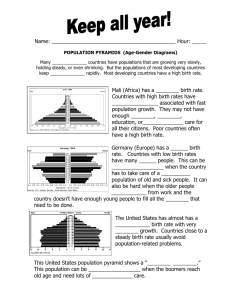
6-55 flow rate of cold water is to be determined.
advertisement
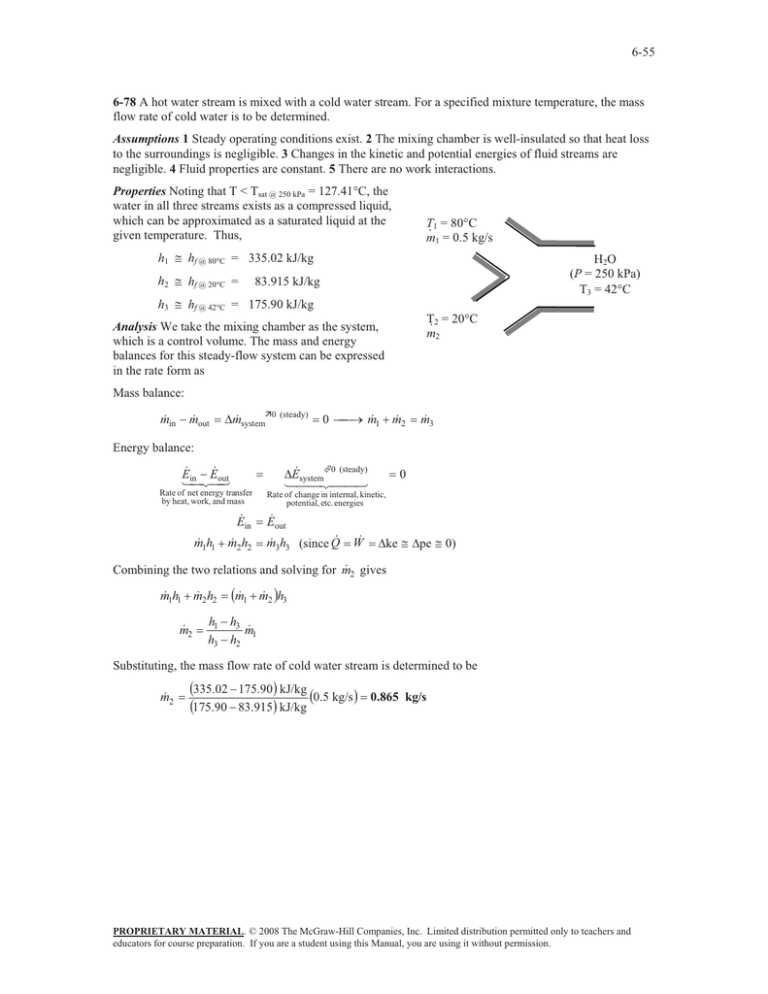
6-55 6-78 A hot water stream is mixed with a cold water stream. For a specified mixture temperature, the mass flow rate of cold water is to be determined. Assumptions 1 Steady operating conditions exist. 2 The mixing chamber is well-insulated so that heat loss to the surroundings is negligible. 3 Changes in the kinetic and potential energies of fluid streams are negligible. 4 Fluid properties are constant. 5 There are no work interactions. Properties Noting that T < Tsat @ 250 kPa = 127.41°C, the water in all three streams exists as a compressed liquid, which can be approximated as a saturated liquid at the given temperature. Thus, T1 = 80qC · m1 = 0.5 kg/s h1 # hf @ 80qC = 335.02 kJ/kg h2 # hf @ 20qC = H2O (P = 250 kPa) T3 = 42qC 83.915 kJ/kg h3 # hf @ 42qC = 175.90 kJ/kg T2 = 20qC · m2 Analysis We take the mixing chamber as the system, which is a control volume. The mass and energy balances for this steady-flow system can be expressed in the rate form as Mass balance: m in m out 'm systemÊ0 (steady) 0 o m 1 m 2 m 3 Energy balance: E E out in Rate of net energy transfer by heat, work, and mass E in m 1h1 m 2 h2 'E system©0 (steady) 0 Rate of change in internal, kinetic, potential, etc. energies E out m 3h3 (since Q W 'ke # 'pe # 0) 2 gives Combining the two relations and solving for m m 1h1 m 2h2 2 m m 1 m 2 h3 h1 h3 1 m h3 h2 Substituting, the mass flow rate of cold water stream is determined to be m 2 335.02 175.90 kJ/kg 0.5 kg/s 175.90 83.915 kJ/kg 0.865 kg/s PROPRIETARY MATERIAL. © 2008 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.