added, and the maximum gas pressure and temperature are to... an ideal gas with constant specific heats. 9-54
advertisement
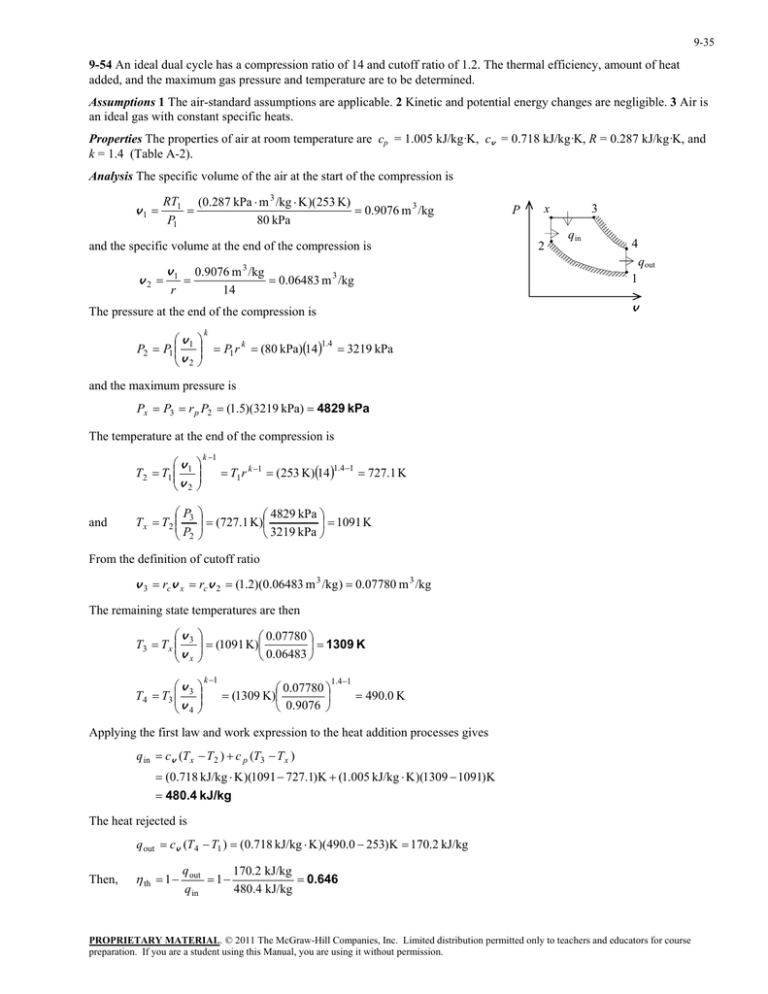
9-35 9-54 An ideal dual cycle has a compression ratio of 14 and cutoff ratio of 1.2. The thermal efficiency, amount of heat added, and the maximum gas pressure and temperature are to be determined. Assumptions 1 The air-standard assumptions are applicable. 2 Kinetic and potential energy changes are negligible. 3 Air is an ideal gas with constant specific heats. Properties The properties of air at room temperature are cp = 1.005 kJ/kg·K, cv = 0.718 kJ/kg·K, R = 0.287 kJ/kg·K, and k = 1.4 (Table A-2). Analysis The specific volume of the air at the start of the compression is v1 RT1 (0.287 kPa m 3 /kg K )(253 K) 0.9076 m 3 /kg P1 80 kPa and the specific volume at the end of the compression is v2 v1 r x 2 3 qin 4 qout 0.9076 m 3 /kg 0.06483 m 3 /kg 14 1 v The pressure at the end of the compression is v P2 P1 1 v 2 P k P1 r k (80 kPa)141.4 3219 kPa and the maximum pressure is Px P3 r p P2 (1.5)(3219 kPa) 4829 kPa The temperature at the end of the compression is and k 1 v T2 T1 1 v 2 P T x T2 3 P2 4829 kPa (727.1 K) 1091 K 3219 kPa T1 r k 1 (253 K)14 1.4 1 727.1 K From the definition of cutoff ratio v 3 rcv x rcv 2 (1.2)(0.06483 m 3 /kg ) 0.07780 m 3 /kg The remaining state temperatures are then v T3 T x 3 v x 0.07780 (1091 K) 1309 K 0.06483 v T4 T3 3 v 4 k 1 0.07780 (1309 K) 0.9076 1.4 1 490.0 K Applying the first law and work expression to the heat addition processes gives q in cv (T x T2 ) c p (T3 T x ) (0.718 kJ/kg K )(1091 727.1)K (1.005 kJ/kg K )(1309 1091)K 480.4 kJ/kg The heat rejected is q out cv (T4 T1 ) (0.718 kJ/kg K )(490.0 253)K 170.2 kJ/kg Then, th 1 q out 170.2 kJ/kg 1 0.646 480.4 kJ/kg q in PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.







