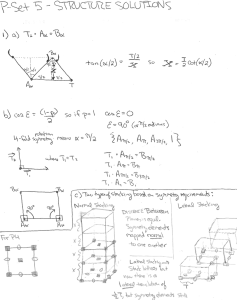
8-122 temperature, the mass flow rate of the steam and the... 8-168
advertisement
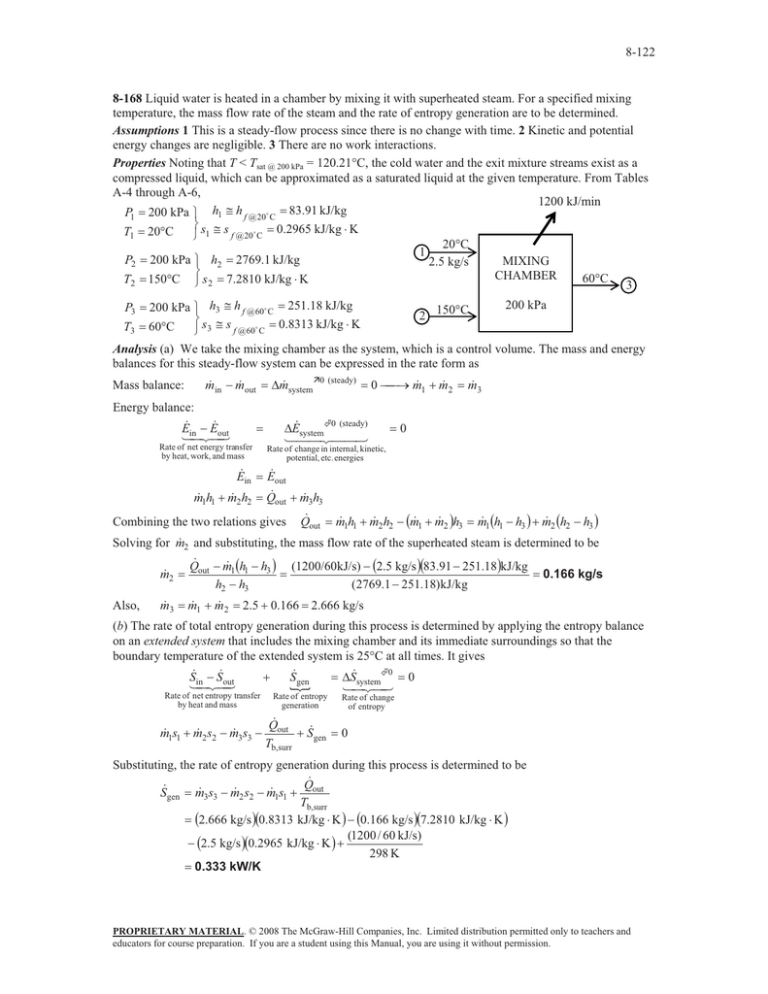
8-122 8-168 Liquid water is heated in a chamber by mixing it with superheated steam. For a specified mixing temperature, the mass flow rate of the steam and the rate of entropy generation are to be determined. Assumptions 1 This is a steady-flow process since there is no change with time. 2 Kinetic and potential energy changes are negligible. 3 There are no work interactions. Properties Noting that T < Tsat @ 200 kPa = 120.21qC, the cold water and the exit mixture streams exist as a compressed liquid, which can be approximated as a saturated liquid at the given temperature. From Tables A-4 through A-6, 1200 kJ/min P1 200 kPa ½ h1 # h f @ 20$ C 83.91 kJ/kg ¾s # s 0.2965 kJ/kg K T1 20qC f @ 20$ C ¿ 1 20qC 1 P2 200 kPa ½ h2 2769.1 kJ/kg MIXING 2.5 kg/s ¾ CHAMBER 60qC T2 150qC ¿ s 2 7.2810 kJ/kg K 3 P3 T3 200 kPa ½ h3 # h f @60$ C 251.18 kJ/kg ¾s # s 0.8313 kJ/kg K 60qC f @ 60$ C ¿ 3 2 200 kPa 150qC Analysis (a) We take the mixing chamber as the system, which is a control volume. The mass and energy balances for this steady-flow system can be expressed in the rate form as Mass balance: m in m out 'm systemÊ0 (steady) Energy balance: E E out in Rate of net energy transfer by heat, work, and mass E in m 1h1 m 2 h2 0 o m 1 m 2 'E system©0 (steady) m 3 0 Rate of change in internal, kinetic, potential, etc. energies E out Q out m 3h3 Combining the two relations gives Q out m 1h1 m 2 h2 m 1 m 2 h3 m 1 h1 h3 m 2 h2 h3 2 and substituting, the mass flow rate of the superheated steam is determined to be Solving for m Also, m 2 Q out m 1 h1 h3 h2 h3 m 3 m 1 m 2 (1200/60kJ/s) 2.5 kg/s 83.91 251.18kJ/kg (2769.1 251.18)kJ/kg 0.166 kg/s 2.5 0.166 2.666 kg/s (b) The rate of total entropy generation during this process is determined by applying the entropy balance on an extended system that includes the mixing chamber and its immediate surroundings so that the boundary temperature of the extended system is 25qC at all times. It gives Sin Sout Sgen 'Ssystem©0 0 , Rate of net entropy transfer by heat and mass Rate of entropy generation m 1s1 m 2 s2 m 3s3 Q out Sgen Tb,surr Rate of change of entropy 0 Substituting, the rate of entropy generation during this process is determined to be Q Sgen m 3s3 m 2 s2 m 1s1 out Tb,surr 2.666 kg/s 0.8313 kJ/kg K 0.166 kg/s 7.2810 kJ/kg K (1200 / 60 kJ/s) 2.5 kg/s 0.2965 kJ/kg K 298 K 0.333 kW/K PROPRIETARY MATERIAL. © 2008 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.