8-105 entropy generation within the heat exchanger are to be determined.
advertisement

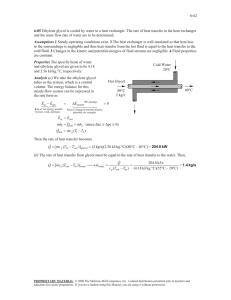
8-105 8-150 Water is heated by hot oil in a heat exchanger. The outlet temperature of the oil and the rate of entropy generation within the heat exchanger are to be determined. Assumptions 1 Steady operating conditions exist. 2 The heat exchanger is well-insulated so that heat loss to the surroundings is negligible and thus heat transfer from the hot fluid is equal to the heat transfer to the cold fluid. 3 Changes in the kinetic and potential energies of fluid streams are negligible. 4 Fluid properties are constant. Properties The specific heats of water and oil are given to be 4.18 and 2.3 kJ/kg.qC, respectively. Analysis (a) We take the cold water tubes as the system, which is a control volume. The energy balance for this steady-flow system can be expressed in the rate form as E E out in Rate of net energy transfer by heat, work, and mass E in Q in m h1 Q in 'E systemÊ0 (steady) 0 Oil 170qC 10 kg/s 70qC Water 20qC 4.5 kg/s Rate of change in internal, kinetic, potential, etc. energies E out m h2 (since 'ke # 'pe # 0) m c p (T2 T1 ) Then the rate of heat transfer to the cold water in this heat exchanger becomes Q [m c p (Tout Tin )]water (4.5 kg/s)(4.18 kJ/kg.qC)(70qC 20qC) = 940.5 kW Noting that heat gain by the water is equal to the heat loss by the oil, the outlet temperature of the hot oil is determined from Q [m c p (Tin Tout )]oil o Tout Tin Q m c p 170qC 940.5 kW (10 kg/s)(2.3 kJ/kg.qC) 129.1qC (b) The rate of entropy generation within the heat exchanger is determined by applying the rate form of the entropy balance on the entire heat exchanger: S Sout in Rate of net entropy transfer by heat and mass Sgen , Rate of entropy generation m 1s1 m 3s3 m 2 s2 m 3s4 Sgen m water s1 m oil s3 m water s2 m oil s4 Sgen Sgen 'Ssystem©0 (steady) Rate of change of entropy 0 (since Q 0) 0 m water ( s2 s1 ) m oil ( s4 s3 ) Noting that both fluid streams are liquids (incompressible substances), the rate of entropy generation is determined to be Sgen m water c p ln T T2 m oilc p ln 4 T3 T1 (4.5 kg/s)(4.18 kJ/kg.K)ln 129.1 + 273 70 + 273 (10 kg/s)(2.3 kJ/kg.K)ln 170 + 273 20 + 273 0.736 kW/K PROPRIETARY MATERIAL. © 2008 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.