multiple QTLs were detected for each type of resistance. The principal stripe rust resistance QTL was on the short arm of chromosome 5 and the principal BYDV resistance QTL was on the long arm of chromosome 1, linked in repulsion phase with the leaf rust resistance gene. Additional QTLs and QTL x QTL interactions were detected. The majority of the qualitative and quantitative resistance loci detected in the Shyri x Galena population coincided with Resistance Gene Analog Polymorphisms (RGAPs) mapped in the same population. These RGAPs were based on degenerate primers derived from cloned resistance gene sequence motifs. These associations should be useful for efficient resistance gene mapping and provide an approach for ultimately isolating and describing quantitative and qualitative resistance genes. The second chapter describes a molecular marker assisted selection (MMAS) effort to introgress stripe rust resistance QTLs on chromosomes 4 and 7 into susceptible germplasm. DH lines were derived form a MMAS backcross-one (BC-1) population, extensively phenotyped for stripe rust resistance, and genotyped for the introgressed QTLs and background genome. The resistance QTLs that were introgressed were significant determinants of resistance in the new genetic background. Additional resistance QTLs were also detected. Together, these chapters describe an integrated approach to disease resistance gene characterization and utilization. Mapping and Introgression of Disease Resistance Genes in Barley (Hordeum vulgare L.) by Theerayut Toojinda A THESIS submitted to Oregon State University In partial fulfillment of the requirement for the degree of Doctor of Philosophy Presented December 9, 1998 Commencement June 1999 Doctor of Philosophy thesis of Theerayut Toojinda presented on December 9, 1998 APPROVED: Redacted for privacy Major Professor, representing Crop fence Redacted for privacy ead of Department of Crop and Soil Science Redacted for privacy can of Gradua 1 chool I understand that my thesis will become part of the permanent collection of Oregon State University libraries. My signature below authorizes release of my thesis to any reader upon request. Redacted for privacy Theerayut Toojinda, Author ACKNOWLEDGEMENTS I would like to thank my major professor, Dr. Patrick M. Hayes, for his advice, encouragement and support during my Ph.D. program. I thank the members of my graduate committee, Dr. Steve J. Knapp, Dr. Christopher E. Mundt, Dr. Tony Chen, Dr. Hugo Vivar, Dr. Warren Kronstad, and Dr. Leslie Fuchigami for their advice and assistance. I would also like to thank my colleagues at OSU, CIMMYT and Scottish Crop Research Institute (SCRI) for their help and friendship. I would like to thank the Barley Project at Oregon State University for sponsoring my Ph.D. studies. I would like to thank the North American Barley Genome Mapping Project, and the Oregon, Idaho, and Washington Barley Commissions for their support of my research. Many thanks to Dr. Somvong Tragoonrung, Dr. Chalermlarb Chuiprasit, Ajan Anong Junsrikul and all my dear friends at LARTC for encouraging me to pursue this degree. Most of all, I extend special thanks to my father (Prajob Toojinda), my mother (Sawang Toojinda), my aunt (Samran Juntanee) and my sisters (Panjaree, Busaba and Katekanda Toojinda), not only for love and understanding, but also for their constant support and encouragement. Finally, I would like to express my appreciation to my wife, Kanokrat Tiyapun, for her love, patience, and companionship. CONTRIBUTION OF AUTHORS This dissertation has benefited from advice and suggestions kindly offered by Dr. Patrick M. Hayes, my major professor and advisor. Dr. Steve J. Knapp, Dr. Christopher E. Mundt, Dr. Tony Chen and Dr. Hugo Vivar provided detailed critiques of a draft of this dissertation. Dr. Patrick M. Hayes proposed the subject of this research and was involved in all genetic and statistical analysis. Dr. Hugo Vivar and his colleagues at CIMMYT were involved in phenotype data collection. Dr. A. Kleinhofs and D. Kudrna were involved in generating RFLPs. Xianming Chen was involved in generating RGAPs. John Korte was involved in providing all materials and expertise needed in the lab and also helped in development of the SSR and RGA techniques used in these experiments. TABLE OF CONTENTS Page CHAPTER 1. INTRODUCTION Molecular Markers 1 1 Quantitative and Qualitative Disease Resistance 4 Molecular Breeding 6 CHAPTER 2. ASSOCIATION OF QUANTITATIVE AND QUALITATIVE DISEASE RESISTANCE GENES IN BARLEY (Hordeum vulgare) WITH RESISTANCE GENE ANALOG POLYMORPHISMS (RGAPs) 8 Abstract 9 Introduction 9 Materials and Methods Plant Material Genotyping and Map Construction Disease Resistance Phenotyping Stripe Rust Leaf Rust Barley Yellow Dwarf Virus (BYDV) QTL Analysis Results and Discussion 13 13 13 18 18 19 20 20 21 Linkage Map Construction 21 QTL Detection 22 References 38 TABLE OF CONTENTS (Continued) Page CHAPTER 3. INTROGRESSION OF QUANTITATIVE TRAIT LOCI (QTLs) DETERMINING STRIPE-RUST RESISTANCE IN BARLEY: 45 AN EXAMPLE OF MARKER-ASSISTED LINE DEVELOPMENT Abstract 46 Introduction 46 Materials and Methods 50 Germplasm Genotyping Phenotyping 50 52 55 Data Analysis 56 Results 58 Discussion 64 References 71 CHAPTER 4. CONCLUSION 74 BIBLIOGRAPHY 78 LIST OF FIGURES Page Figure 2.1a 2.1b 2.1c Linkage maps of chromosomes 1, 2, and 3 based on 94 DH progeny from the cross of Shyri x Galena 16 Linkage maps of chromosome 4, 5, and 6 based on 94 DH progeny from the cross of Shyri x Galena 17 Linkage maps of chromosome 7 based on 94 DH progeny from the cross of Shyri x Galena 18 Average stripe-rust severity (%) in the DH progeny of Shyri x Galena cross 22 SIM test statistic from the QTL analysis of stripe-rust severity (%) on chromosome 2 and 3 in DH population of Shyri x Galena 24 SIM test statistic from the QTL analysis of stripe-rust severity (%) on chromosome 5 and 6 in DH population of Shyri x Galena 24 Mean and 95% LSD intervals for the two locus interactions between the QTLs on chromosomes 2 and 5 determining stripe-rust severity (%) 27 Mean and 95% LSD intervals for the two locus interactions between the QTLs on chromosomes 2 and 6 determining stripe-rust severity (%) 28 2.7 Average leaf-rust index in the DH progeny of Shyri x Galena 30 2.8 SIM test statistic from the QTL analysis of leaf-rust index on chromosome 1 in the DH population of Shyri x Galena and showing the coincident QTL peak and position of the Rphx, locus 31 Plant height reduction (cm) after infection with the BYDV-MAV serotype in the DH progeny of Shyri x Galena 32 Dwarfness score (0-9) in the DH progeny of Shyri x Galena infected with the BYDV-MAV serotype 33 Tillering score (0-9) in the DH progeny of Shyri x Galena infected with BYDV-MAV serotype 33 2.2 2.3 2.4 2.5 2.6 2.9 2.10 2.11 LIST OF FIGURES (Continued) Figure 2.12 2.13 2.14 2.15 3.1 3.2 3.3 Page Dwarfness score (0-9) in the progeny of Shyri x Galena infected with the BYDV-PAV serotype 34 Tillering score (0-9) in the progeny of Shyri x Galena infected with the BYDV-PAV serotype 34 SIM test statistics from the QTL analysis of plant height reduction (cm), dwarfness score, and tillering score after infected with the BYDV-MAV and BYDV-PAV serotypes on chromosome 1 in DH population of Shyri x Galena 35 SIM test statistics from the QTL analysis of plant height reduction (cm), dwarfness score, and tillering score after infection with the BYDV-MAV and BYDV-PAV serotypes on chromosome 4 and 5 in DH population of Shyri x Galena 36 Derivation of double haploid (DH) germplasm from a marker-assisted selection program for adult plant stripe rust resistance 51 Average stripe rust disease severity (%) in DH lines derived from one cycle of marker-assisted backcrossing using BRB41 and Steptoe as the donor and recurrent parents, respectively 58 Percentage of donor parent genome in DH lines derived from one cycle of marker assisted backcrossing as measured by 120 markers 63 LIST OF TABLES Table 2.1 2.2 Page Resistance Gene Analog (RGA) primers used to develop Resistance Gene Analog Polymorphism (RGAP) markers for the Shyri x Galena cross 15 Chromosomal location, allele phase, and effect (expressed as percentage of phenotypic variance explained) for stripe rust, leaf rust, and BYDV resistance loci in the Shyri x Galena population 25 Percentage of phenotypic variance explained (R2p) in multilocus models involving main effect QTLs and their interactions for stripe rust and BYDV resistance in the Shyri x Galena population 29 Chromosome location, slope, p-value, and R2 for markers significantly associated with stripe rust severity in single locus regression. Chromosome locations in italics are putative 60 Markers with significant effects in multi-locus regression models of stripe rust severity in individual environments and in the average of four environments 61 Average stripe rust severities in 1995 and 1996 for the ten most resistant and susceptible doubled haploid lines derived from one cycle of marker assisted selection 62 the Rep 2.3 3.1 3.2 3.3 MAPPING AND INTROGRESSION OF DISEASE RESISTANCE GENES IN BARLEY (Hordeum vulgare) CHAPTER 1 INTRODUCTION Molecular markers are tools for simultaneously advancing our understanding of plant genome organization and increasing the efficiency of plant breeding. Quantitatively and qualitatively inherited disease resistance genes are logical targets for applying molecular marker technologies because these genes are of tremendous economic importance. While recent advances have revealed commonalties in cloned qualitative resistance genes, little is known about the structure and function of quantitative resistance genes. However, quantitative resistance is attractive from the standpoint of probable durability. Barley is an excellent model system for molecular marker analysis. This diploid species has seven cytologically distinct chromosomes and a haploid genome size of approximately 5 x 109 by (Bennet and Smith, 1976). The economic importance of the crop makes it a model system of practical utility. Molecular Markers Molecular markers are abundant and phenotypically neutral. As a consequence, molecular markers have been used in an array of organisms, including crop plants in general, and barley in particular, for DNA fingerprinting (Bassam and Bentley 1994; Becker et al. 1995), phylogenetic analysis (Saghai-Maroof et al. 1995), genome mapping (Hayes et al. 1996), map-based cloning (Zhang et al. 1994; Tanksley et al. 1995; Dean and Schmidt 1995; Kilian et al. 1997; Bennetzen and Freeling 1997), candidate gene 2 analysis (Touzet et al. 1995), and markerassisted breeding (Mazur and Tingey 1995; Han et al. 1997; Toojinda et al. 1997). With particular reference to traits showing complex inheritance, molecular markers can be used to dissect the genetic basis of complex phenotypes into Mendelian components in order to obtain information about gene dosage, epistasis, pleiotropy and genotype x environment interaction (Hayes et al. 1993; Bezant et al. 1997; Mather et al. 1997). Information regarding the number, effect, and chromosomal location of genes determining a phenotype is of immediate practical utility. This information can be used to design matings and to select target genotypes in segregating progeny. In barley, QTL analysis has moved beyond the descriptive phase to validation (Hayes et al. 1996) and use in cultivar development (Toojinda et al. 1997). The four most common types of markers used in applied plant genomics in general, and implemented in barley, are Restriction Fragment Length Polymorphisms (RFLPs), Random Amplified Polymorphic DNAs (RAPDs), Amplified Fragment Length Polymorphisms (AFLPs), and Simple Sequence Repeats (SSRs). RFLPs have been the mainstay of North American Barley Genome Mapping Project (NABGMP) and other maps (Kleinhofs A. 1991; Heun et al. 1991; Graner et al. 1991). RFLPs are based on the differential hybridization of cloned DNA to DNA fragments in a sample of restriction enzyme- digested DNA. The marker is specific to a single clone/restriction enzyme combination. RFLPs are usually codominant and often multiallellic. They are excellent framework markers, as they have locus identity. They are excellent markers for comparative mapping, across divergent taxonomic groups, such as barley: wheat, barley:rice, etc. However, RFLP are tedious and inefficient to assay. 3 RAPD markers are based on the differential PCR amplification of a random sample of DNA with short oligonucleotide sequences. RAPDs are usually dominant. The primary advantages of RAPDs are simplicity and cost, and have been used widely in barley genome mapping (Barua et al. 1993; Giese et al. 1994; Noli et al. 1997; Dahleen et al. 1997). However, these advantages can be outweighed by low reproducibility. Furthermore, RAPDs do not have defined locus identity, and it can be difficult to relate RAPD loci between different experimental populations of the same species. AFLP markers are generated by a combination of restriction digestion and PCR amplification. They are visually dominant, biallelelic and extremely high throughput. AFLPs have been useful in developing several barley linkage maps (Becker et al. 1995; Powell et al. 1997; Qi et al. 1998) and for whole genome screening in marker-assisted selection experiments. A principal drawback to AFLPs is that the assay is time consuming, as it is based on sequencing protocols. The issue of locus identity has been explored with AFLPs (Waugh et al. 1997; Qi et al. 1998). However, identity needs to be established on a case by case basis. An additional drawback to AFLPs is that they are reported to cause map expansion (Becker et al. 1995). Linkage map expansion is usually attributable to poor quality data. SSR markers are a molecular marker based on the PCR amplification of tandem repeats of one to six nucleotide motifs. The polymorphism among individuals is due to the variation in the number of repeat units. SSR markers are codominant, often multiallelic which allow the unambiguous identification of alleles. The multi-allelism can be impressive (Maroof et al. 1994; Russell et al. 1997). SSRs are excellent 4 framework markers (Becker and Heun 1995; Liu et al. 1996) with locus identity. They can be multiplexed to achieve higher throughput (Mitchell et al. 1997). Two disadvantages of SSRs are the high cost of discovery and the lack of transferability across genera and even distantly-related species (Roder et al. 1995). Quantitative and Qualitative Disease Resistance Disease resistance is commonly the result of a biochemical pathway from perception to defense response (Beynon 1997). H.H. Flor (1955) demonstrated the interaction of host and pathogen genes in determining resistance and susceptibility in what is known as the gene-for-gene concept. J.E. Vanderplank introduced the ideas of horizontal and vertical resistance (Thresh 1998). However, the genetic basis and relationship between genes determining vertical (qualitative) resistance and those determining horizontal (quantitative) resistance is still unclear. Advances in molecular biology are revealing that many qualitative resistances following the gene-for-gene systems are actually quite complex (Crute 1985; Jorgensen 1992). On the other hand, only one or few loci have been shown to determine quantitative resistance (Parlevliet 1978; Geiger and Heun 1989; Chen et al. 1994; Thomas et al. 1995). The genes that are determinants of quantitative resistance, and quantitative phenotypes in general, are termed quantitative trait loci (QTLs). QTL analysis has been used for dissecting quantitative characters into discrete genetic loci (Tanksley 1993; Wang et al. 1994; Thomas et al. 1995; Powell et al. 1997) and for defining the roles of individual genes in quantitative disease resistance (Hayes et al. 1996). To date, the primary outcomes of QTL resistance mapping projects have been informed on the number and location of principal determinants (Steffenson et al. 1996; Moharramipour et al. 1997). The 5 availability of reference linkage maps is allowing for systematic intra-specific mapping of resistance genes and comparative mapping of resistance genes in related taxa (Young 1996). These efforts will ultimately lead to a clearer picture of gene number, relationship, structure, and function. With particular reference to qualitative resistance genes, genes that are induced in response to pathogens have been identified in several host:pathogen systems (Ferreira et al. 1995; Lefebvre and Palloix 1996). In many cases, genes conferring resistance to different specificities of the same pathogen, or resistance to different pathogens, are found in clusters (Michelmore 1995; Ellis et al. 1998). This led to the hypothesis that some resistance genes are related in function and evolution and that individual member of these multigene families diverged to confer different specificities (Michelmore et al 1987; Pryor 1987). The presence of common motifs among cloned genes the leucine rich repeat (LRR), nucleotide binding sites (NBS), serine/threonine kinase- supports this hypothesis. Based on structure, disease resistance genes can be classified into five groups: detoxifying enzymes, kinases, NBS/LRR proteins, extracellular receptors and receptor kinases (Lamp 1994; Dangl 1995; Martin 1996; Buschges et al. 1997). Although many candidate disease resistance genes have been identified, it has been difficult to demonstrate that these are truly defense genes (Bowles 1990). The candidate gene approach combining genome mapping and sequence information to integrate molecular analysis of host-pathogen interactions and gene mapping (Concibido et al. 1996; Dahleen 1997; Han et al. 1997). The conserved motifs of cloned resistance genes have been used to design degenerate primers known as "Resistance Gene Analogs" (RGAs) for isolating unknown disease resistance gene homologous in different plant 6 taxa. The RGA approach has proven to be an efficient tool for isolating resistance genes (Kanazin et al 1996; Yu et al 1996; Leister et al.1996; Marek and Shoemaker RC; Leister et al.1998). The RGA approach can also be used to generate Resistance Gene Analog Polymorphisms (RGAPs) for linkage mapping in populations (Chen et al.1998). Linkage or coincidence of RGAPs with resistance genes would support the hypothesis that these polymorphisms are revealing disease resistance loci. To date, these molecular tools have not been focussed on quantitative disease resistance. Molecular Breeding Molecular markers can be used to increase the efficiency with which qualitative and quantitative disease resistance genes are manipulated in breeding programs (Horvath et al. 1995; Toojinda et al. 1997). Backcrossing, a technique that has been used extensively to introgress disease resistance loci into adapted backgrounds, can be fraught with linkage drag (Tanksley et al. 1989). Molecular markers can increase the efficiency of the process in several ways. Flanking markers can be used to identify the backcross lines that are heterozygous for resistance loci regions. Advancing only these selected lines will also have the effect of reducing linkage drag (Young and Tanksley 1989; Tanksley and Nelson 1996). Singlecopy, or low copy, markers with defined map locations, such as RFLPs, SSRs, and RGAPs, are ideal for this step. Molecular markers could also increase the efficiency of backcrossing by allowing for selection of genotypes with maximum percentage of the recurrent parent genome. Markers with higher information content per reaction, such as AFLPs and RAPDs, are ideal for this step (Waugh et al. 1997). However, manipulation of QTLs can be 7 problematic due to loss of target loci through recombination, incorrect information regarding the location of the QTLs, and/or negatively altered expression of the QTLs in new genetic background (Hayes et al. 1996). Therefore, a marker-assisted QTL backcrossing scheme might (1) use flanking markers to select progeny with a probability of earring the target QTL allele (s), (2) confirm the target phenotype in the selected progeny, and (3) use multiplex markers to identify those selections with the maximum percentage of the recurrent parent genome. A series of experiments were conducted to integrate molecular markers, qualitative and quantitative disease resistance, and molecular breeding. These experiments were conducted in the context of the very serious stripe rust problem facing barley growers from the Pacific Northwest of the United States to the Andean region of South America. Quantitative resistance may be the best tool for dealing with stripe rust, caused by Puccinia striiformis f. sp. hordei. The first chapter of this thesis describes mapping qualitative and quantitative disease resistance loci using a range of molecular markers, including RFLPs, AFLPs, SSRs, and RGAPs. The second chapter describes markerassisted introgression of QTLs determining stripe rust resistance. 8 CHAPTER 2 ASSOCIATION OF QUANTITATIVE AND QUALITATIVE DISEASE RESISTANCE GENES IN BARLEY (Hordeum vulgare) WITH RESISTANCE GENE ANALOG POLYMORPHISMS (RGAPs) Theerayut Toojinda, Xianming Chen, Patrick M. Hayes, Hugo Vivar A. Kleinhofs 9 Abstract Stripe rust, leaf rust, and Barley Yellow Dwarf Virus (BYDV) are important diseases of barley (Hordeum vulgare L). Using 94 doubled haploid lines (DH) from the cross of Shyri x Galena, multiple disease phenotype data sets, and a 99-marker linkage map, we determined the number, genome location, and effects of genes conferring resistance to these diseases. We also demonstrated associations of these resistance genes with Resistance Gene Analog Polymorphism (RGAP) loci based on degenerate motifs of cloned disease resistance genes. Leaf rust resistance was determined by a single gene on chromosome 1. QTLs on chromosomes 2, 3, 5, and 6 were the principal determinants of resistance to stripe rust. Two-locus QTL interactions were significant determinants of resistance to this disease. Resistance to the MAV and PAV serotypes of BYDV was determined by coincident QTLs on chromosomes 1, 4, and 5. QTL interactions were not significant for BYDV resistance. The associations of RGAPs with qualitative and quantitative resistance loci provide a powerful tool for efficiently mapping disease resistance genes. These relationships should be useful in answering fundamental questions regarding quantitative and qualitative disease resistance genes. Introduction The genetic resistance-of plants to diseases is an area of intense and important research activity. Genetic resistance is the most cost-effective and environmentally appropriate approach to disease management. Plant breeders have, in general, made excellent use of genetic resistance. Disease resistance breeding remains a principal objective of many breeding programs. While genetic variation for disease resistance to many diseases is still available within the cultivated germplasm pool of many crop species, 10 in many cases restricted genetic variance has led to searches for new resistance genes in crop ancestors and relatives (Conner et al. 1989; Ordon and Friedt, 1993; Reuvein et al. 1997; Veremis et al. 1997). Introgression of exotic genes is an expensive and difficult process (Hoffbeck et al. 1995; Tanksley and Nelson 1996). Resistance genes are, accordingly, precious commodities. In this context, the durability of resistance is of great importance. While durability can only be demonstrated in hindsight, theory and some historical evidence support the contention that quantitative resistance is often more durable than qualitative resistance (Browning et al. 1977; Johnson 1981; and Line 1993). Qualitative resistance genes have been extensively studied in terms of genome location (Giese et al. 1993; El-Kharbotly et al. 1994; Graner and Tekauz 1996), specificity (Thomas et al. 1995; Ori et al. 1997), and most recently structure and function (Lamp 1994; Martin 1996; Dangl 1995; Buschges et al. 1997). Until the recent development of quantitative trait locus (QTL) analysis tools, the study of quantitative resistance genes focused on biometrics and epidemiology. QTL tools allow for the systematic dissection of quantitative resistance into estimates of locus number, location, effect, and interaction (Michelmore 1995; Young 1996; Powell et al. 1997). Disease resistance QTLs have been described for a number of host pathogen systems (Williamson et al. 1994; El-Kharbotly et a. 1994; Maisonneuve et al. 1994; Zaitlin et al. 1993), including barley (Barua et al. 1993; Giese et al. 1993; Graner and Bauer 1993; Chen et al. 1994; Hayes et al. 1996). However, the structure and function of quantitative resistance genes is still a matter of conjecture. They could represent the effects of alternative alleles at qualitative resistance loci (Dingerdissen et al. 1996) or they could represent an entirely different class of genes (Pryor and Ellis 1993). 11 Advances in molecular biology are revealing that many "simple" gene-for-gene systems are actually quite complex (Crate, 1985; Jorgensen, 1992). QTL studies are revealing in many systems that one or a few loci are principal determinants of trait expression (Thomas et al. 1995; Chen et al. 1994). The availability of reference linkage maps is allowing for systematic intra-specific mapping of resistance genes and comparative mapping of resistance genes in related taxa, and these efforts will ultimately lead to a clearer picture of gene number, relationship, structure, and function. Genes conferring resistance to different specificities of the same pathogen, and to different pathogens, are known to cluster in a range of plants (Saxena et al. 1968; Giese et al. 1981; Hooker 1985; Paran et al. 1991; Michelmore 1995; Ellis et al. 1998). These clusters are particularly dynamic regions of the genome, although the mechanisms leading to variation are still a matter of debate (Borst and Greaves, 1987; Pryor 1987; Pryor and Ellis, 1993). The discovery of common motifs in cloned resistance genes leucine rich repeats (LRR), nucleotide binding sites (NBS), serine/threonine kinase has served as a basis for a generalized approach to resistance gene analysis. Degenerate primers based on these motifs can be used to amplify specific genomic DNA sequences known as resistance gene analogs (RGAs). The RGA approach has proven to be an efficient tool for isolating resistance genes (Kanazin et al 1996; Yu et al 1996; Leister et al 1996 ; Leister et al. 1998). The technique can also be used to generate polymorphisms (RGAPs) for linkage mapping in populations (Chen et al. 1998). Qualitative and quantitative resistance to rust fungi (Puccinia sp.) has been an area of extensive study in the Triticeae. In barley (Hordeum vulgare L.), there is an especially rich literature on resistance to leaf rust (Puccinia hordei) (Alemayehu and Parlevliet 1996; 12 Feuerstein et al. 1990; Jin et al. 1996; Qi 1998). The impetus for this research was the fact that race-specific qualitative resistance genes lacked durability to this pathogen of worldwide importance. There is less information on the basis of genetic resistance to stripe rust (Puccinia striiformis, fsp. hordei). Due to the relatively recent arrival and importance of this pathogen in the Americas, we have been systematically mapping resistance in a range of germplasm (Chen et al. 1994; Hayes et al. 1996). Barley Yellow Dwarf Virus (BYDV) is an aphid-vectored luteovirus of worldwide importance (Harder and Harber 1992; D'Arcy 1995; Collin et al. 1996). These diseases tend to be episodic in response to environmental conditions, but in any given environment at least one disease is usually a principal production constraint. In many situations, the use of cultivars resistant to multiple diseases is a necessity. Thus, the ideal variety should be resistant to multiple diseases, assuming resistance genes per se do not have a cost. Development of such varieties can be expedited by information on the number, location and effect of the determinants of resistance. We reasoned that if many disease resistance genes contain conserved motifs, then the RGAP approach could serve as tool for efficient mapping of multiple resistance genes in a single population. A portion of the total number of RGAPs scored in a linkage mapping population should correspond to resistance genes. Furthermore, if quantitative resistance genes are related to qualitative resistance genes, then resistance QTLs should be detected in association RGAPs. To test these hypotheses, we mapped RGAPs in a doubled haploid population of barley, together with a qualitative resistance gene conferring resistance to leaf rust (caused by the fungus Puccinia hordei), QTLs associated with resistance to stripe rust (caused by the fungus Puccinia striifarmis fsp. hordei), and QTLs 13 associated with resistance to two serotypes (MAV and PAV) of Barley Yellow Dwarf Virus (BYDV). Materials and Methods Plant Material One hundred doubled haploid (DH) lines were derived from the F1 of the cross Shyri/Galena, using the Hordeum bulbosum technique, as described by Chen and Hayes (1989). Shyri is a two-rowed feed barley developed by ICARDA/CIMMYT (Mexico) and released by INIAP (Ecuador). Shyri is a source of resistance to a range of diseases, including stripe rust, leaf rust, scald and net blotch. Galena is proprietary two-rowed malting barley belonging to the Coors Brewing Company Inc. Genotyping and Map Construction Ninety-four of the DH lines were genotyped with 41 Restriction Fragment Length Polymorphism (RFLP), 51 Simple Sequence Repeat (SSR), 562 Amplified Fragment Length Polymorphism (AFLP), 144 Resistance Gene Analog Polymorphism (RGAP) markers, and one morphological marker. The RFLP marker nomenclature follows that employed by the North American Barley Genome Mapping Project (Kleinhofs et al., 1993; Hayes et al., 1996). RFLPs were assayed as described by Chen et al. (1994) and Kleinhofs et al. (1993). Two sources of SSRs were used: database-derived repeats (described by Becker and Heun 1995; Liu et al. 1996) and repeats derived from an enriched genomic library. Fifty-one SSR markers (designated as BMAC, EBMAC, HVM, HVC, HVP, HTT plus an arbitrary number) were assayed as described by Morgante et al. (1994); Liu et al. (1996) and Russell et al. (1997). 14 The AFLP assays were performed using 16 Pstl/Msel and 16 EcoRl/Msel primer combinations as described by Zabeau and Vos (1993). A total of 562 AFLP markers (designated as E M for EcoRI /Msel, T M for Pstl/Msel) were scored. The RGAP markers were generated with a set of degenerate primers derived from resistance gene homologs (Table 2.1) as described by Chen et al. (1998). PCR amplification was performed in a Perkin Elmer 9600 thermal cycler. The reaction mixture and the polyacrylamide gel electrophoresis procedures were as described by Liu et al (1996) and Chen et al (1998). The morphological marker, rachilla hair length (mSrh), was scored under a dissecting microscope. The eight-hundred and nine markers were used for linkage map construction. The base map (Figure 2.1) was constructed using a subset of the total markers to achieve a target interval distance of 10-15 cM. The procedure for constructing the base map was as follows. Markers in common with published maps were retained, when possible, in order to facilitate map integration. Markers showing significant segregation distortion, markers with missing observations, and markers causing map expansion were discarded. Linkage analysis was performed on the remaining subset of 138 markers using Gmendel 3.0 (Hollaway and Knapp, 1994). Linkage groups were first calculated using a maximum allowable recombination percentage (rmax) of 0.25 and a LOD score of 7. Cosegregating and tightly linked markers were then dropped. The markers with the most complete data were retained. Linkage groups were then calculated using LOD 3.8 and rmax 0.35. Marker order was checked by Monte Carlo and Bootstrap simulations, using annealing temperatures of 300 inner and 200 outer. 15 Table 2.1 Resistance Gene Analog (RGA) primers used to develop Resistance Gene Analog Polymorphism (RGAPs) markers for the Shyri X Galena cross. Primer' Sequence (5' - 3')° No. of polymorphic markers LM637 LM638 ARIGCTARIGGIARICC GGIGGIGTIGGIAAIACIAC NBS -F1 GGAATGGGNGGNGTNGGNAARAC NBS-R1 YCTAGTTGTRAYDATDAYYYTRC NLRR-for TAGGGCCTCTTGCATCGT NLRR-rev TATAAAAAGTGCCGGACT NLRR-IN1 TGCTACGTTCTCCGGG NLRR-IN2 TCAGGCCGTGAAAAATAT Nkin2 GTAACTAAGGATAGA Nploop TCAATTAATGTTTGAGTTATTGTA Ptokinl GCATTGGAACAAGGTGAA Ptokin3 TAGTTCGGACGTTTACAT Ptokin2 AGGGGGACCACCACGTAG Ptokin4 AGTGTCTTGTAGGGTATC PtoFen-S ATGGGAAGCAAGTATTCAAGGC Pto Fen-AS TTGGCACAAAATTCTCATCAAGC RLK-for GAYGTNAARCCIGARAA RLK-rev TCYGGYGCRATRTANCCNGGITGICC RLRR-for CGCAACCACTAGAGTAAC RLRR-rev ACACTGGTCCATGAGGTT SI GGTGGGGTTGGGAAGACAACG AS3 IAGIGCIAGIGGIAGICC S2 GGIGGIGTIGGIAAIACIAC AS3 IAGIGCIAGIGGIAGICC S2-INV CAICAIAAIGGITGIGGIGG AS3-INV CCIGAIGGIGAICGIG XLRR-for CCGTTGGACAGGAAGGAG XLRR-rev CCCATAGACCGGACTGTT XLRR-INV1 TTGTCAGGCCAGATACCC XLRR-INV2 GAGGAAGGACAGGTTGCC Total polymorphic markers 4 3 11 13 9 13 12 6 1 13 12 17 16 7 7 144 The primers LM637 and LM638 was designed by Kanazin et al. (1996). LM637 was based on a second region of amino acid identity which in the RPS2 protien is proposed to reside in a transmembrane region and LM638 was based on the conserved Ploop sequence of genes N, RPS2, and L6. The primer pair, NBS Fl and NBS R1, were designed by Yu et al. (1996) based on the amino acid sequences of two highly conserved motifs of the nucleotide-binding site in N and RPS2 genes. The primer Nkin2 was based on the second kinase region and primer NPloop was based on the P-loop of the N gene (Naweed Naqvi, IRRI). The primers Ptokinl, Ptokin2, Ptokin3, and Ptokin4 were designed based on the DNA sequence encoding for protein kinase in the tomato Pto gene conferring resistance to Pseudomonas syringae pv tomato (Naweed Naqvi, IRRI). The primers Pto/Fen-S and Pto/Fen-AS were based on a member of Pto gene family conferring sensitivity to fenthion (Leister et al. 1996). The primers Si, S2, AS1, and AS3 were designed by Leister et al. (1996) based on the resistance genes RPS2 of Arabidopsis thaliana and N of tobacco. The primer pairs, RLK for and RLK rev, were designed by Feuillet et al. (1997) to amplify serine/threonine kinase sequence subdomains II to VIII of the wheat Lr10 gene conferring resistance to Puccinia recondita. The primer pairs, NLRR-for and NLRR-rev, RLRR-for and RLRR-rev, and XLRR-for and XLRR-rev were designed based on leucine-rich repeat regions of genes RPS2, Xa21, N, and C19, respectively (Naweed Naqvi, IRRI). The primers NLRR-INV1, NLRR-INV2, S2-INV, AS3-INV, XLRR-INV1, and XLRR-INV2 were designed based on the inverse sequences of NLRR-for, NLRR-rev, S2, AS3, XLRR-for, and XLRR-rev, respectively. All primers were made by Operon (Alameda, California, USA). b Codes for mixed bases: Y = C/T, N = A/G/C/T, R. = A/G, and D = A/G/ 16 The final map, consisting of 99 markers, is shown in Figure 2.1 and was used, together with the phenotype data sets, for QTL analysis. The assignment of linkage groups to chromosomes was based on markers in common with previously published maps (Heun et al. 1991; Kleihofs et al. 1993; Qi et al. 1996). Chromosome 1 Chrormsome 2 ABG704 Owormsonm 3 BCD307 F1R2-5 ABO:68 5.8 RoFc6-3 EACIvb2f 7.7 223 HvIAC1861 XLRF62 FLFR-10 288 >21:12112-3 XLRRIN4 H1PFB S2AS31N.5 271 144:3889 HvI.C222a F2121.1 113 789 1-1 KRIN40 14 CMA 123 BMAC187 15.7 122 BMA054 31.8 E42M17e 1F21.37/8-4 87 S1AS3-16 S2AS311440 EBMAC525 Ra/3-1 BMAC144f S2AS311,L1 SR HVIvE0 FK1/3-9 KRIM 3).1 P104 20.12-5 N.R8.8 M.28.7 E33M12e 14112..7 224 4.8 2U1145 200 KFP190 BYDV BMaC144a 10.0 11.1 ABC253 1118.2 RLR4 S2ASIN-3 106 E44M3611 RON FK13-10 142 RI.R84 B88431,11 82122.7 13346 HVMD RI.Rfr-a 17.8 1.6/1537/8-1 S2A..811144 R.LK-2 184 EHMAC539b HV1AC218a SIAS3-7 DAK642 /4Bt-8 RIAS3-10 SI-1A.%41 SlAS3-L2 81RIR-1 122M521( <1 F1R24 FLRIN3 SR BYDV 5.3 31 14.3 19.8 S2AS31N-11 LR -2 HVM54 BCD135 87 12YR-3 HVM49 Thal HVPRP 10.5 7k0 22 = 1 FIC2146 8441/4-5 IMT3G 10.7 XLRRFR-1 BG1Zb 203.3 cM 233 cM 193.1 cM Figure 2.1a Linkage maps of chromosome 1, 2, and 3 based on 94 doubled haploid (DH) progeny from the cross of Shyri x Galena. Marker loci indicated on the left side of each map were used for linkage map construction. Distances are in Kosambi cM units. Markers on the right side of each linkage group are RGAPs mapping to the region within the corresponding brackets. QTLs for stripe rust (SR), leaf rust (LR), and barley yellow dwarf virus (BYDV) resistance are shown "bold". 17 Clinansotre4 2440334 Chumsonr5 F2R26 Ilv137/8-3 =8 33.7 MEV MAG137 Ea44C213 145 32 5.6 -/ _\ thrusososre6 )1142H7.3 MA440 1,1R1N6 N_RR.45 WINS 98 N3-1 IWW° N3SM1 43 EM4C316 ASS-7 XLM1R2 15.4 amorx OX1542 1103 -2 2L4 S2AS3-9 S2AS3-8 HV1VB N(19 B3MCh 11.8 139/3.6 P102 55 1\111N7 PKV3-3 13M4C30 106 165 106 AEC160 N.111N1 E42M34o 21.5 S2ASMN6 80 STAMM KsAlH2 F1R26 ECRU S2ASV12 line S1AS33 HVM74 111126-3 19.2 /4111N9 147 RIM 1L6 WIVES MAG552A 34 42rz 80 T22Ivf2f Parent 98 SIA.S3-9 BM/ FM-3 143 N.BRO 68 ER2-3 131 218b 69 66 P2R2-5 HEM BIZvB4d RE1W-10 FIRM PIOS FA44C3C8z1 A1EG472 167 BM4C1446 9'1 /4.112.-6 119/3-7 as S1AS36 Rcan-1 PK2/4-1 121 206 KFF221 F19/3-11 17.5 111414 HvHVA1 35.0 IsKR2 AF3397 15.1 NLBP.-4 360 SMIN13 aryl SYS3-17 S2AS34 IvINGNS SASS SZAS3B415 81 R1.RFR.6 T2Z 1vP4g Ficy4-11 SZAS34 RLRE-12 45 S2AS3-3 RI11-11 Ftfen4 120 AE 387 1662dvl 1769cM 1427cM Figure 2.1b Linkage maps of chromosome 4, 5, and 6 based on 94 DH progeny from the cross of Shyri x Galena. Marker loci indicated on the left side of each map were used for linkage map construction. Distances are in Kosambi cM units. Markers on the right side of each linkage group are RGAPs mapping to the region within the corresponding brackets. QTLs for stripe rust (SR), leaf rust (LR), and barley yellow dwarf virus (BYDV) resistance are shown "bold". 18 Chromosome 7 HMSO 21.2 F2R2-2 21.8 XLRAFR-7 /4111N-4 ABC302 5.4 BCD298 199 mErh F2R2-4-1 7.8 ABG59 24.4 DEMAC539a HVEHN7 1VCEIN9 9.5 RIREC2a 24.1 N.RIN12 £3354124 25.3 H.F.114-12 24.F -3 N.1114.13 2321%641 10.8 S2ARD3-2 243 201.3 cM Figure 2.1c Linkage maps of chromosome 7 based on 94 DH progeny from the cross of Shyri x Galena. Marker loci indicated on the left side of each map were used for linkage map construction. Distances are inKosambi cM units. Markers on the right side of each cartoon are RGAPs mapping to the region within the corresponding brackets. Disease Resistance Phenotyping Stripe Rust The 94 DH lines and parents were assessed for adult plant resistance in four tests at Toluca, Mexico and one test at Celaya, Mexico. At Celaya, the DH lines and parents were grown in uni-replicate hill plots in the winter of 1995. A field epidemic was initiated by 19 inoculating spreader rows (formed from a mixture of 15 extremely susceptible genotypes) with a stripe rust isolate whose virulence pattern corresponds to the race 24 VarundaMazurka type described by Dubin and Stubbs (1986). Stripe rust severity was rated at DGS59 (Feekes stage 10.5) as percent severity on a plot basis by the modified Cobb Scale (Melchers and Parker, 1992) and reaction type was scored using a 0 to 9 scale (0= immune, 9 = susceptible). At Toluca, the DH lines and the parents were planted in one-row, 3-m plots in 1994, 1995 and at two planting dates in 1996. Spreader rows, planted at 5.25-m intervals and consisting of a mixture of 15 susceptible genotypes, were inoculated twice with infected plants placed in the foliage, and with applications of spores suspended in oil. Infected plants and spores were collected locally. The race composition of this inoculum was not determined. Stripe rust was rated as percent severity on a plot basis. Leaf Rust The 94 DH lines and parents were assessed for adult plant leaf rust resistance in field tests at Ciudad Obregon, Mexico in 1994, 1995 and 1996 using two-row, 3-m plots. Epidemics were initiated by inoculating spreader rows, a mixture of several very susceptible lines, with fresh spores of a mixture of leaf rust races 8, 19, and 30 collected from a susceptible variety. The inoculum was applied by two methods: (1) a water and surfactant suspension was injected by syringe into the stems of boot-stage plants in spreader rows and (2) a talc carrier was blown into the nursery with a backpack duster multiple times during the growing cycle. Disease was rated by infection type - resistant (R), moderately resistant (MR), moderately susceptible (MS) and susceptible (S) - and percent severity on a plot basis. For mapping purposes, a rust index was calculated by 20 equating the infection type rating with a numerical score (R =1, MR = 2, MS = 3 and S = 4) and multiplying this value by the percentage severity. Bark, Yellow Dwarf Virus (BYDV) The population and parents were assessed for resistance to the BYDV-MAV-Mex and BYDV-PAV-Mex serotypes. In all tests, one replication of two row, 1-m plots was used. The infected plots were infested twice with 5-10 aphids harboring the MAV and PAV serotypes respectively, at three weeks intervals. Aphids were killed by applying an insecticide one week after the second inoculation. Control plots were chemically protected against aphid attack during the entire growing cycle. MAV resistance was described with a dwarfness score (0-9) and a tillering score (0-9) in four tests at Toluca, Mexico (two planting dates in each of 1995 and 1996). In 1996, plant height reduction (control - infected) was also calculated. PAV resistance was measured in one test at Toluca in 1996. The same dwarfing and tillering scores used for MAV were used to measure PAV resistance. OTL Analysis QTLs were mapped using the interval mapping (SIM) and simplified composite interval mapping (sCIM) procedures of MQTL (Tinker and Mather, 1995) and regression procedures. For MQTL, each data set was analyzed with 1,000 permutations, a 5-cM walk speed, and a Type I error rate of 5%. For sCIM, eighteen background markers with approximately even spacing were specified, with a maximum of three background markers per linkage group. Approximate estimates of heritability were computed by substituting environments for replications as: 21 H2 a2 , 2 g/ 15 62e/r where 62g is the variance among DH lines, 62e is the error variance among DH lines, and r is the number of environments. Results and Discussion Linkage Map Construction The base map of 1316.5 cM is shown in Figure 2.1. Single linkage groups were assigned to each chromosome, except for chromosomes 3 and 7. There are also gaps on the long arm of chromosomes 3; the long and short arms of 7; and the long arm of chromosomes 5 and 6. Locus ordering and distance are in agreement with published maps (Heun et al. 1991; Graner et al. 1991; Kleinhofs et al. 1993; Qi et al. 1996). Based on comparisons of markers in common with the Steptoe/Morex map (Kleinhofs et al. 1993) and the merged barley map (Qi et al. 1996), the gap on the short arm of chromosome 7 is approximately 40 cM long, and the gap on chromosome 3 is approximatly 39 cM long. Telomeric RFLP loci on chromosome 5 and 6 (ABG387 and MWG798, respectively) were not associated with the corresponding linkage groups. Twolocus recombination distances between these telomeric markers and nearest markers (HvHVA1 and PK2/4-1) were 36 and 35 cM respectively. RFLPs and SSRs served as anchor markers and the higher-throughput AFLPs and RGAPs were used to fill gaps. However, only 16 AFLPs are included in the final base map, due to AFLP clustering, a phenomenon reported in the literature (Becker et al. 1995). Most (87%) of the RGAP markers followed expected segregation ratios. One-hundred and ten RGAPs mapped to high density (<5 cM) clusters and were therefore excluded from the base-map. Therefore, only 12 RGAPs are included. Markers used for base-map 22 construction are shown on the left-hand side of each linkage group, while additional RGAPs are shown on the right hand side of each linkage group (Figure 2.1a, 2.1b, 2.1c QTL Detection The phenotypic distribution of stripe rust severity, averaged over the five environments, did not show discrete classes allowing for Mendelian analysis (Figure 2.2). Similar distributions were observed for each of the individual environments (data not shown). Only five of the DH lines were as resistant as Shyri (1.5 + 0.9%) while there were 33 susceptible transgressive segregants with severities higher than Galena (62.5±7.4). These data suggest that unique configurations of multiple alleles may be required for high levels of resistance and that the susceptible parent has some resistance alleles. 20 16 0 -o 12 8 4 1 0 20 40 60 80 100 Disease severity (% ) Figure 2.2 Average stripe-rust disease severity (%) in the DH progeny of Shyri x Galena Cross The consistency of the disease severity ratings (h2= 94%) confirms that resistance was stable in the face of the stripe rust virulence present during the years these tests were conducted. While we do not have extensive data on pathogen virulence in these tests, studies in the U.S.A. have shown considerable variation in stripe rust populations (Chen et 23 al. 1995). We have observed comparable levels of resistance in Shyri in over ten years of testing at multiple locations throughout the Americas (data not shown). This may be preliminary evidence for durability of stripe rust resistance. The QTL data confirm the multi-locus control of stripe rust resistance in Shyri and provide some evidence for a resistance allele in Galena. Shyri contributed resistance alleles at QTLs on chromosomes 3, 5, and 6, while Galena contributed the resistance allele at a QTL on chromosome 2 that approached, but did not reach, the significance threshold (Figures 2.3 and 2.4). The largest-effect QTL on the short arm of chromosome 5 (act8BMAC213) was significant in all five tests. This QTL accounted for 28% to 50% of the variation in phenotypic expression (PVE) in the individual environments and 47% PVE in the average of the five tests (Table 2.2). We mapped this QTL to approximately the same position as the Yr4 locus (von Wettstein-Knowles, 1992) and a stripe rust resistance QTL reported by Thomas et al. (1995). The relationship of the Shyri chromosome 5 QTL to these other loci remains to be determined, although the Yr4 locus is reported to confer resistance to race 23, while the virulence in the Americas is broadly defined as race 24 (Marshall and Sutton 1995; Chen et al. 1995). The QTLs on chromosomes 3 and 6 mapped to the ABG004-T22M3 1 i and KsuAl H2-Linka intervals, respectively. The QTL main effect on chromosome 3 was significant only in the Celaya data set, while the main effect on chromosome 6 was significant only in the Toluca 1994 and Toluca 1995 data sets. 24 Chromosome 2 Chromosome 3 Environments - Cclaya 1995 ----- Toluca 1994 Toluca 1995 10 ----- Toluca 1996-1 Toluca 1996-2 E42.111470-EBMAC525 ABG004-T22M3 Figure 2.3 SIM test statistics from the QTL analysis of stripe-rust severity (%) on chromosomes 2 and 3 in DH population of Shyri x Galena. QTL peak intervals are shown on the x-axis. The horizontal bar indicates the maximum significant threshold (P = 0.05). Environments 70 - Cclaya 1995 60 Toluca 1994 50 Toluca 1995 Toluca 1996-1 40 Toluca 1996-2 F. 30 20 10 acte-BMAC213 Kso A 1K2 -Links Figure 2.4 SIM test statistics from the QTL analysis of stripe-rust severity (%) on chromosomes 5 and 6 in DH population of Shyri x Galena. QTL peak intervals are shown on the x-axis. The horizontal bar indicates the maximum significant threshold (P = 0.05). Table 2.2 Chromosome location, allele phase, and effect (expressed as the R2p - percentage of phenotypic variance explained) for stripe rust, leaf rust, and BYDV resistance loci in the Shyri x Galena population. Resistance Phenotype Environments or Measurments Type of inheritance Chromosome Stripe rust Celaya 1995 Toluca 1996-1 Celaya 1995 All tests Toluca 1994 Toluca 1995 All tests Height reduction Dwarfness score Tillering score Height reduction Height reduction Dwarfness score Height reduction Dwarfness score Tillering score QTL 2 E42M47e-EBMAC525 QTL QTL QTL 3 Stripe rust Stripe rust Stripe rust Leaf rust MAV MAV, PAV MAV, PAV MAV MAV MAV, PAV MAV MAV, PAV MAV, PAV Qualitative QTL Closest RGAP Resistance allele R2p NLFR4 G+ 12%, ABG004-T22M3 I i act8-BMAC213 KsuA 1H2-Linka XLRR-5 S2AS3-7 XLRRFR3, NLR1N6 S S S 12% Rphxs ABC253-HVM49 S2AS31N- 1 I S2AS31N-11 marker 5 6 I 1 ,, QTL QTL Marker interval 48++ 4b QTL 5 If II ,, 9% S G If KFP221-ABG397 MWG634-CD0542 ABC160-BMAC303d NKP-2 F2R2-6 G G RLRFR- I 0 S PP 1> PP + "G" refers to resistance allele from Galena and "S" refers resistance allele from to Shyri ++ 4a and 4b denote the two distinct QTLs on the short and long arms of chromosome 4, respectively ,, ,, 28%-50% 23%, 15% 91-96% 18% 20%, 11% 22%, 11% 10% 7% 7%, 6% 15% 13%, 3% 10%, 7% 26 The individual effects of these QTLs were much smaller than the chromosome 5 QTL. The maximum PVE's for the chromosome 3 and 6 QTLs were 12% and 23% respectively. The relationships of these QTLs to the chromosome 3 QTLs reported by Toojinda et al. (1998) and the Yr4 locus on chromosome 6B of wheat (Triticum aestivum) reported by Chen et al. (1995) will need to be resolved by comparative mapping. Galena contributed the resistance allele at a QTL that approached the significance threshold on chromosome 2 (E42M47e-EBMAC525). This trend was observed in only two of the data sets (Celaya 1995 and Toluca 1996-1). The significant main-effect QTLs, when considered in multi-locus models, accounted for 39-52% and 41-55% of the variation in phenotypic and genotypic expression, respectively. These QTLs should be of considerable value and utility, as they are different from those mapped on chromosomes 4 and 7 by Chen et al. (1994) and Hayes et al. (1996). The genetic variance that is not accounted for by the QTLs have mapped in this population could be due to additional QTLs with small-effects and/or to QTL interactions (epistasis). Some of these QTLs could be located in regions of the genome not covered by our current linkage maps. As pointed out by Melchinger et al. (1998), our estimates of QTL effect may be biased by the use of the same population for mapping and estimating QTL effects. These authors also point out that larger populations are required for estimating higher order QTL x QTL interactions. Recognizing the limitations of our population of 94 DH lines, we did, however, proceed to test for interactions between significant or nearly significant, main effects QTLs. Significant interactions between the chromosome 2 and chromosome 5 QTLs, and between the chromosome 2 and chromosome 6 QTLs, were detected in all five 27 environments. The PVE's for the QTLch2 x QTLch5 interaction, after accounting for QTL main effects, ranged from 3 to 12 % in the analysis of individual environment data. In this interaction, the source of the allele on chromosome 2 was not important, but Shyri always contributed the resistance allele on chromosome 5. 80 60 40 20 0 SS SG GS GG Two locus interaction of QTL and QTLch5 Figure 2.5 Mean and 95% LSD intervals for the two locus interactions between the QTLs on chromosomes 2 and 5 determining stripe-rust severity (%). SS, SG, GS, and GG refer to the allelic composition of DH lines at QTLs on chromosomes 2 and 5, respectively. The SQTLch2 x SQTLch5 and Gotch2 x SQ1Lch5 phenotypes showed the same resistance levels (Figure 2.5). The PVE for the QTL62 x QTLch6 interaction, after accounting for QTL main effects, was 4%. In the case of this interaction, Galena contributed the resistance allele on chromosome 2 and Shyri contributed the resistance allele on chromosome 6. The average disease severity of the GQTheh2 x SQTLch6 genotypes was 28% 28 (Figure 2.6). The significant main-effect QTLs and their two locus interactions, when considered in multi-locus models accounted for 44-59% and 47-63% of the variation in phenotypic and genotypic expression, respectively (Table 2.3). 80 04 60 4/1 40 20 0 SS SG GS GG Two locus interaction of QTLch, and QTL,s6 Figure 2.6 Mean and 95% LSD intervals for the two locus interactions between the QTL on chromosomes 2 and 6 for stripe-rust severity (%). SS, SG, GS, and GG refer to the allelic composition of DH lines at QTLs on chromosomes 2 and 6, respectively. As shown in Figure 2.1 and Table 2.2, RGAP markers mapped in proximity with the large-effect QTL on chromosome 5, the chromosome 6 QTL, and the region on chromosome 2 where the susceptible parent (Galena) may have a resistance allele. The NLFR4 locus mapped to the region on chromosome 2. The XLRR-5 locus, 9 cM distal to T22M31i, mapped near the resistance QTL on chromosome 3. The S2AS3-7 locus co-segregated with act8, which flanked the chromosome 5 resistance QTL. The QTL peak on chromosome 6 mapped to the KsuAl H2NLRIN9 interval. These associations of RGAPs and stripe rust resistance QTLs have immediate utility for marker assisted selection and germplasm characterization. 29 Table 2.3 Percentage of phenotypic variance explained (R2p) in multilocus models involving main effect QTLs and their interactions (if significant) for stripe rust and BYDV resistance in the Shyri x Galena population. Disease resistance phenotype Stripe rust Environment Main effect QTLs Toluca 1994 QTLchs Two locus interaction (P<0.01) QT1,,h2xgrLas Stripe rust Celaya 1995 QTLch3, QT1,65 QTLch2xQTLa6 QTL,h2xQT1,65 QTLch2xQIIch6 Stripe rust Toluca 1995 QTLchs QT1,,h2xQTLchs Multilocus R2 55°10 44% 52% QTL,h2xgrLch6 Stripe rust Toluca 1996-1 QTLchs QT1,c1,2xqn-Ths 59% QTLch2xQnch6 Stripe rust Stripe rust Toluca 1996-2 Average QTL,h5 QT1,62xQlichs 53% QTLchs QTLch2xQTLch6 QT1,,h2xQTLchs QTLch2xQT1,66 61% BYDV-MAV Toluca 1996 QTLchI, QTLch4a , Height reduction QTLchab, QTLchs BYDV-MAV Toluca 1996 QTLchi, glichab, Dwarfness score QT1,65 BYDV-MAV Toluca 1996 QTLchi, QTLchs Tillering score BYDV-PAV Toluca 1996 QTLchi, Q11.-ch4b, Dwarfness score QTLchs BYDV-PAV Toluca 1996 QTLchi, QTLchs Tillering score ch = chromosome and #, see Table 2 for details ++4a, 4b refer to the KFP221-ABG397 and MWG634-CD0542 intervals, respectively 43% 39% 32% 18% 17% Assuming a subset of the RGAPs correspond to disease resistance genes (Kanazin et al 1996; Yu et al 1996; Leister et al 1996; Leister et al. 1998; Chen et al. 1998), it is intriguing to find qualitative and quantitative resistance genes in proximity. In contrast to stripe rust resistance, leaf rust resistance in this population is clearly qualitatively inherited. The phenotypic distribution for the leaf rust severity index, averaged over the three environments, is discrete (Figure 2.7). This 1:1 ratio (chi square = 0.68 with P-value < 0.01) is clear evidence for monogenic inheritance. The two parents lie at opposite ends of the frequency distribution. The heritability of the rust severity index 30 was 97%. When the quantitative severity index data were mapped using the procedures of MQTL, a single QTL mapped to the long arm of chromosome 1 (7H) in the ABC253-Tha2 interval. Shyri contributed the resistance allele. 50 ca 40 30 20 10 0 50 100 150 200 250 300 Leaf rust index Figure 2.7 Average leaf-rust index in the DH progeny of Shyri x Galena. The single locus accounted for 84 % of the PVE. When the data were treated as bivariate scores (1,0), the single locus (Rphxs), mapped 3.2 cM proximal to That (Figure 2.8). Rphxs may be allelic with the Rph3 locus reported by Jin et al (1993) and the Rphxc locus reported by Hayes et al (1996). Rph genes have been used extensively in barley breeding programs, but these genes often lack durability (Feuerstein et al. 1990; Jin et al. 1996; Qi 1998). 31 400 Environments - Ciudad 0 brcgon 1994 ---- Ciudad 0 bregon 1995 Ciudad 0 brcgon 1996 Figure 2.8 SIM test statistics from the QTL analysis of leaf-rust index on chromosome 1 in DH population of Shyri x Galena and showing the coincident QTL peak and position of the Rphxs locus. The horizontal bar indicates the maximum significant thresholds (P = 0.05). When Shyri was released in Ecuador in 1989, it was resistant to leaf rust, with a maximum rating of 20MS. It now has ratings as high 90S (Vivar personal communication), underscoring the danger of reliance on single qualitative resistance genes. The RGAP marker S2AS3IN-11 was 5.8 cM proximal to the Rphxs locus. Thus, RGAP markers were found in association with both quantitatively and qualitatively inherited genes conferring resistance to Puccinia species. We also found RGAP markers in association with quantitatively inherited resistance to two serotypes of the aphid-vectored viral pathogen, BYDV. The phenotypic distributions for BYDV-MAV and BYD-PAV did not show discrete classes for any of the three traits used to measure resistance - plant height reduction, dwarfing score or tillering score (Figures 2.9-2.13). 32 Shyri Galena 30 25 20 15 7 10 5 0 0 10 20 30 40 Plant H eight Reduction (cm ) Figure 2.9 Plant height reduction (cm) after infection with the BYDV-MAV serotype in the DH progeny of Shyri x Galena. These distributions suggest that resistance in this population is not due to the Ryd2 locus on chromosome 3, which gives a clear distribution of resistance vs. susceptible classes when resistant and susceptible alleles are segregating in a DH mapping population (Hayes et al. 1996). There were large numbers of positive phenotypic transgressive segregants for all measures of resistance, suggesting that both parents contributed resistance alleles. The QTL data support the presence of resistance genes other than Ryd2, the contribution of resistance alleles from both parents, a common genetic basis for the three measures of resistance, and a common basis of resistance to the two serotypes. QTLs exceeded significance threshold only in the Toluca 96 date 1 data set. Although trends were apparent in the other data sets, the following discussion is based on 1 environment of MAV and PAV data. Four QTLs were detected for BYDV-MAV resistance and three for PAV resistance. None mapped to the centromeric region of chromosome 3, the site of the Ryd2 locus (Collins et al. 1996). 33 Shyri Galena 50 40 30 20 10 0 0 1 2 3 4 5 Dwarfness Score ( 0-9 ) Figure 2.10 Dwarfness score (0-9) in the DH progeny of Shyri x Galena infected with the BYDV-MAV serotype. 50 40 30 20 z 10 0 0 2 3 4 5 Tillering Score ( 0-9 ) Figure 2.11 Tillering score (0-9) in the DH progeny of Shyri x Galena infected with BYDV-MAV serotype 34 Shyri G alena 40 30 0 .0 £ 20 0 z 10 0 0 2 4 6 10 8 Dwarfness Score (0-9) .Figure 2.12 Dwarfness score (0-9) in the progeny of Shyri x Galena infected with the BYDV-PAV serotype. alen a 24 S h y ri 20 16 .0 z 8 4 0 0 2 4 6 T illering Score (0-9) 10 Figure 2.13 Tillering score (0-9) in the progeny of Shyri x Galena infected with the BYDV-PAV serotype. Coincident large-effect QTLs for all three measures of MAV and the two measures of PAV resistance mapped to chromosome 1 in the ABC253 -HVM49 interval (Figure 2.14). 35 Phenotypes 3 M AY- height 25 redaction M A V -dw arta ess store 20 scare ----- PA V -dw arfaese 15 111 BC 233- H V31 49 Figure 2.14 SIM test statistics from the QTL analysis of plant height reduction (cm), dwarfness score, and filleting score after infected with the BYDV-MAV and BYDV-PAV serotypes on chromosome 1 in DH population of Shyri x Galena. QTL peak intervals are shown on the x-axis. The horizontal bar indicates the maximum significant thresholds (P = 0.05). In all cases Galena contributed the resistance allele. The PVE values for MAV resistance ranged from 18% to 21% (Table 2.2). The PVEs for the two measures of PAV resistance were 11%. Two regions on chromosome 4 were associated with MAV resistance and one with PAV resistance. In all cases Galena contributed the resistance allele. A MAV dwarfing score QTL mapped to KFP221-ABG397 interval (PVE = 10%). Plant height reduction and dwarfing score QTLs for MAV and a PAV dwarfing score QTL coincided in the MWG634-CD0542 interval. The MAV resistance QTLs had PVE's of 7%, while the PAV QTL had a PVE of 6% (Table 2.2). Shyri contributed resistance alleles for all measures of resistance to the two serotypes at coincident QTLs on chromosome 5 in the ABC160-BM_AC303d interval (Figure 2.15). 36 20 Phenotypes 1 Chromosome 4 Chromosome 5 M AV-height reduction MAV - dwarfness 15 score ../. \ M AV- tillering r,.. 10 \ ---___ PAV- tillering score ..../ % .... VA, f-N..-ki ,\ I. 0 _____. PA Y-dw arfness score 1 \I 5 score 1.-,. . . i ;71i .. A \ ' ',. \ \s,...../7 '-';',1: ....., 1/ \-:. : ABC 160-BMAC303d Figure 2.15 SIM test statistics from the QTL analysis of plant height reduction (cm), dwarfness score, and tillering score after infected with the BYDV-MAV and BYDV-PAV serotypes on chromosomes 4 and 5 in DH population of Shyri x Galena. QTL peak intervals are shown on the x-axis. The horizontal bar indicates the maximum significant thresholds (P = 0.05). The MAV resistance QTLs accounted for PVEs ranging from 10 - 15%, while the PAV resistance QTL accounted for 3 - 7% of the PVE. The multi-locus PVE values for MAV dwarfing score, plant height reduction, and tillering score were 43, 39, and 32%, respectively. The multi-locus PVE values for PAV dwarfing score and tillering score were 17% and 18%, respectively (Table 2.3). No two-locus QTL interactions were significant for resistance to either serotype, indicating that the phenotypic variance that remains unaccounted for may be due to genes in unmapped regions of the genome or to higher order interactions. The chromosome 1 BYDV-MAV/PAV resistance QTL may be homoeologous with Bdvl, a BYDV resistance gene in wheat. This gene, mapped to chromosome 7D, 37 was reported to be tightly linked to the Lr34 and Yr18 genes for resistance to leaf rust and stripe rust, respectively (Singh, 1993; Sharma et al 1995). Barley chromosome 1 (7H) and wheat chromosome 7 are homoeologous. In this population, the BYDV resistance QTL and the Rphxs loci are linked, although in repulsion phase. Additional evidence for homoeology of these resistance clusters is the presence of a qualitative stripe rust resistance gene in the same region of the genome in CI10587 (Hayes personal communication). As in the cases of qualitative and quantitative resistance to fungal pathogens, we found RGAPs associated with quantitative resistance to the viral BYDV pathogen. As described in the preceding section on leaf rust resistance, two RGAPs mapped to the same region on chromosome 1 as the BYDV resistance QTL. RGAP clusters mapped to the same regions of the genome as both BYDV resistance QTLs on chromosome 4. The RLRFRJO mapped distal to the chromosome 5 BYDV resistance QTL. In summary, we mapped a qualitatively inherited gene conferring resistance to a fungal disease (leaf rust) and determinants of quantitative resistance to a fungal disease (stripe rust) and a viral pathogen (BYDV). The leaf rust resistance gene may be allelic with a gene mapped in other germplasm (Jin et al. 1993; Hayes et al. 1996), and its linkage relationship with a BYDV resistance QTL mirrors a homoeologous relationship in wheat (Singh, 1993; Sharma et al. 1995). The largest-effect stripe rust resistance QTL coincides with a previously reported QTL in other germplasm (Thomas et al. 1995) and a qualitative resistance gene (von Wettstein-Knowles 1992). We have demonstrated linkage relationships of RGAPs with genes conferring qualitative and quantitative resistance to two fungal pathogens and 38 quantitative resistance to a viral pathogen. RGAPs are also linked with QTLs for resistance to two other fungal pathogens - scald (caused by Rhyncosporium secalis) and net blotch (caused by Pyrenophora teres) in this same population, as will be shown in a subsequent report. RGAPs are clearly a powerful tool for mapping qualitative and quantitative disease resistance genes. These linkage relationships support clustering of disease resistance genes in barley, as reported in other species (Saxena et al. 1968; Giese et al. 1981; Hooker 1985; Paran et al. 1991; Ellis et al. 1998) and may also be evidence for commonalties of qualitative and quantitative resistance genes. The availability of abundant RGAP markers should facilitate isolation and characterization of both qualitative and quantitative resistance and provide tools for answering fundamental questions regarding the genetic basis of the two classes of resistance. References Alemayehu F, Parlevliet JE (1996) Variation for resistance to Puccinia hordei in Ethiopian barley landraces. Euphytica 90:365-370. Barua UM, Chalmers KJ, Hackett CA, Thomas WTB, Powell W, Waugh R (1993) Identification of RAPD markers linked to a Phynchosporium secalis resistance locus in barley using near-isogenic lines and bulked segregant analysis. Heredity 71:177-184. Becker J, Heun M (1995) Barley microsatellites: allele variation and mapping. Plant Mol Biol 27:835-845. Becker J, Vos P, Kuiper M, Salamini F, Huen M (1995) Combined mapping of AFLP and RFLP markers in barley. Mol. Gen. Genet. 249:65-73. Borst P, Greaves DR (1987) Programmed gene rearrangements altering expression. Science 235:658-667. 39 Browning JA, Simons MD, Torres E (1977) Managing host genes:epidemiologic and genetic concepts. In Horsfall JG, Cowling EB (eds). Plant Disease:an advanced treatise. Vol.l. Academic Press, New York. Buschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J, Topsch S, Vos P, Salamini F, Schulze-Lefert P (1997) The barley Mlo gene: A novel control element of plant pathogen resistance. Cell 88:695-705. Chen F, Hayes PM (1989) A comparison of Hordeum bulbosum-mediated haploid production efficiency in barley using in vitro floret and tiller culture. Theor. Appl. Genet. 77:701-704. Chen F, Prehn D, Hayes PM, Mulrooney D, Corey A, Vivar H (1994) Mapping genes for resistance to barley stripe rust (Puccinia striiformis f.sp hordei). Theor Appl Genet 88:215-219. Chen XM, Line RF, Leung H (1995) Virulence and polymorphic DNA relationships of Puccinia striiformis f.sp. hordei to other rusts. Phytopathology 85:1335-1342. Chen XM, Line RF, Leung H (1998) Genome scanning for resistance-gene analogs in rice, barley, and wheat by high-resolution electrophoresis. Theor. Appl. Genet 97:345-355. Crute IR (1985) The genetic basic of relationships between microbial parasites and their host. Pages 62 in: Mechanisms of resistance in plant diseases. Kluwer Academic Press. Collins NC, Paltridge NG, Ford CM, Symons RH (1996) The Yd2 gene for barley yellow dwarf virus resistance maps close to the centromere on the long arm of barley chromosome 3. Theor. Appl. Genet 92:858-864. Conner RL, Whelan EDP, MacDonald MD (1989) Identification of sources of resistance to common root rot in wheat-alien amphiploid and chromosome substitution lines. Crop Science 29:916-919. D'Acry CJ (1995) Symptomatology and host range of barley yellow dwarf. Pages 9-28 in: Barley Yellow Dwarf: 40 Years of Progress. D'Acry CJ and Burnett PA, eds. The American Phytopathologal Society, St. Paul, MN. Dangl JL (1995) Piece de resistance: novel classes of plant disease resistance genes. Cell 80:363-366. 40 Dingerdissen AL, Geiger HH, Lee M, Schechert A, We lz HG (1996) Interval mapping of genes for quantitative resistance of maize to Setoria turcica, cause of norther leaf blight, in a tropical environment. Molecular Breeding 2:143-156. Dubin HJ, Stubbs RW (1986) Epidemic spread of barley stripe rust in South America. Plant Dis 70:141-144. El-Kharbotly A, Leonards-Schippers C, Huigen DJ, Jacobsen E, Pereira A (1994) Segregation analysis and RFLP mapping of the RI and R3 alleles conferring race-specific resistance to Phytohthora infestans in progeny of dihaploid potato plants. Mol.Gen.Genet. 242:749-754. Ellis JG, Lawswnce GJ, Peacock WJ, and Pryor AJ (1998) Approaches to cloning plant genes conferring resistance to fungal pathogens. Annual Review of Phytopathology 26:245-263. Feuerstein U, Brown AHD, Burdon JJ (1990) Linkage of rust resistance genes from wild barley (Hordeum spontaneum) with isozyme markers. Plant Breed 104:318-324. Graner A, Jahoor A, Schondelmaier J, Siedler H, Pillen K, Fischbeck G, Wensel G, Herrmann G (1991) Construction of an RFLP map of barley. Theor.Appl. Genet. 83:250-256. Graner A, Bauer E (1993) RFLP mapping of the ym4 virus resistance gene in barley. Theor.Appl.Genet. 86:689-693. Garner A, Tekauz A (1996) RFLP mapping in barley of a dominant gene conferring resistance to scald (Rhynchosporium secalis). Theor. Appl. Genet. 93:421-425. Giease H, Jorgensen J, Jensen HP, Jensen J (1981) linakge relationships of ten powdery mildew resistance genes on barley chromosome 5. Hereditas 95:43-50. Giease H, Holm-Jensen AG, Jensen HP, Jensen J (1993) Localization of the Laevigatum powdery mildew resistance gene to barley chromosome 2 by the use of RFLP markers.Theor.Appl.Genet. 85:897-900. Harder DE, Harber S (1992) Barley yellow dwarf virus. Pages 379 in: Oats Science and Technology. Marshall HG, Sorrells ME, eds. American Society of Agronomy, Inc, Madison, WI. Hayes PM, Prehn D, Vivar H, Blake T, Comeau A, Henry I, Johnston M, Jones B, Steffenson B (1996) Multiple disease resistance loci and their relationship to agronomic and quality loci in a spring-barley population. J.QTL http: / /probe.nalusda.gov: 8000 /otherdocs /j QTL/index.htm 41 Heun M, Kennedy AE, Anderson JA, Lapitan NLV, Sorrels ME, Tanks ley SD (1991) Construction of a restriction fragment length polymorphism map for barley (Hordeum vulgare). Genome 34:437-447. Hoffbeck MD, Openshaw SJ, Geadelmann JL, Peterson RH, Stuthman DD (1995) Backcrossing and intermating in an exotic X adapted cross of maize. Crop Science 35:1359-1364. Holloway J, Knapp SJ (1994) Gmendel 3.0 Users Guide. Knapps@css.orst.edu. Hooker AL (1985) Corn and sorghum rust. In "The cereal rusts. Volume 2. Diseases, Distribution, Epidemiology, and Control" (Roelfs AP and Bushnell WR, eds) pp 207-233. Jin Y, Statler GD, Franckowiak JD, Steffenson BJ (1993) Linkage between leaf rust resistance genes and morphological markers in barley. Phytopathology 83:230233. Jin Y, Cui GH, Steffenson BJ, Franckowiak JD (1996) New leaf rust resistance in barley and their allelic and linkage relationships with other Rph genes. Phytopathology 86:887-890. Johnson R (1981) Durable resistance: definition of, genetic control, and attainment. Phytopathology 71:567-568. Jorgensen JH (1992) Discovery, characterization and exploitation of Mbo powdery mildew resistance in barley. Euphytica 63:141-152. Kanazin V, Marex LF, Shoemaker RC (1996) Resistance gene analogs are conserved and clustered in soybean. Proc. Natl. Acad. Sci. USA 93:1174611750. Kleinhofs A, Kilian A, Saghai Maroof M, Biyashev R, Hayes PM, Chen F, Lapitan A, Fenwick A, Blake T, Kanazin V, Ananiev E, Dahleen L, Kudrna D, Bollinger J, Knapp SJ, Liu B, Sorrells M, Heun M, Franckowiak J, Hoffman D, Skadsen R, Steffension B (1993) A molecular, isozyme, and morphological map of the barley (Hordeum vulgare) genome. Theor. Appl. Genet. 86:705-712. Lamb CJ (1994) Plant disease resistance genes in signal perception and transduction. Cell 76:419-422. Leister D, Ballvora A, Salamini F, Gebhardt C (1996) A PCR-based approach for isolsting pathogen resistance genes from potato with potential for wide application in plants. Nature Genetics 14:421-429. 42 Leister D, Kurth J, Laurie DA, Yano M, Sasaki T, Devos K, Graner A, SchulzeLefert P (1998) Rapid reorganization of resistance gene homologues in cereal genomes. Proc. Natl. Acad. Sci. USA 95:370-375. Line RF (1993) Durability of resistance to Puccinia striiformis in North American wheat cultivars. In: Jacobs TH, Parlevliet JE (eds) Durability of disease resistance, Kluwer Academic Press. p 332. Liu ZW, Biyashev RM, Saghai Maroof MA (1996) Development of simple sequence repeat markers and their integration into a barley linkage map. Theor. Appl. Genet. 93:869-876. Maisonneuve B, Bellec Y, Anderson P, Michelmore RW (1994) Rapid mapping of two genes for resistance to downy mildew from Lactuca serriola to existing clusters of resistance genes. Theor.Appl.Genet. 89:96-104. Martin GB (1996) Molecular cloning of plant disease resistance genes. In: Plant microb interactions vol.1. Martin GB, Herrera-Hestrella L, Rosales LS, RiveraBustamante R, Neuenschwander U, Lawton K, Ryals J, Schardl CL, Kronstad JW, Thomashow LS, Weller DM, Philips DA, Streit WR, Prome JC, Demont N, Stacey G (eds).Chapman & Hall; New York; USA. Marshall D, Sutton RL (1995) Epidemiology of stripe rust, virulence of Puccinia striiformis f. sp. hordei, and yield loss in barley. Plant Dis 70:732-737. Melchers LE, Parker JH (1992) Rust resistance in winter wheat varieties. US Dep Agric Bull 1046 Melchinger AE, Utz HF, Schon CC (1998) Quantitative trait locus (QTL) mapping using testers and independent population samples in maize reveals low power of QTL detection and large bias in estimates of QTL effects. Genetics 149:383403. Michelmore RW (1995). Molecular approaches to manipulation of disease resistance genes. Ann. Rev. Phytopath 33:393-428. Morgante M, Rafalski A, Bidle P, Tingey S, Oliveri AM (1994) Genetic mapping and variability of seven soybean simple sequence repeat loci. Genome 37:763769. Ordon F, Friedt W (1993) Mode of inheritance and genetic diversity of BaMMV resistance of exotic barley germplasms carrying genes different from ym4. Theor. Appl. Genet. 86:229-233. 43 Ori N, Eshed Y, Paran I, Presting G, Aviv D, Tanks ley S, Zamir D, Fluhr R (1997) The 12C family from the wilt disease resistance locus 12 belongs to the nucleotide binding, leucine-rich repeat superfamily of plant resistance genes. Plant Cell 9:521-532. Paran I, Kesseli R, Michelmore R (1991) Identification of restriction fragment length polymorphism and random amplified polymorphic DNA markers linked to downy mildew resistance genes in lettuce, using near-isogenic lines. Genome 34:1021-1027. Powell W, Thomas WTB, Baird E, Lawrence P, Booth A, Harrower B, McNicol JW, Waugh R (1997) Analysis of quantitative traits in barley by the use of Amplified Fragment Length Polymorphisms. Heredity 79:48-59. Pryor T (1987) The origin and structure of fungal disease resistance genes in plants. Trends in Genetics 3:157-161. Pryor T, Ellis J (1993) The complexity of fungal resistance genes in plants. Advances in Plant Pathology 10:281-304. Qi X, Stam P, Lindhout P (1996) Comparison and integration of four barley genetic maps. Genome 39:379-394. Qi X (1998) Identification and mapping of genes for partial resistance to Puccinia hordei Otth in barley. Thesis Wageningen Agricultural University. Reuvein R, Dudai N, Putievsky E, Elmer WH, Wick RL (1997) Evaluation and identification of basil germplasm for resistance to Fusarium oxysporum f sp. Basilicum. Plant Disease 81:1077-1081. Russell J, Fuller J, Young G, Thomas B, Taramino G, Macaulay M, Waugh R, Powell W (1997) Discriminating between barley genotypes using microsatellite markers. Genome 40:442-450. Saxena KMS, Hooher AL (1968) On the structure of a gene for disease resistance in maize. Proc. Natl. Acad. Sci. USA 61:1300-1305. Sharma H, Ohm H, Goulart L, Lister R, Appels R, Benlhabib 0 (1995) Introgression and characterization of barley yellow dwarf virus resistance from Thinopyrum intermedium into wheat. Genome 38:406-413. Singh RP (1993) Genetic association of gene Bdvl for tolerance to barley yellow dwarf virus with genes Lr34 and Yr18 for adult plant resistance to rusts in bread wheat. Plant Dis 77:1103-1106. 44 Tanks ley SD, Nelson JC (1996) Advanced backcross QTL analysis: a method for the simultaneous discovery and transfer of valuable QTLs from unadapted germplasm into elite breeding lines. Theor. Appl. Genet. 92:191-203. Thomas WTB, Powell W, Waugh R, Chalmers KJ, Barua UM, Jack P, Lea V, Forster BP, Swanston JS, Ellis RP, Hanson PR, Lance RCM (1995) Detection of quantitative trait loci for agronomic, yield, grain and disease characters in spring barley (Hordeum vulgare L.). Theor Appl Genet 91:1037-1047. Tinker NA, Mather D (1995) Methods for QTL analysis with progeny replicated in multiple environments. J.QTL:http://probe.nalusda.gov:8000/otherdocs/jqt1/ Toojinda T, Baird E, Booth A, Broers L, Hayes PM, Powell W, Thomas W, Vivar H, Young G (1997) Introgression of quantitative trait loci (QTLs) determing stripe rust resistance in barley: an example of marker-assisted line development. Theor Appl Genet 96:123-131. Veremis JC, Cap GB, Robert PA (1997) A search for resistance in Lycopersicon spp. to Nacobbus aberrans. Plant Disease 81:217-221. Von Wettstein-Knowles P (1992) Barley: genetics, biochemistry, molecular biology and biotechnology. CAB Int, Wallingord, UK, pp 73-98. Williamson VM, Ho JY, Wu FF, Miller N, Kaloshian I (1994) A PCR-based marker tightly linked to nemathod resistance gene, Mi, in tomato. Theor.Appl.Genet. 87:757-763. Young ND (1996) QTL mapping and quantitative disease resistance in plants. Aim. Rev. Phytopath. 34:479-501. Yu YG, Buss GR, Saghi Maroof MA (1996) Isolation of a superfamily of candidate disease-resistance genes in soybean based on a conserved nucleotide-binding site. Proc. Natl. Acad. Sci. USA 93:11751-11756. Zabeau M, Vos P (1993) European patent application publication no. EP 0534858 Zaitlin D, DeMars S, Ma Y (1993) Linkage of Rhm, a recessive gene for resistance to southern corn leaf blight, to RFLP marker loci in maize (Zea mays) seedlings. Genome 36:555-564. 45 CHAPTER 3 INTROGRESSION OF QUANTITATIVE TRAIT LOCI (QTLs) DETERMINING STRIPE-RUST RESISTANCE IN BARLEY: AN EXAMPLE OF MARKER-ASSISTED LINE DEVELOPMENT Theerayut Toojinda, Patrick M. Hayes, Hugo Vivar 46 Abstract Genome analysis tools are useful for dissecting complex phenotypes and manipulating determinants of these phenotypes in breeding programs. Quantitative Trait Locus (QTL) analysis tools were used to map QTLs conferring adult plant resistance to stripe rust (caused by Puccinia striiformis f.sp. hordei) in barley. The resistance QTLs were introgressed into a genetic background unrelated to the mapping population with one cycle of marker-assisted backcrossing. Doubled haploid lines were derived from selected backcross lines, phenotyped for stripe rust resistance, and genotyped with an array of molecular markers. The resistance QTLs that were introgressed were significant determinants of resistance in the new genetic background. Additional resistance QTLs were also detected. The susceptible parent contributed resistance alleles at two of these new QTLs. We hypothesize that favorable alleles were fixed at these new QTLs in the original mapping population. Genetic background may, therefore, have an important role in QTL transfer experiments. A breeding system is described that integrates single copy and multiplex markers with confirmation of the target phenotype in doubled haploid lines phenotyped in field tests. When resources are limited, this approach may be useful for simultaneously producing agronomically useful germplasm and contributing to an understanding of quantitatively inherited traits. Introduction Genome analysis based on DNA polymorphisms can reveal the genetic determinants of complex phenotypes and provide tools for manipulating these determinants to maximize selection response. We are using molecular markers in barley (Hordeum vulgare L.) in an attempt to rapidly develop cultivars adapted to the Pacific 47 Northwest of the United States that are resistant to barley stripe rust. Barley stripe rust is caused by Puccinia striiformis f.sp. hordei. The disease was first reported in the Americas in 1975 (Dubin and Stubbs 1985). It was first reported in the United States in 1991 (Marshall and Sutton 1995). By 1995, the disease was reported throughout the western United States, where localized epidemics have caused severe losses in yield and quality. While the disease can be controlled by fungicides, economic and environmental considerations favor genetic resistance. There is limited information on the genetics of resistance to stripe rust in barley. Bakshi and Luthra (1971) described a dominant resistance gene in Indian germplasm but did not map it. Three recessive resistance genes (Yr, Yr2, and Yr3) are reported in European spring barley germplasm, and one dominant resistance gene is reported in European winter barley. These genes have not been mapped (Lehmann et al., 1975). The only stripe rust resistance gene showing Mendelian inheritance that has been mapped in barley is Yr4. This locus is on located on chromosome 5 (1H). It does not confer resistance to race 24 (von Wettstein-Knowles 1992). The virulence of stripe rust in the Americas was first described in terms of race 24 (Dubin and Stubbs 1986). Chen et al. (1994) and Hayes et al. (1996c) reported adult plant stripe rust resistance QTLs on chromosomes 4 (4H) and 7 (5H). The race composition of the field inoculum was not known. Thomas et al. (1995) reported QTLs for adult plant stripe rust resistance to uncharacterized field inoculum on chromosomes 1 (7H), 5 (1H), and 7 (5H). The chromosome 5 (1H) QTL was hypothesized to be due to the Yr4 locus and the chromosome 7 (5H) QTL to be the same QTL reported by Chen et al. (1994) and Hayes et al. (1996c). In contrast, the genetics of stripe rust in wheat is an 48 area of extensive study (reviewed by Line et al. 1993). Based on the homoeology of the two crop species, there are likely to be parallels in the two host: pathogen interaction systems. For example, quantitative, adult plant resistance will probably be more durable than race-specific resistance (Line 1993). We have, therefore, focused our attention on introgressing resistance genes from genotypes that, under field conditions, allow limited disease development on adult plants. This type of disease reaction may indicate durable, adult plant resistance (Parlevliet and Van Ommeren 1975). When such genotypes, developed by the International Center for Agricultural Research in the Dry Areas (ICARDA) program based at the International Maize and Wheat Improvement Center (CIMMYT) in Mexico, are crossed with susceptible genotypes, the progeny show a range of disease reaction phenotypes that do not fall into discrete classes. This quantitative inheritance can be studied through the techniques of quantitative trait locus (QTL) analysis (Hayes et al., 1996c). Based on restriction fragment length (RFLP) linkage data and adult plant disease reaction phenotype data, we reported stripe rust resistance QTLs on chromosomes 4 (4H) and 7 (511) of Calicuchima-sib (Chen et al. 1994; Hayes et al. 1996c). Backcrossing is an approach to introgressing target loci, such as stripe rust resistance QTLs, into adapted backgrounds. The contribution of the donor parent is reduced by half with each generation of backcrossing, assuming no linkage. Molecular markers can increase the efficiency of the process in several ways. Flanking markers can be used to identify the backcross lines that are heterozygous for target genome regions. Advancing only these selected lines will also have the effect of reducing linkage drag 49 (Young and Tanks ley 1989; Tanks ley and Nelson 1996). Single-copy, or low-copy, markers with defined map locations, such as RFLPs and simple sequence repeats (SSRs) , are ideal for this step. Molecular markers could also increase the efficiency of backcrossing by allowing selection of genotypes with the maximum percentage of recurrent parent genome. Markers with higher information content per reaction, such as amplified fragment length polymorphisms (AFLPs), are ideal for this step (Waugh et al. 1997). Manipulation of QTLs can be problematic due to loss of target loci though recombination, incorrect information regarding the location of the QTLs, and/or negatively altered expression of the QTLs in new genetic backgrounds (Hayes et al. 1996b). Therefore, a marker-assisted QTL backcrossing scheme for a self-pollinated crop, such as barley, might (i) use flanking markers to select progeny with a probability of carrying the target QTL allele(s), (ii) confirm the target phenotype in the selected progeny, and (iii) use multiplex markers to identify those selections with the maximum percentage of recurrent parent genome. In crops where a rapid approach to homozygosity (such as a doubled haploid technique) is possible, the efficiency of the second step can be increased by phenotyping on a plot, rather than an individual line basis. See Powell et al. (1996) for additional detail on the idea of integrating single and multi-locus markers in barley breeding. Our long-term practical objective is to develop six-row, spring habit germplasm adapted to the Pacific Northwest of the United States that has durable resistance to stripe rust. In doing so, we sought to (i) validate the effects of mapped stripe rust resistance QTLs, (ii) determine if there were different resistance QTLs in an unrelated genotype, 50 and (iii) pilot a marker-assisted backcrossing scheme incorporating RFLP, AFLP, and random amplified polymorphic DNA (RAPD) markers, doubled haploids, and field phenotyping. Materials and Methods Germplasm The germplasm derivation process is shown in Figure 3.1. BSR41, a 6-row mapline from the Calicuchima-sib x Bowman mapping population (Chen et al. 1994; Hayes et al. 1996c) was used as the donor parent in a single backcross to the variety Steptoe. Steptoe is the most widely-grown six-row feed barley in the Pacific Northwest United States. It has been the subject of intensive genome mapping efforts by the North American Barley Genome Mapping Project (see Hayes et al. 1996b for a review). Steptoe is susceptible to stripe rust. Four RFLP markers bracketing the stripe rust resistance QTLs on chromosomes 4 (4H) and 7 (5H) described by Chen et al. (1994) and Hayes et al. (1996c) were screened in a population of 66 backcross-one (BC1) generation plants. Only a subset of the RFLP markers available at the time this experiment was conducted showed polymorphism between Steptoe and BSR41. The target regions on chromosome 4 (4H) and chromosome 7 (5H) were poorly populated with markers when this work was carried out, and they remain sparsely populated on the current barley consensus map of Qi et al. (1996). ABG366 and Bmyl flank the resistance QTLs on chromosome 4 (4H). ABG366 was not mapped in the reference mapping population, Calicuchima-sib x Bowman, where 51 the resistance QTL peak was detected between Bmyl and ABG397, a distance of 28.1 cM. ABG366 and Bmyl are 32.7 cM apart on the Steptoe x Morex map (Mather 1995). In the Steptoe x Morex cross, ABG366 is 3.7 cM proximal to ABG397 (Mather 1995). WG530 and CD057 flank the resistance QTL on chromosome 7 (5H). WG530 was not mapped in Calicuchima-sib x Bowman, where the resistance QTL peak was detected in the Ale-CD057 interval, a distance of 20.3 cM. WG530 and CD057 are 31.4 cM apart on the Steptoe x Morex map (Mather 1995). As shown in Figure 3.1, eleven of the sixty six BC1 plants (plant numbers 6, 7, 20, 21, 22, 28, 40, 50, 55, 56, and 58) were selected as heterozygotes for the target BSR 41 x STEPTOE Fl x STEPTOE BC 1 1 20. 21. 22 6. 7 28 40 55. 56....58 50 n= 66 It DH 6 17 15 I SELECTIONS 2 8 14 3 10 19 6 17 19 n=134 I 2 n= 10 ir e4ir 4/ Figure 3.1 Derivation of doubled haploid (DH) germplasm from a markerassisted selection program for adult plant stripe rust resistance. BC1 refers to the first backcross generation and the numbers identify the BC1 plants that were selected based on their genotypes at marker loci flanking stripe rust resistance QTLs on chromosomes 4 (4H) and 7 (5H). DH refers to doubled haploid and the numbers indicate the number of DH lines produced from each BC1 plant. Selections refers to the ten most resistant DH lines advanced to extensive phenotyping. See text for additional details on line derivation and genotyping procedures. 52 flanking markers. Doubled haploid (DH) lines were derived from these BC1 plants, using the Hordeum bulbosum technique, as described by Chen and Hayes (1989). A total of 134 DH lines were produced, with varying numbers of DHs per BC1 plant, as shown in Figure 3.1. For example, six DHs were produced from BC1 plant no. 6, 17 DHs were produced from BC1 plant no. 7, etc. Genotyping The 134 DH lines were genotyped for the four RFLP markers used for resistance QTL introgression and an additional RFLP on chromosome 4 (4H) (ABG54); two morphological markers on chromosome 7 (5H), mSrh (rachilla hair length) and mR (awn texture); 106 AFLPs; and eight RAPDs. RFLPs were assayed as described by Chen et al. (1994). The morphological markers were scored under a dissecting microscope. RAPDs were assayed as described by Barua et al. (1993). The AFLP methodology was essentially as described by Zabeau and Vos (1993) with the following modifications. Template DNA was prepared the using restriction enzyme combination EcoRIIMsel (Boehringer Mannheim/New England Biolabs). 2.512g genomic DNA was digested as outlined by Zabeau and Vos (1993) and two specific double stranded adapters were ligated to the fragment ends. Neither of the adapters was biotinylated and the selection step using streptavidin coated magnetic beads was omitted. Adapter sequences were: EcoRI 5' CTCGTAGACTGCGTACC 5' AATTGGTACGCAGTC MseI 5' GACGATGAGTCCTGAG 5' TACTCAAGGACTCAT 53 The digested and ligated DNA was preamplified using an EcoRI-directed primer and an MseI-directed primer. The primers did not have additional selective nucleotides at the 3' end. The sequences of the primers were E00 5' GACTGCGTACCAATTC MOO 5' GATGAGTCCTGAGTAA Preamplification was performed in a total volume of 25111 containing 75ng each of primers E00 and MOO, 0.2mM of all four dNTPs (Pharmacia), 1 x PCR buffer (Perkin Elmer Cetus), 1U Amplitaq DNA Polymerase LD ( Perkin Elmer Cetus) and 3Ong of the digested and ligated DNA. The cycle profile used was as follows; denaturation for 30s at 94°C, annealing for 30s at 60°C, and extension for 60s at 72°C, for 30 cycles. After preamplification, the product was diluted by the addition of 55111 of buffer (10mM Tris pH8, 0.1mM EDTA). This mixture was used as a template for selective amplification. Selective amplification was carried out using adapter-directed primers. Nine different primer combinations were used, each combination consisted of one 'Eco' primer and one 'Mse' primer. All of the primers had three selective nucleotides at the 3' end. Primer combinations and base extensions were: Primer combination EcoRI Msel e32m34 AAC AAT e35m42 ACA AGT e36m33 ACC AAG e36m36 ACC ACC e36m50 ACC CAT e38m31 ACT AAA 54 Primer combination EcoRI Msel e38m50 ACT CAT e39m61 AGA CTG e41m33 AGG AAG In each case the 'Eco' primer was radiolabelled using 33P -ATP as described by Vos et al. (1995). The amplification reactions were carried out in a total volume of 20111, comprising 6.7ng labeled EcoRI primer, 25ng unlabelled EcoRI primer, 3Ong Msel primer, 0.2 mM of all four dNTPs, 1 x PCR buffer (Perkin Elmer Cetus), 0.5U Amplitaq DNA Polymerase (Perkin Elmer Cetus) and 2111 of template DNA. Reactions were carried out using the cycle profile described by Vos et al. (1995). All PCR reactions were performed using a Perkin Elmer 9600 thermocycler. Reactions were stopped by the addition of an equal volume of formamide loading buffer (98% formamide, 10mM EDTA pH8, Bromophenol Blue, Xylene Cyanol). The samples were denatured at 90°C for 5 minutes. Then, 3.51.11 of each sample was loaded onto a 40cm, 6% denaturing polyacrylamide gel (Easigel, Scotlab) which had been preheated by running at 80W for 30mins. The samples were then electrophoresed at a constant power of 80W for lhr 45mins. Gels were transferred to Whatmann 3MM paper and dried for 2 hrs at 80°C on a gel drier (Biorad). They were then exposed to autoradiographic film (X-OMAT S, Kodak) to visualize the results. Results were scored manually. The AFLP loci were named based on enzyme, primer sequence, and band size. For example, e38m311 refers to band "1" revealed in this germplasm, using primer sequence 38 with EcoRI and primer sequence 31 with Msel. 55 Phenotyping The DH lines were evaluated in four field tests. In the first test, lines and parents were grown in uni-replicate hill plots at Celaya, Mexico in the winter of 1995. To initiate a field epidemic, spreader rows (formed from a mixture of 15 extremely susceptible genotypes) were inoculated with a stripe rust isolate whose virulence pattern corresponds to the race 24 Varunda- Mazurka type described by Dubin and Stubbs (1986). Stripe rust severity was rated at DGS 59 (Feekes stage 10.5) as percent severity on a plot basis. Percent severity was estimated visually as the percentage of the total plant canopy in each plot that was covered with stripe rust pustules. DH lines and parents were then assessed at three planting dates at Toluca, Mexico in the summer of 1995. A single replicate was grown at each planting date, using three-m, one row plots. Spreader rows, planted at 5.25 m intervals and consisting of a mixture of 15 susceptible genotypes, were inoculated twice with infected plants placed in the foliage and with applications of spores suspended in oil. Infected plants and spores were collected locally. The race composition of this inoculum was not determined. Genotypes inoculated in this fashion will never escape rust infection (H. Vivar, personal communication). Stripe rust was rated as percent severity on a plot basis. At the time of rating, genotypes at the three planting dates were at growth stages DGS 75, DGS 59, and DGS 49, respectively. Multiple planting dates were used in an attempt to determine the effect of maturity on the expression of stripe rust resistance. In the summer of 1996 the ten most resistant and ten most susceptible lines (as measured by average performance in the previous four tests), and the two parents, were grown at Toluca, Mexico. The phenotyping and rating procedures were the same as those employed in 1995. 56 Data Analysis Of the 134 DH lines that were produced, there was sufficient seed to include 96 in all four 1995 phenotyping experiments. Subsequent analyses were based on these 96 genotypes. Each of the four experiments grown in 1995 were considered replicates for the purposes of calculating an estimate of the heritability of stripe rust severity. This heritability estimate was calculated as: H2 cy2g/(0.2g 62e /r)) where 62g is the variance among DH lines, 62e is the error variance, and r = 4, the number of environments sampled. Alleles at the 120 marker loci were scored as 0 (Steptoe) or 1 (BSR41) and were considered independent variables. Stripe rust severity data were considered dependent variables and were used for estimating genotype: phenotype associations via simple and multiple regression. These associations were determined for each of the 1995 environments, and from the mean of the four 1995 environments. Individual markers that were significant (p < .05) determinants of trait expression were included in multiple regression models. Determining the joint effects of multiple loci could be biased if linked loci are included in a multi-locus model. Therefore, only the most significant single locus from each group of linked loci was included in the multi-locus model. Procedures for grouping linked loci are described in the next paragraph. Multiple regression models were evaluated using Sawa's Bayesian Information Criterion (BIC), as available in the Statistical Analysis System (SAS, 1988). The sign of the slope was used to identify the value of stripe rust reaction alleles contributed by each parent. Negative and positive effects indicate that BSR41 57 contributed resistant and susceptibility QTL alleles, respectively. The R2 value was used as a measure of the total phenotypic variance accounted for by each marker and by the joint analysis of multiple markers. A genotypic coefficient of variation was calculated as R2p/H2, where Rep is the proportion of phenotypic variance accounted for by a marker or set of markers, and H2 is the heritability. Putative map positions of the AFLP and RAPD loci were established as follows. Homologous AFLP products, identified by fragment sizes, have been shown to map to the same regions in the barley genome in a study of a number of barley crosses (Waugh et al., 1997). Some of the primer combinations used in the current study had also been used in extending the Dicktoo x Morex map (Hayes et al., 1996a) and inclusion of Dicktoo and Morex on the AFLP gels for the current study enabled the identification of 12 markers also segregating in the Dicktoo x Morex population. The chromosomal locations of these markers were therefore established by reference to the Dicktoo x Morex map. In order to establish tentative linkage group assignments for AFLP and RAPD markers that could not be directly related to mapped loci in other mapping populations, we employed multivariate analysis of all of the marker data. Similarities between markers in a backcross population are equivalent to 1-r, where r = the recombination value (Ramsay and Thomas, 1992). For an unselected backcross of 66 individuals, markers showing similarities >0.74 and >0.82 are significantly linked at the 0.05 and 0.01 probability levels respectively. Groups showing similarities of >0.82 were therefore formed and given a tentative chromosomal assignment if they contained markers mapped in other populations. Because the population assayed with molecular markers is based on 11 selected BC1 58 genotypes, we cannot be completely sure of the chromosome location of these groups. However, the putative locations provided an objective basis for identifying a single locus from each group of loci for the multilocus regression models, as described in the previous paragraph. Results The phenotypic distribution of stripe rust severity in the DH lines, averaged over the four environments sampled in 1995, did not show discrete classes (Figure 3.2). 15 Steptoe 81.6+1-4.5 BSR41 12.1+/-1.5 12 i) 9 0 a) E 6 z 3 0 I 1 I 10 I I 1 I- 111111111 1 1 I 1 1 I I 1111 11 N 1 20 I 30 40 50 60 Disease severity (% ) 70 90 100 Figure 3.2 Average stripe rust disease severity (%) in doubled haploid lines derived from one cycle of marker-assisted backcrossing using BSR41 and Steptoe as the donor and recurrent parents, respectively. 59 Similar distributions were observed for each of the individual environments (data not shown). The standard errors for disease severity for BSR41 and Steptoe were + 1.5 and + 4.5%, respectively. The heritability of stripe rust severity, calculated using environments as replicates, was 0.95. A total of 120 data points were generated on the 96 genotypes. Of these, four were RFLPs, two were morphological markers, one-hundred and six were AFLPs and eight were RAPDs. The individual markers that were significant determinants of stripe rust severity are shown in Table 3.1. These include markers used for introgression of stripe rust resistance QTLs on chromosome 4 (4H) (Bmyl) and chromosome 7 (5H) (CD057). The chromosome 4 markers ABG54 and ABG366 were not significantly associated with stripe rust severity. WG530, which is proximal to CD057, did not have a significant association with stripe rust severity, while mSrh, which is distal to CD057, did have a significant association with stripe rust severity. Effects for these flanking loci were negative, indicating that BSR41 contributed the resistance alleles. Nine of the AFLPs and one of the RAPDs also showed significant associations with stripe rust severity. Of these, three were positive effects, indicating that Steptoe contributed the favorable allele. Of the markers that were significant determinants of stripe rust severity in single locus regressions, five were significant in the multi-locus model for the average of the four 1995 environments (Table 3.2). These included the CD057 and Bmyl markers used for introgression of resistance QTLs and three AFLP markers: e36m36a, e38m311, and e36m50i. Based on the relationship with the Dicktoo x Morex map, the e36m36a marker is on chromosome 3 (3H). The chromosome positions of the e36m50i, and e38m311 60 markers can only be inferred by the multivariate analysis. The e38m311 marker was grouped with the e39m61k locus, which is equivalent to the e39m61s locus on the Dicktoo x Morex map (Hayes et al. 1996a). The e39m61s locus is 48.8 cM distal to the mSrh locus on the Dicktoo x Morex map. Therefore, with reference to the current study, the e38m311 locus would be expected to be unlinked with CD057. Table 3.1 Chromosome location, slope, p-value, and R2 for markers significantly associated with stripe rust severity in single locus regressions. Chromosome locations in italics are putative. Negative and positive slopes indicate that BSR41 contributed resistance and susceptibility QTL alleles, respectively. Marker Chromosome location Slope p-value e36m36a e41m33j e41m33p e36m33j e36m36h OPD8a 3 3 3 3 3 3 -17.5 -13.1 -13.5 -12.0 -12.6 -12.9 0.000 0.001 0.001 0.009 0.002 0.005 10.4 10.8 8.3 9.7 9.6 Bmyl e32m24q 4 4 -11.1 -9.4 0.006 0.042 7.9 5.2 CD057 7 7 ? ? ? -15.6 -13.9 0.000 0.000 0.011 0.041 0.063 15.6 12.1 MSrh e36m36d e36m50i e38m311 13.6 12.1 11.9 19.1 8.1 5.4 4.5 On the Dicktoo x Morex map, the e39m61 s locus is 16.5 cM from the mR locus. In the current study, there was no consistent pattern of association between alleles at the mR and e38m311loci, indicating a lack of linkage. The e36m50i locus was grouped with the chromosome 3 (3H) loci in the multivariate analysis. 61 In the average of the four environments, the CD057, e36m36a, Bmyl, e38m311, and e36m50i loci accounted for 54% of the phenotypic, and 57% of the genotypic, variation in stripe rust severity. As shown in Table 3.2, CD057 was the only locus significant in all four environments. The e36m33d locus effect was unique to the Celaya environment. The e36m36a and e38m311loci were common to all of the Toluca environments. The e36m50i and Bmyl loci were significant in the second and third Toluca environments. Table 3.2 Markers with significant effects in multi-locus regression models of stripe rust severity in individual environments and in the average of four environments. Negative and positive slopes indicate that BSR41 contributed resistance and susceptibility QTL alleles, respectively. Environment Marker Slope p-Value CD057 e36m36a Bmyl -17.7 -14.3 -12.4 e38m311 e36m501 13.9 10.4 0.0001 0.0001 0.0008 0.0032 0.0206 0.54 Celaya CD057 -14.6 DGS 59 e36m33d e36m50i 0.0100 0.0400 0.0300 0.29 0.0001 0.0001 0.0129 0.41 0.0005 0.0005 0.0001 0.0012 0.0170 0.52 0.0001 0.0051 0.0001 0.0017 0.0649 0.60 Average 14.1 16.3 Toluca 1 CD057 DGS 75 e36m36a -22.2 -19.4 e38m311 15.0 CD057 e38m311 -17.1 -17.4 -19.8 20.9 e36m50i 14.6 Toluca 2 DGS 59 Toluca 3 DGS 49 e36m36a Bmyl CD057 -20.91 e36m36a Bmyl -10.26 -15.90 e38m311 e36m50i 14.36 8.24 62 Table 3.3 Average stripe rust severities in 1995 and 1996 for the ten most resistant and susceptible doubled haploid lines derived from one cycle of marker assisted selection. Allelic structure for each line is shown at marker loci significant in multi-locus regressions. Alleles from BSR41 are coded as "1", and this genotype is expected to contribute resistance alleles at QTL linked to e36m36a, Bmyl, and CD057. Alleles from Steptoe are coded as "0" and this genotype is expected to contribute resistance alleles at QTL linked to e36m50i and e38m311. Marker allele genotypes contrary to these expectations are shown in italics. Line number Mean stripe rust severity Mean stripe rust severity 1995 1996 e36m36a CD057 Bmyl e36m50i e38m311 10 most resistant lines SR33 SR35 SR43 SR47 SR66 SR80 SR116 SR123 SR125 SR127 13 8 1 1 1 0 0 18 18 1 0 1 0 23 29 1 0 1 1 1 1 14 22 22 27 0 0 0 18 22 18 14 7 7 18 17 20 13 0 0 1 1 1 1 0 0 1 1 0 0 1 1 1 1 1 1 0 0 0 0 1 1 1 0 0 1 0 1 0 0 0 0 0 0 0 0 10 most susceptible lines SR5 SR8 SR34 SR46 77 90 0 73 63 75 91 0 0 0 1 1 0 1 0 0 0 0 88 0 0 1 SR50 SR72 SR85 SR93 SR97 SR120 0 0 1 1 83 85 87 93 95 0 0 0 1 0 65 90 65 78 70 72 72 90 0 0 1 1 0 0 0 1 0 0 0 0 0 0 0 0 0 0 1 82 92 0 0 0 0 0 12 8 1 1 1 1 1 1 Parents Steptoe BSR41 Marker genotypes for loci significant in the average multi-locus regression model of the ten most resistant and ten most susceptible genotypes, together with their average 63 stripe rust severity ratings in the 1995 and 1996 field trials, illustrate the phenotype: genotype associations (Table 3.3). The ten most resistant genotypes traced to six different backcross plants (Figure 3.1). Three of the four resistant lines selected for accelerated assessment as potential varieties (SR123, SR125, and SR127) traced to BC1 plant no. 58 (Figure 3.1). The first three of these selections have the favorable marker genotypes at all loci. SR127 has the susceptibility marker allele at the chromosome 4 (4H) marker locus. There were five deviations from the predicted favorable allele genotype among the most resistant lines and 20 deviations from the predicted unfavorable genotype in the susceptible lines. 7 93 127 6 85 123 43 5 _8 34 116 125 12 72 35 80 47 66 33 97 4 50 5 46 .n 3 2 1 Donor genome (%) Figure 3.3 Percentage of donor parent genome in doubled haploid lines derived from one cycle of marker assisted backcrossing as measured by 120 markers. The numbers in standard and italic font are line numbers (see Table 3.3) of the ten most resistant and susceptible genotypes, respectively. 64 Based on 120 data points, the percentage of donor parent genome in the population ranged from seven to 56 percent, with an average of 32.6% (Figure 3.3). Considering the ten most resistant genotypes, the percentage of donor genome ranged from 19 to 46%, with an average of 34.8%. The percentage of donor genome in the ten most susceptible lines ranged from 8 - 43%, with an average of 24.6%. The four resistant genotypes selected for accelerated regional assessment as potential varieties were selected before the AFLP data were available. Selection was based on phenotypic resemblance to the recurrent parent (Steptoe). The percentage of donor parent genome in these genotypes ranged from 32 - 39%. Discussion The continuous distribution of disease severity phenotypes (Figure 3.2) is probably due to the quantitative expression of the genetic determinants of resistance rather than to experimental error in phenotyping. The heritability of 0.95 is an approximation since environments were used as replicates. However, this high value indicates a high degree of consistency, or repeatability, in the measurement of the stripe rust severity phenotype. The single locus regressions underscore the importance of resistance QTLs on chromosomes 7 (5H) and 4 (4H) that were introgressed from BSR41 into the Steptoe background. The significance of mSrh, and lack of significance of WG530, indicates that the resistance QTL on chromosome 7 (5H) is most likely distal to CD057. Multiple QTL peaks were observed in the original mapping population (Chen et al. 1994). On chromosome 4 (4H), the higher R2 value for Bmyl indicates the resistance locus is closer 65 to this marker than to the proximal markers ABG366 and ABG54. The significance and sign of the chromosome 4 (4H) and 7 (5H) markers confirms that in these regions of the genome there are loci determining adult plant reaction to stripe rust. We recognize that experiment-wise error rates complicate single locus regression procedures with large marker data sets and that a selected population is not as appropriate for QTL detection as a defined mapping population. However, the presence of loci significant in both the single locus and multi-locus models indicates that other regions of the genome were potentially important in determining reaction to stripe rust in the Steptoe background. There is an important resistance QTL, tentatively mapped to chromosome 3, where BSR41 contributes the favorable allele. Steptoe contributed resistance QTL alleles at markers that could not be assigned genome positions. We hypothesize that favorable (resistance) alleles were fixed at these additional resistance loci in the original mapping population (Calicuchima-sib x Bowman). This underscores the lack of predictability that may be encountered when QTLs detected in a mapping population are transferred to other genetic backgrounds. Determining the joint effects of multiple resistance loci could be biased if linked marker loci are included in a multi-locus model. In our data, some linkage relationships are documented, as in the case of the marker loci used to introgress resistance QTLs on chromosomes 4 (4H) and 7 (5H). In other cases, we used multivariate analysis to establish tentative linkage groups. In this way, five AFLP and one RAPD locus (Table 3.1) group together and were tentatively assigned to chromosome 3 (3H). One of these loci (e36m36a) was significant in the multi-locus model of average stripe rust severity. BSR41 contributed the resistance allele at this locus. 66 In the case of the e38m311 and e36m50i loci, we could not assign map positions to these AFLPs that showed associations with stripe rust resistance. These associations are particularly intriguing because the susceptible parent (Steptoe) contributed resistance alleles. For the purposes of simultaneous locus discovery and advance of breeding material through backcrossing, as proposed by Tanksley and Nelson (1996), the ideal marker would have a defined map position. AFLPs are an excellent type of marker for rapidly generating large amounts of polymorphism data (Becker et al. 1995; Waugh et al. 1997). AFLP products of the same size assayed in different genotypes may represent the same locus. However, this needs to be demonstrated on a case-by-case basis. Five markers accounted for 54% of the variation in phenotypic expression of stripe rust severity, averaged over the four environments. Using the heritability estimate of 95%, these markers accounted for 57% of the genetic variation in trait expression. The fact that the marker loci do not account for a higher proportion of phenotypic and genotypic variance is probably attributable to recombination between marker loci and the target QTLs, and the effects of additional loci that contribute to the expression of resistance. The individual effects of these additional loci cannot be detected at the level of resolution afforded by the experiment. This raises the question of how much variance can be accounted for by QTLs, because the magnitude of the phenotypic, or genotypic, R2 may be used to determine the likelihood that all loci that are important determinants of trait expression have been detected. By way of perspective, the mV locus determines fertility of lateral florets in barley. Two-row barley genotypes, with sterile lateral florets, typically have higher 67 kernel weights than six-row barley genotypes. When kernel weight was mapped as a QTL in the doubled haploid progeny of Calicuchima-sib x Bowman a two-row x sixrow cross, the mV locus accounted for 77% of the variation in phenotypic trait expression (Hayes et al. 1996c). Therefore, having detected 54% and 57% of the phenotypic and genotypic variance, respectively, in the expression of adult plant stripe rust resistance, we believe that we have located the principal genes determining stripe rust resistance in this germplasm. Two are the QTLs that were introgressed, and these are located on chromosomes 4 (4H) and 7 (5H). One is likely on chromosome 3 (3H), and the map positions of the remaining loci cannot be determined at this time. The multi-locus analyses of the individual environment data underscore the importance of using multiple measures of phenotype and, potentially, the importance of measuring disease reaction at different growth stages. The heritability estimate of stripe rust severity was 0.95. This indicates a consistency of response across the four environments. Therefore, the average of the four environments is one appropriate measure of the stripe rust reaction phenotype. However, individual environment data may also be useful. For example, the Celaya environment was useful in revealing the significance of the resistance QTL linked to e36m33d. This locus was not significant in any of the Toluca environments, or in the multi-locus model based on average severity. Markers accounted for the lowest percentage of phenotypic variance at Celaya, and we attribute this to the use of hill plots. Iyamabo and Hayes (1995), in a comparison of hill and row plots for QTL detection, reported that hill plots were best suited to detection of characters determined by largeeffect QTLs. 68 In terms of growth stage, the chromosome 4 (4H) marker Bmyl was significant only at growth stages DGS 49 and DGS 59. If assessments were based only at DGS 75, the effect of the chromosome 4 (4H) resistance QTL would not have been detected. The chromosome 4 (4H) effect may be due to maturity or it may reflect a resistance locus important at earlier stages of plant growth. The Sh locus is 2.6 cM from Bmyl (Laurie et al. 1995) and a heading date QTL was detected at the same position in the Calicuchima- sib x Bowman population (Hayes et al. 1996c). On the other hand, in a controlled environment seedling test of the Calicuchima-sib x Bowman mapping population, a stripe rust resistance QTL was detected proximal to Bmyl (Hayes et al. 1996c). The patterns of association of genotype and phenotype in the ten most resistant genotypes are additional evidence for the importance of resistance QTLs linked to markers on chromosomes 3 (3H), 4 (4H), and 7 (5H) and to the two unmapped loci (Table 3.3). The ten most resistant selections traced to different BC 1 plants, but three of the selected genotypes traced to a single backcross plant (Figure 3.1). The first three of these selections have the favorable marker genotypes at all loci (Table 3). The fourth selection may represent a crossover event between the resistance locus and the Bmyl marker locus on chromosome 4 (4H) , or it may lack the chromosome 4 (4H) QTL. This implies that in marker-assisted backcross projects it may be advisable to advance material derived from multiple backcross plants. This would be even more important when multiple cycles of marker assisted backcrossing are used without the benefit of phenotypic assessment for the target phenotype. The higher degree of correspondence between marker genotype and phenotype in the resistant vs. the susceptible lines suggests epistasis, as one would expect similar 69 frequencies of crossovers between marker and resistance loci in the two groups. That is, the resistant phenotype resulted only when appropriate resistance alleles were configured at multiple loci, while the susceptible phenotype resulted from the presence of only one or a few susceptibility alleles. SR97, for example, was susceptible to stripe rust but had resistant marker alleles at four out of five loci. This suggests that if a complex phenotype, such as adult plant resistance, is the consequence of a complex multi-locus pathway, it would be relatively easy to disrupt the pathway with susceptible alleles at various points in the pathway. The resistant phenotype would result only with resistant alleles at all, or most, points in the pathway. AFLP markers were useful for identifying additional resistance loci and they provided information on the genetic structure of the BC1-derived DH population. The population average of 32.6% donor parent genome is, as would be expected, higher than 25%, as the BC1 plants were selected (Figure 3.3). As shown in this Figure, percentages of recurrent parent genome ranged from seven to fifty-six percent. The resistant genotypes had a higher average percentage of donor parent genome (34.8%) than the susceptible genotypes (24.6%), but the difference was not that great, considering that portions of the genome on at least three chromosomes were introgressed into the resistant lines. Four DH lines were selected, based on their phenotypic resemblance to the recurrent parent, for accelerated assessment as potential varieties before the AFLP data were available. The percentage of donor parent genome in these lines ranged from 32 39%. If resources are available, larger populations of BC1-derived lines could be used. In this case, the percentage of recurrent parent genome would be a more useful selection criterion. 70 In summary, marker-assisted mapping and transfer of stripe rust resistance QTLs allowed us make use of limited resources to rapidly develop barley germplasm with potential for commercial production. Markers that were targets for transfer were significantly associated with resistance in a genetic background different from the reference mapping population. The use of high throughput markers - primarily AFLPs allowed us to detect additional resistance QTLs, including QTLs where the susceptible parent contributed resistance alleles. Our findings may be useful in view of the many ongoing efforts in a number of crop species to introgress QTLs. When QTLs mapped in a reference population are introgressed into new genetic backgrounds, the anticipated selection responses may not be achieved. Precautions can be taken during the introgression process to minimize the loss of QTLs through double crossovers between flanking markers and to guard against the consequences of imprecise positioning of the QTLs in the original mapping population. However, the configuration of alleles in the breeding population at loci where alleles were fixed in the mapping population may not be known. We hypothesize that this was the case with the new resistance QTLs resistance alleles we detected in the Steptoe background. An intriguing, but unanswered, question is the relationship between disease resistance QTLs and race-specific resistance genes. At this point we know nothing regarding the kinds of genes that are detected as adult plant stripe rust resistance QTLs. Syntenic relationships in the Triticeae should allow for useful comparative mapping and extension of findings from one genus to another. For example, the stripe rust resistance QTL on chromosome 7 (5H) may be homoeologous with one of the durable stripe rust resistance loci in wheat described by Law and Worland (1997). 71 References Bakshi JS, Luthra JK (1971) Inheritance of resistance to stripe rust (Puccinia striiformis west.) in barley. In: Nilan RA (ed.) Proc Symp Barley Genet II. Washington State University Press, Pullman, Washington, pp 478-483 Barua UM, Chalmers KJ, Hackett CA, Thomas WTB, Powell W, Waugh R (1993) Molecular mapping of genes determining height, time to heading, and growth habit in barley (Hordeum vulgare). Heredity 71:177-184 Becker J, Vos P, Kuiper M, Salamini F, Heun M (1995) Combined mapping of AFLP and RFLP markers in barley. Mol Gen Genet 249:65-73 Chen F, Prehn D, Hayes PM, Mulrooney D, CoreyA, Vivar H (1994) Mapping genes for resistance to barley stripe rust (Puccinia striiformis f. sp. hordei). Theor Appl Genet 88:215-219 Chen F, Hayes PM (1989) A comparison of Hordeum bulbosum - mediated haploid production efficiency in barley using in vitro floret and tiller culture. Theor Appl Genet 77:701-704 Dubin HJ, Stubbs RW (1986) Epidemic spread of barley stripe rust in South America. Plant Disease 70:141-144 Hayes PM, Chen FQ, Corey A, Pan A, Chen THH, Baird E, Powell W, Thomas W, Waugh R, Bedo Z, Karsai I, Blake T, Oberthur L (1996a) The Dicktoo x Morex population: a model for dissecting components of winterhardiness in barley. In: Li PH, Chen TH(ed.) Plant Cold Hardiness. Plenum Press, New York, USA Hayes PM, Chen FQ, Kleinhofs A, Kilian A, Mather D (1996b) Barley genome mapping and its applications. In: Jauhar PP (ed) Methods of Genome Analysis in Plants: CRC Press. Boca Raton, USA Hayes PM, Prehn D, Vivar H, Blake T, Comeau A, Henry I, Johnston M, Jones B, Steffenson B (1996c) Multiple disease resistance loci and their relationship to agronomic and quality loci in a spring barley population. J.QTL http ://probe.nalusda.gov:8000/otherdocs/j QTL /index.htm Iyamabo OE, Hayes PM (1995) Effects of plot type on detection of quantitative trait locus effects in barley (Hordeum vulgare L.). Plant Breeding 114:55-60 Laurie DA, Pratchett N, Bezant JH, Snape JW (1995) RFLP mapping of five major genes and eight quantitative trait loci controlling flowering time in a winter x spring barley cross. Genome 38:575-585 72 Law CN, Worland AJ (1997) The control of adult-plant resistance to yellow rust by the translocated chromosome 5SB-7BS of bread wheat. Plant Breeding 116:59-63 Lehmann CO, Nover I, Scholz F (1975) The Gatersleben barley collection and its evaluation. In Gaul H (ed) Proc Symp Barley Genet III. Verlag Karl Thiemig, Munich, pp 64-69 Line RF (1993) Durability of resistance to Puccinia striiformis in North American wheat cultivars. p. 332. In Durability of Disease Resistance. Jacobs TH,. Parlevliet JE (eds) Kluwer Academic Publishers 375 pp Marshall D, Sutton RL (1995) Epidemiology of stripe rust, virulence of Puccinia striiformis f. sp. hordei, and yield loss in barley. Plant Disease 70:732-737 Mather D (1995) Barley Steptoe x Morex and Harrington x TR306 basemaps. gopher://greeng enes. cit. cornell. edu:70/1ftp%3Agnome. agrenv.mcgill.ca@pub/ genetics/data/basemaps/ Parlevliet JE, Van Ommeren A (1975) Partial resistance of barley to leaf rust, Puccinia hordei. II. Relationship between field trials, micro plot test and latent period. Euphytica 24:293-303 Powell W, Baird E, Booth A, Lawrence P, MacAulay M, Bonar N, Young G, Thomas WTB, McNicol JW, Waugh R (1996) Single and multi-locus molecular assays for barley breeding and research. In: Barley Genetics VII Proc of Intl Symp, Saskatoon, Canada Qi X, Stam P, Lindhout P (1996) Comparison and integration of four barley genetic maps. Genome 39:379-394 Ramsay G, Thomas WTB (1992) Two-dimensional representations of linkage groups using similarity matrices. Abstracts of the 11th International Chromosome Conference, Edinburgh, UK, 4 -4 8 August, 1992 Statistical Analysis Institute (1988) SAS/STAT User's Guide, 6.03. SAS Institute, Cary, NC, USA Tanksley SD, Nelson JC (1996) Advanced backcross QTLs analysis: a method for the simultaneous discovery and transfer of valuable QTLs from unadapted germplasm into elite breeding lines. Theor Appl Genet 92:191-203 73 Thomas WTB, Powell W, Waugh R, Chalmers KJ, Barua UM, Jack P, Lea V, Forster BP, Swanston JS, Ellis RP, Hanson PR, Lance RCM (1995) Detection of quantitative trait loci for agronomic, yield, grain and disease characters in spring barley (Hordeum vulgare L.). Theor Appl Genet 91:1037-1047 von Wettstein-Knowles P (1992) Cloned and mapped genes:current status. In Shewry PR (ed) Barley:genetics, biochemistry, molecular biology, and biotechnology. CAB Int, Wallingford UK, pp 73-98 Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Homes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucl Acids Res 23: 4407-4414 Waugh, R, Bonar N, Baird E, Hayes P, Graner A, Thomas WTB, and Powell W (1997) Homology of AFLP products in three mapping populations of barley. Mol. Gen. Genet. 255:311-321. Young ND, Tanksley SD (1989) RFLP analysis of the size of chromosomal segments retained around the Tm-2 locus of tomato during backcross breeding. Theor Appl Genet 77:353-359 Zabeau M, Vos P (1993) European Patent Application publication no. EP 0534858 74 CHAPTER 4 CONCLUSION The first objective of this research was to map genes responsible for resistance to multiple diseases - stripe rust (Puccinia striiformis f.sp hordei)), leaf rust (Puccinia hordei), and Barley Yellow Dwarf Virus (BYDV) - in the doubled haploid (DH) progeny of Shyri x Galena. We generated eight hundred and nine polymorphism using RFLP, SSR, AFLP and RGAP markers. Our strategy was to use RFLPs and SSRs as anchor . markers and to use AFLPs and RGAPs to fill the gaps. A subset of the total markers was used to construct the base map, which consists of 99 markers with an average interval distance of 10-15 cM. This map covers approximately 92 % of the barley genome. Locus ordering and distance were in agreement with published maps. SSR markers are a powerful tool for constructing a skeleton map, due to their high informative content and the ease of genotyping. However, the catalog of SSRs does not yet provide full genome coverage. RFLPs markers were of crucial importance for extending our linkage maps into some regions of the genome. The high throughput AFLP and RGA markers are useful for rapidly generating abundant markers to fill gaps. In our hands, AFLPs were not as useful as we had hoped. Although data scoring was not an issue - band patterns on autoradiographs were clear and consistent the resulting AFLPs data, when added to established linkage groups of SSR and RFLP markers, usually led to significant map expansion. AFLPs maybe useful in cases where marker saturation is required, and the resulting AFLP products will be cloned and converted to a more stable format. RGAP markers, on the other hand, were 75 generally high quality data points. The RGAP marker technology is attractive from a number of standpoints, and research with this class of markers should be continued. Using the 99-point base map, we determined the number, genome location, and effects of loci determining resistance to the three diseases. QTLs were identified on chromosomes 2, 3, 5 and 6 for stripe rust resistance; on chromosome 1 for leaf rust resistance; and chromosomes 1, 3, 4, and 5 for BYDV resistance. Leaf rust resistance showed qualitative inheritance and subsequently mapped as single locus. Two-locus epistasis was important in determining resistance to stripe rust, but epistasis was not significant in the case of leaf rust or BYDV resistance. Shyri clearly has a different major different QTL for stripe resistance than Calicuchima-sib (Chen et al. 1994) and CMB643 (Hayes et al. 1998). The smaller-effect stripe rust resistance QTL on chromosome 3 was coincident with QTLs detected in CMB643 (Hayes et al. 1998), Kold (Hayes et al.1998), and Steptoe derivatives (Toojinda et al. 1997). The smaller-effect QTLs on chromosomes 4 and 6 were only detected in Calicuchima-sib (Chen et al. 1994) and Shyri respectively. We have observed significant two locus interactions between small-effect QTLs and large effect QTLs in multiple mapping populations: Calicuchima-sib x Bowman (Chen et al.1994), Gobernadora x CMB643 (Hayes et al. 1998) , Kold x Colter (Hayes et al. 1998), and now in Shyri x Galena. These significant interactions indicate the importance of epistasis in determining stripe rust resistance. Epistasis of loci determining stripe rust resistance was also reported by Chen and Line (1998). The chromosome 1 QTL was the single largest determinant for leaf rust resistance in Shyri. When the qualitative data were treated as a single locus effects, we mapped the 76 Rphxs locus to approximately the same location as the Rph3 (Jin et al. 1993) and Rphxc (Hayes et al. 1996) loci. We identified three major QTLs, and one secondary QTL, for serotype non-specific resistance to BYDV. Resistance was determined by additive effects at these loci. The contribution of two major QTLs on chromosome 1 and 5 was consistent with all trait measurements. The secondary QTL on chromosome 3 was coincident with a QTL determining plant height. At the level of resolution afforded by this mapping population, we cannot determine if this coincidence is due to linkage or pleiotropy. However, the genome location of this QTL is different from Yd2 locus. Therefore, BYDV resistance in this germplasm is clearly not due to the Yd2 locus. The chromosome 4 QTL was detected with the dwarfness score and plant height reduction, but it was not significant with the tillering score data. We demonstrated linkage relationships of RGAPs with genes conferring quantitative and qualitative resistance to two fungal pathogens: Puccinia striiformis fsp. hordei and Puccinia hordei, and quantitative resistance to a viral pathogen, BYDV. The coincidence of RGAPs with QTL peaks, or loci, and a qualitative locus determining disease resistance demonstrates the power of the RGAP technique for mapping qualitative and quantitative disease resistance genes. The clustering of RGAPs, and their linkage relationships with qualitative and quantitative resistance genes, is evidence for the existence of resistance gene clusters in barley and may be based on commonalities of qualitative and quantitative resistance genes. The availability of abundant RGAP markers should facilitate isolation and characterization of both qualitative and quantitative resistance. This will provide tools for answering fundamental questions regarding the genetic basis of the two classes of resistance. 77 We found that quantitative stripe rust resistance was more complex than the single large-effect QTLs that are detected in reference mapping populations. When chromosome 4 and 7 stripe rust resistance QTLs mapped in Cali-sib x Bowman (Chen et al., 1994) were introgressed into an unrelated genetic background (Steptoe), the resistance QTLs that were introgressed were significant determinants of resistance, as were previously undetected QTLs. Therefore, small-effect QTLs should be considered in resistance QTL introgression and pyramiding experiments. This experiment demonstrated the efficiency of marker-assisted disease resistance breeding in barley. Molecular markers also increase the efficiency of backcrossing, by allowing for selection of genotypes with maximum percentage of the recurrent parent genome. In our case, the experimental population was not large enough to make effective use of such information. With this series of experiments, we have demonstrated the power of applied genomics to locate and use disease resistance genes. We are optimistic that the RGAP technique, with some modifications, will allows us to increase the efficiency of resistance gene mapping. It should also enable us to systematically pyramid multiple loci, determining resistance to multiple diseases, in agronomically useful germplasm. 78 BIBLIOGRAPHY Alemayehu F, Parlevliet JE (1996) Variation for resistance to Puccinia hordei in Ethiopian barley landraces. Euphytica 90:365-370. Bakshi JS, Luthra JK (1971) Inheritance of resistance to stripe rust (Puccinia Striiformis west.) in barley. In: Nilan RA (ed.) Proc Symp Barley Genet II. Washington State University Press, Pullman, Washington, pp 478-483 Barua UM, Chalmers KJ, Hackett CA, Thomas WTB, Powell W, Waugh R (1993) Molecular mapping of genes determining height, time to heading, and growth habit in barley (Hordeum vulgare). Heredity 71:177-184 Barua UM, Chalmers KJ, Thomas WTB, Hackett CA, Lea V, Jack P, Forster BP, Waugh R, Powell W (1993) Molecular mapping of genes determining height, time to heading, and growth habit in barley (Hordeum vulgare). Genome 36: 1080-1087. Bassam BJ, Bentley S (1994) DNA fingerprinting using arbitrary primer technology (APT): a tool or a torment. Australasian Biotechnology 4: 232-236 Becker J, Heun M (1995) Barley microsatellites: allele variation and mapping. Plant Mol Biol 27:835-845. Becker J, Vos P, Kuiper M, Salamini F, Huen M (1995) Combined mapping of AFLP and RFLP markers in barley. Mol. Gen. Genet. 249:65-73. Bennetzen JL, Freeling M (1997) The unified grass genome: synergy in synteny. Genome Research 7: 301-306. Bezant J, Laurie D, Pratchett N, Chojecki J, Kearsey M (1997) Mapping QTL controlling yield and yield components in a spring barley (Hordeum vulgare L.) cross using marker regression. Molecular Breeding 3: 29-38. Borst P, Greaves DR (1987) Programmed gene rearrangements altering expression. Science 235:658-667. Bowles DJ (1990) Defense-related proteins in higher plants. Annu. Rev. Biochem. 59: 873-907. Browning JA, Simons MD, Torres E (1977) Managing host genes:epidemiologic and genetic concepts. In Horsfall JG, Cowling EB (eds). Plant Disease:an advanced treatise. Vol.1 . Academic Press New York. 79 Buschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J, Topsch S, Vos P, Salamini F, Schulze-Lefert P (1997) The barley Mlo gene: A novel control element of plant pathogen resistance. Cell 88:695-705. Chen F, Hayes PM (1989) A comparison of Hordeum bulbosum mediated haploid production efficiency in barley using in vitro floret and tiller culture. Theor Appl Genet 77:701-704. Chen F, Prehn D, Hayes PM, Mulrooney D, CoreyA, Vivar H (1994) Mapping genes for resistance to barley stripe rust (Puccinia striiformis f. sp. hordei). Theor Appl Genet 88:215-219. Chen XM, Line RF, Leung H (1998) Genome scanning for resistance-gene analogs in rice, barley, and wheat by high-resolution electrophoresis. Theor. Appl. Genet 97:345-355. Chen XM, Line RF, Leung 1-1 (1995) Virulence and polymorphic DNA relationships of Puccinia striiformis f.sp. hordei to other rusts. Phytopathology 85:1335-1342. Collins NC, Paltridge NG, Ford CM, Symons RH (1996) The Yd2 gene for barley yellow dwarf virus resistance maps close to the centromere on the long arm of barley chromosome 3. Theor. Appl. Genet 92:858-864. Concibido VC, Young ND, Lange DA, Denny RL, Danesh D, Orf JH (1996) Targeted comparative genome analysis and qualitative mapping ofa major partial-resistance gene to the soybean cyst nematode. Theor. Appl.Genet. 93:234-241. Conner RL, Whelan EDP, MacDonald MD (1989) Identification of sources of resistance to common root rot in wheat-alien amphiploid and chromosome substitution lines. Crop Science 29:916-919. Crute IR (1985) The genetic basic of relationships between microbial parasites and their host. Pages 62 in: Mechanisms of resistance in plant diseases. Kluwer Academic Press. D'Acry CJ (1995) Symptomatology and host range of barley yellow dwarf. Pages 9-28 in: Barley Yellow Dwarf: 40 Years of Progress. D'Acry CJ and Burnett PA, eds. The American Phytopathologal Society, St. Paul, MN. Dahleen LS (1997) Mapped clone sequences detecting differences between 28 North American Barley cultivars. Crop Science 37:952-957. 80 Dahleen LS, Hoffman DL, Dohrmann J, Gruber R, Franckowiak J (1997) Use of a subset of double-haploid lines for RAPD interval mapping in barley. Genome 40: 626-632. Dangl JL (1995) Piece de resistance: novel classes of plant disease resistance genes. Cell 80:363-366. Dean C and Schmidt R (1995) Plant genome: a current molecular description. Annual Review of Plant Physiology and Plant Molecular Biology 46: 395-418. Dingerdissen AL, Geiger HH, Lee M, Schechert A, Welz HG (1996) Interval mapping of genes for quantitative resistance of maize to Setoria turcica, cause of norther leaf blight, in a tropical environment. Molecular Breeding 2:143-156. Dubin HJ, Stubbs RW (1986) Epidemic spread of barley stripe rust in South America. Plant Dis70:141-144. El-Kharbotly A, Leonards-Schippers C, Huigen DJ, Jacobsen E, Pereira A (1994) Segregation analysis and RFLP mapping of the RI and R3 alleles conferring race-specific resistance to Phytohthora infestans in progeny of dihaploid potato plants. Mol.Gen.Genet. 242:749-754. Ellis JG, Lawswnce GJ, Peacock WJ, and Pryor AJ (1998) Approaches to cloning plant genes conferring resistance to fungal pathogens. Annual Review of Phytopathology 26:245-263. Ferreira ME, Rimmer SR, Williams PH, Osborn TC (1995) Mapping loci controlling Brassica napus resistance to Leptosphaeria maculans under different screening conditions. Phytopathology 85:213-217. Feuerstein U, Brown AHD, Burdon JJ (1990) Linkage of rust resistance genes from wild barley (Hordeum spontaneum) with isozyme markers. Plant Breed 104:318-324. Garner A, Tekauz A (1996) RFLP mapping in barley of a dominant gene conferring resistance to scald (Rhynchosporium secalis). Theor. Appl. Genet. 93:421-425. Geiger HH, Heun M (1989) Genetics of quantitative resistance to fungal diseases. Annu. Rev. Phytopathol. 27:317-341. Giease H, Holm-Jensen AG, Jensen HP, Jensen J (1993) Localization of the Laevigatum powdery mildew resistance gene to barley chromosome 2 by the use of RFLP markers.Theor.Appl.Genet. 85:897-900. 81 Giease H, Jorgensen J, Jensen HP, Jensen J (1981) linakge relationships of ten powdery mildew resistance genes on barley chromosome 5. Hereditas 95:4350. Giese H, Holm-Jensen AG, Mathiassen H, Kjaer B, Rasmussen SK, Bay H, Jensen J (1994) Distribution of RAPD markers on a linkage map of barley. Hereditas Landskrona 120: 267-273. Graner A, Bauer E (1993) RFLP mapping of the ym4 virus resistance gene in barley. Theor.Appl.Genet. 86:689-693. Graner A, Jahoor A, Schondelmaier J, Siedler H, Pillen K, Fischbeck G, Wensel G, Herrmann G (1991) Construction of an RFLP map of barley. Theor.Appl. Genet. 83:250-256. Han F, Kleinhofs A, Kilian A, Ullrich SE (1997) Cloning and mapping of a putative barley NADPH-dependent HC-toxin reductase. Molecular Plant Microbe Interactions 10: 234-239. Han F, Romagosa I, Ullrich SE, Jones BL, Hayes PM, Wesenberg DM (1997) Moleular marker-assisted selection for malting quality traits in barley. Molecular Breeding 3: 427-437. Harder DE, Harber S (1992) Barley yellow dwarf virus. Pages 379 in: Oats Science and Technology. Marshall HG, Sorrells ME, eds. American Society of Agronomy, Inc, Madison, WI. Hayes PM, Chen FQ, Corey A, Pan A, Chen THH, Baird E, Powell W, Thomas W, Waugh R, Bedo Z, Karsai I, Blake T, Oberthur L (1996a) The Dicktoo x Morex population: a model for dissecting components of winterhardiness in barley. In: Li PH, Chen TH(ed.) Plant Cold Hardiness. Plenum Press, New York, USA Hayes PM, Chen FQ, Kleinhofs A, Kilian A, Mather D (1996) Barley genome mapping and its applications. In:Jauhar PP (ed) Methods of genome analysis in plants. CRC Press. Boca Raton, USA Hayes PM, Liu BH, Knapps SJ, Chen F, Jones B, Blake T, Franckowiak J, Rasmusson D, Sorrells M, Ullrich SE, Wesenberg D, Kleinhofs A (1993) Quantitative trait locus effects and environmental interaction in a sample of North American barley germplasm. Theor. Appl. Genet. 87: 392-401. Hayes PM, Prehn D, Vivar H, Blake T, Comeau A, Henry I, Johnston M, Jones B, Steffenson B (1996) Multiple disease resistance loci and their relationship to agronomic and quality loci in a spring-barley population. J.QTL http://probe.nalusda.gov:8000/otherdocs/jQTL/index.htm 82 Heun M, Kennedy AE, Anderson JA, Lapitan NLV, Sorrels ME, Tanks ley SD (1991) Construction of a restriction fragment length polymorphism map for barley (Hordeum vulgare). Genome 34:437-447. Hoffbeck MD, Openshaw SJ, Geadelmann JL, Peterson RH, Stuthman DD (1995) Backcrossing and intermating in an exotic X adapted cross of maize. Crop Science 35:1359-1364. Holloway J, Knapp SJ (1994) Gmendel 3.0 Users Guide. Knapps@css.orst.edu. Hooker AL (1985) Corn and sorghum rust. In The cereal rusts. Vol.2. Diseases, Distribution, Epidemiology, and Control (Roelfs AP and Bushnell WR, eds) pp 207-233. Horvath DP, Dahleen LS, Stebbing JA, Penner G (1995) A co-domonant PCRbased marker for assisted selection of durable stem rust resistance in barley. Crop Science 35: 1445-1450. Iyamabo OE, Hayes PM (1995) Effects of plot type on detection of quantitative trait locus effects in barley ( Hordeum vulgare L.). Plant Breeding 114:55-60 Jin Y, Cui GH, Steffenson BJ, Franckowiak JD (1996) New leaf rust resistance in barley and their allelic and linkage relationships with other Rph genes. Phytopathology 86:887-890. Jin Y, Statler GD, Franckowiak JD, Steffenson BJ (1993) Linkage between leaf rust resistance genes and morphological markers in barley. Phytopathology 83:230-233. Johnson R (1981) Durable resistance: definition of, genetic control, and attainment. Phytopathology 71:567-568. Jorgensen JH (1992) Discovery, characterization and Exploitation of MTh powdery mildew resistance in barley. Euphytica 63:141-152. Kanazin V, Marex LF, Shoemaker RC (1996) Resistance gene analogs are conserved and clustered in soybean. Proc. Natl. Acad. Sci. USA 93:1174611750. Kilian A, Chen J, Han F, Steffenson B, Kleinhofs A (1997) Towards map-based cloning of the barley stem rust resistance gene Rpgl and Rpg4 using rice as an intergenomic cloning vehicle. Plant Molecular Biology 35:187-195. Kleinhofs A (1991) The NABGMP mapping progress report, spring 1992. Barley Genetics Newsletter 21: 38-48. 83 Kleinhofs A, Kilian A, Saghai Maroof M, Biyashev R, Hayes PM, Chen F, Lapitan A, Fenwick A, Blake T, Kanazin V, Ananiev E, Dahleen L, Kudrna D, Bollinger J, Knapp SJ, Liu B, Sorrel ls M, Heun M, Franckowiak J, Hoffman D, Skadsen R, Steffension B (1993) A molecular, isozyme, and morphological map of the barley (Hordeum vulgare) genome. Theor. Appl. Genet. 86:705712. Lamb CJ (1994) Plant disease resistance genes in signal perception and transduction. Cell 76:419-422. Laurie DA, Pratchett N, Bezant JH, Snape JW (1995) RFLP mapping of five major genes and eight quantitative trait loci controlling flowering time in a winter x spring barley cross. Genome 38:575-585. Law CN, Worland AJ (1997) The control of adult-plant resistance to yellow rust by the translocated chromosome 5SB-7BS of bread wheat. Plant Breeding 116:59-63. Lefebvre V, Palloix A (1996) Both epistatic and additive effects of QTLs are involved in polygenic induced resistance to disease: a case study, the interaction pepper-Phytophthora capsici Leonian. Theor. Appl.Genet. 93:503511. Lehmann CO, Nover I, Scholz F (1975) The Gatersleben barley collection and its evaluation. In Gaul H (ed) Proc Symp Barley Genet III. Verlag Karl Thiemig, Munich, pp 64-69. Leister D, Ballvora A, Salamini F, Gebhardt C (1996) A PCR-based approach for isolsting pathogen resistance genes from potato with potential for wide application in plants. Nature Genetics 14:421-429. Leister D, Kurth J, Laurie DA, Yano M, Sasaki T, Devos K, Graner A, SchulzeLefert P (1998) Rapid reorganization of resistance gene homologues in cereal genomes. Proc. Natl. Acad. Sci. USA 95:370-375. Line RF (1993) Durability of resistance to Puccinia striiformis in North American wheat cultivars. p. 332. In Durability of Disease Resistance. Jacobs TH., Parlevliet JE (eds) Kluwer Academic Publishers 375 pp. Liu ZW, Biyashev RM, Sagai-Maroof MA (1996) Development of simple sequence repeat DNA markers and their integration into a barley linkage map. Theor. Appl.Genet. 93: 869-876. Liu ZW, Biyashev RM, Saghai Maroof MA (1996) Development of simple sequence repeat markers and their integration into a barley linkage map. Theor. Appl. Genet. 93:869-876. 84 Maisonneuve B, Bellec Y, Anderson P, Michelmore RW (1994) Rapid mapping of two genes for resistance to downy mildew from Lactuca serriola to existing clusters of resistance genes. Theor.Appl.Genet. 89:96-104. Marek LF, Shoemaker RC (1997) BAC contig development by fingerprint analysis in soybean. Genome 40: 420-427. Maroof MAS, Biyashev RM, Yang GP, Zhang Q, Allard RW (1994) Extraordinarily polymorphic microsatellite DNA in barley: species diversity, chromosomal locations, and population dynamics. Proc. Natl. Acad. Sci. USA 91: 5466-5470. Marshall D, Sutton RL (1995) Epidemiology of stripe rust, virulence of Puccinia striiformis f. sp. hordei, and yield loss in barley. Plant Disease 70:732-737. Martin GB (1996) Molecular cloning of plant disease resistance genes. In: Plantmicrobe interactions vol. 1 . Martin GB, Herrera-Hestrella L, Rosales LS, Rivera- Bustamante R, Neuenschwander U, Lawton K, Ryals J, Schardl CL, Kronstad JW, Thomashow LS, Weller DM, Philips DA, Streit WR, Prome JC, Demont N, Stacey G (eds).Chapman & Hall; New York; USA. Mather D (1995) Barley Steptoe x Morex and Harrington x TR306 basemaps. gopher://greengenes.cit.cornell.edu:70/1ftp %3Agnome.agrenv.mcgill.ca @pub/genetics/data/basemaps/ Mather DE, Tinker NA, LaBerge DE, Edney M, Jones BL, Rossnagel BG, Legge WG, Briggs KG, Irvine RB, Falk DE, Kasha KJ (1997) Region of the genome that affect grain and malting quality in a North American two-row barley cross. Crop Science 37: 544-554. Mazur BJ, Tingey SV (1995) Genetic mapping and introgression of genes of agronomic importance. Current Opinion in Biotechnology 6: 175-182. Melchers LE, Parker JH (1992) Rust resistance in winter wheat varieties. US Dep Agric Bull 1046 Melchinger AE, Utz HF, Schon CC (1998) Quantitative trait locus (QTL) mapping using testers and independent population samples in maize reveals low power of QTL detection and large bias in estimates of QTL effects. Genetics 149:383403. Michelmore RW (1995) Molecular approaches to manipulation of disease resistance genes. Ann. Rev. Phytopath 33:393-428. 85 Mitchell SE, Kresovich S, Jester CA, Hernandez CJ, Szewc-McFadden AK (1997) Application of multiplex PCR and fluorescence-based, semi-automated allele sizing technology for genotyping plant genetic resources. Crop Science 37: 617-624. Moharramipour S, Tsumuki H, Sato K, Yoshida H (1997) Mapping resistance to cereal aphids in barley. Theor. Appl. Genet. 94: 592-596. Morgante M, Rafalski A, Bidle P, Tingey S, Oliveri AM (1994) Genetic mapping and variability of seven soybean simple sequence repeat loci. Genome 37:763769. Noli E, Salvi S, Tuberosa R (1997) Comparative analysis of genetic relationships in barley based on RFLP and RAPD markers. Genome 40: 607-616. Ordon F, Friedt W (1993) Mode of inheritance and genetic diversity of BaMMV resistance of exotic barley germplasms carrying genes different from ym4. Theor. Appl. Genet. 86:229-233. Ori N, Eshed Y, Paran I, Presting G, Aviv D, Tanksley S, Zamir D, Fluhr R (1997) The I2C family from the wilt disease resistance locus 12 belongs to the nucleotide binding, leucine-rich repeat superfamily of plant resistance genes. Plant Cell 9:521-532. Paran I, Kesseli R, Michelmore R (1991) Identification of restriction fragment length polymorphism and random amplified polymorphic DNA markers linked to downy mildew resistance genes in lettuce, using near-isogenic lines. Genome 34:1021-1027. Parlevliet JE (1978) Future evidence of polygenic inheritance of partial resistance in barley to leaf rust, Puccinia hordei. Euphytica 27:369-379. Parlevliet JE, Van Ommeren A (1975) Partial resistance of barley to leaf rust, Puccinia hordei. II. Relationship between field trials, micro plot test and latent period. Euphytica 24:293-303. Powell W, Baird E, Booth A, Lawrence P, MacAulay M, Bonar N, Young G, Thomas WTB, McNicol JW, Waugh R (1996) Single and multi-locus molecular assays for barley breeding and research. In: Barley Genetics VII Proc. of Intl. Symp., Saskatoon, Canada. Powell W, Thomas WTB, Baird E, Lawrence P, Booth A, Harrower B, McNicol JW, Waugh R (1997) Analysis of quantitative traits in barley by the use of amplified fragment length polymorphisms. Heredity 79: 48-59. 86 Pryor T (1987) The origin and structure of fungal disease resistance genes in plants. Trends in Genetics 3:157-161. Pryor T, Ellis J (1993) The complexity of fungal resistance genes in plants. Advances in Plant Pathology 10:281-304. Qi X (1998) Identification and mapping of genes for partial resistance to Puccinia hordei Otth in barley. Thesis Wageningen Agricultural University. Qi X, Stam P, Lindhout P (1996) Comparison and integration of four barley genetic maps. Genome 39:379-394. Qi X, Stam P, Lindhout P (1998) Use of locus-specific AFLP markers to construct a high-density molecular map in barley. Theor. Appl. Genet. 96: 376384. Ramsay G, Thomas WTB (1992) Two-dimensional representations of linkage groups using similarity matrices. Abstracts of the 11th International Chromosome Conference, Edinburgh, UK, 4 -4 8 August, 1992. Reuvein R, Dudai N, Putievsky E, Elmer WH, Wick RL (1997) Evaluation and identification of basil germplasm for resistance to Fusarium oxysporum f.sp. Basilicum. Plant Disease 81:1077-1081. Roder MS, Plaschke J, Konig SU, Borner A, Sorrells ME, Tanksley SD, Ganal MW (1995) Abundance, vaiability and chromosomal location of microsatellites in wheat. Molecular and General Genetics 246: 327-333. Russell J, Fuller J, Young G, Thomas B, Taramino G, Macaulay M, Waugh R, Powell W (1997) Discriminating between barley genotypes using microsatellite markers. Genome 40:442-450. Russell JR, Fuller JD, Macaulay M, Hatz BG, Jahoor A, Powell W, Waugh R (1997) Direct comparison of levels of genetic variation among barley accessions detected by RFLPs, AFLPs, SSRs, RAPDs. Theor. Appl. Genet. 95:714-722. Saghai-Maroof MA, Zhang Q, Biyashev R (1995) Comparison of restriction fragment length polymorphisms in wild and cultivated barley. Genome 38: 298-306. Saxena KMS, Hooher AL (1968) On the structure of a gene for disease resistance in maize. Proc. Natl. Acad. Sci. USA 61:1300-1305. 87 Sharma H, Ohm H, Goulart L, Lister R, Appels R, Benlhabib 0 (1995) Introgression and characterization of barley yellow dwarf virus resistance from Thinopyrum intermedium into wheat. Genome 38:406-413. Singh RP (1993) Genetic association of gene Bdv 1 for tolerance to barley yellow dwarf virus with genes Lr34 and Yr18 for adult plant resistance to rusts in bread wheat. Plant Dis 77:1103-1106. Statistical Analysis Institute (1988) SAS/STAT User's Guide, 6.03. SAS Institute, Cary, NC, USA Steffenson BJ, Hayes PM, Kleinhofs A (1996) Genetics of seedling and adult plant resistance to net blotch (Pyrenophora teres f. teres) and spot blotch (Cochiobolus sativus) in barley. Theor. Appl.Genet. 92: 552-558. Tanksley SD (1993) Mapping polygenes. Annu. Rev. Genet 27:205-233. Tanksley SD, Ganal MW, Martin GB (1995) Chromosome landing: a paradigm for map-based gene cloning in plants with large genomes. Trends Genet. 11: 63-68. Tanksley SD, Nelson JC (1996) Advanced backcross QTLs analysis: a method for the simultaneous discovery and transfer of valuable QTLs from unadapted germplasm into elite breeding lines. Theor Appl Genet 92:191-203. Tanksley SD, Young ND, Paterson AH, Bonierbale MW (1989) RFLP mapping in plant breeding: new tools for an old science. Bio/Technology 7:275-264. Thomas WTB, Powell W, Waugh R, Chalmers KJ, Barua UM, Jack P, Lea V, Forster BP, Swanston JS, Ellis RP, Hanson PR, Lance RCM (1995) Detection of quantitative trait loci for agronomic, yield, grain and disease characters in spring barley (Hordeum vulgare L.). Theor Appl Genet 91:1037-1047 Thresh JM (1998) In memory of James Edward Vanderplank 1909-1997. Plant Pathology 47: 114-115. Tinker NA, Mather D (1995) Methods for QTL analysis with progeny replicated in multiple environments. J.QTL:http://probe.nalusda.gov:8000/otherdocs/jqt1/ Toojinda T, Baird E, Booth A, Broers L, Hayes PM, Powell W, Thomas W, Vivar H, Young G (1997) Introgression of quantitative trait loci (QTLs) determing stripe rust resistance in barley: an example of marker-assisted line development. Theor Appl Genet 96:123-131. Touzet P, Winkler RG, Helentjaris T (1995) Combined genetic and physiological analysis of a locus contributing to quantitative variation. Theor. Appl. Genet. 91:200-205.