6-140 allowed to escape slowly such that temperature remains constant. The... 6-167
advertisement

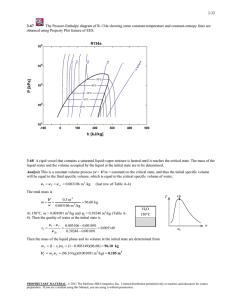
6-140 6-167 A tank initially contains saturated mixture of R-134a. A valve is opened and R-134a vapor only is allowed to escape slowly such that temperature remains constant. The heat transfer necessary with the surroundings to maintain the temperature and pressure of the R-134a constant is to be determined. Assumptions 1 This is an unsteady process since the conditions within the device are changing during the process, but it can be analyzed as a uniform-flow process since the state of fluid at the exit remains constant. 2 Kinetic and potential energies are negligible. 3 There are no work interactions involved. Analysis We take the tank as the system, which is a control volume since mass crosses the boundary. Noting that the microscopic energies of flowing and nonflowing fluids are represented by enthalpy h and internal energy u, respectively, the mass and energy balances for this uniform-flow system can be expressed as Mass balance: min m out 'msystem me m 2 m1 me m1 m 2 Energy balance: E E in out Net energy transfer by heat, work, and mass Qin m e he Qin 'E system Change in internal, kinetic, potential, etc. energies R-134a 0.4 kg, 1 L 25qC m 2 u 2 m1u1 m 2 u 2 m1u1 m e he Combining the two balances: Qin m 2 u 2 m1u1 (m1 m 2 )he The specific volume at the initial state is v1 V m1 0.001 m 3 0.4 kg 0.0025 m 3 /kg The initial state properties of R-134a in the tank are T1 v1 26qC ½° x1 ¾ 3 0.0025 m /kg °¿ u1 v1 v f 0.0025 0.0008313 0.05726 (Table A-11) v fg 0.029976 0.0008313 u f x1u fg 87.26 (0.05726)(156.87) 96.24 kJ/kg The enthalpy of saturated vapor refrigerant leaving the bottle is he h g @ 26qC 264.68 kJ/kg The specific volume at the final state is v2 V m2 0.001 m 3 0.1 kg 0.01 m 3 /kg The internal energy at the final state is T2 v2 26qC ½° x 2 ¾ 3 0.01 m /kg °¿ u2 v 2 v f v fg u f x1u fg 0.01 0.0008313 0.3146 (Table A-11) 0.029976 0.0008313 87.26 (0.3146)(156.87) 136.61 kJ/kg Substituting into the energy balance equation, Qin m 2 u 2 m1u1 ( m1 m 2 )he (0.1 kg )(136.61 kJ/kg ) (0.4 kg )(96.24 kJ/kg ) (0.4 0.1 kg )(264.68 kJ/kg ) 54.6 kJ PROPRIETARY MATERIAL. © 2008 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.