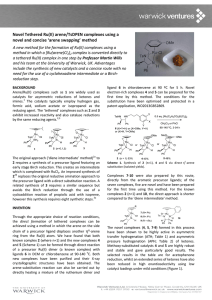
Novel, highly stable, Os(II) arene/TsDPEN complexes for
advertisement

Novel, highly stable, Os(II) arene/TsDPEN complexes for asymmetric hydrogenation of prochiral substrates A novel series of catalytically-active Os(II) complexes has been described by Professors Peter Sadler and Martin Wills and their team at the University of Warwick, UK. The catalysts have been shown to out-perform existing Ru(II) products. The catalysts are highly stable, readily synthesised in under an hour and may be stored for several months before use, without loss of activity. BACKGROUND Ruthenium arene complexes such as 1 are widely used as catalysts for asymmetric reductions of ketones and imines.1 The catalysts typically employ hydrogen gas, formic acid or isopropanol as the reducing agent. Active catalysts 2 and 3 have been fully characterised in the RuII case.2 While other d6 analogues (RhIII and IrIII) of 1 are known,3 the osmium complexes have never before been explored. II The synthesis of the Ru catalyst 1 involves the reaction of a [Ru(arene)Cl2]2 complex with an enantiomerically pure ligand for several hours under basic conditions at high temperature, with an inert atmosphere.1 INVENTION A novel approach, which separates heating and basic conditions, allows access to a new class of osmium compounds. The resulting complexes are highly stable under ambient conditions and may be stored for several months in air without degradation. Interestingly, the complexes can be synthesised via a unique di-chlorido pre-catalyst 4 which, while differing from the ruthenium mono-chlorido species 1, is still active in transfer hydrogenation reactions. Upon treatment with a base, pre-catalyst 4 is converted into active catalyst 5, the OsII analogue of 2. With a synthetic approach optimised from the RuII synthesis,2 careful control of reaction stoichiometry allows the direct isolation of osmium 5 in one step without the need for further purification. Figure 1. Production of osmium di-chlorido pre-catalyst 4 and active catalyst 5 by microwave synthesis compared to the traditional Ru synthesis of 2 (above). A series of complexes with an extended arene system, and interchangeable halides have also been synthesised. The catalysts can be prepared in under an hour. The synthetic procedures and structures have been optimised and protected in UK patent application GB1504281.5. 6 7 Figure 2. Di-iodido analogue 6; biphenyl-capped analogue 7. (S,S) complexes are shown. The (R,R) analogues are also known. The novel complexes (4-7), formed in this process, have been shown to be highly active in asymmetric transfer hydrogenation (ATH; Table 1) of ketones, in some cases surpassing the rates of reduction observed with Ru(II) catalysts. Representative enantioselectivities for the reduction of acetophenone derivatives are detailed in Figure 3. Table 1: Selected ATH reductions of acetophenone (formic acid/triethylamine - FA/TEA used as reductant). 4 5 6 7 Ru mol% 0.5 0.5 0.5 0.5 0.5 T/K 310 310 310 310 310 t/h 24 24 24 24 24 conv./% [a] ee/% [a] 98 98 99 99 96 98 99 95 99 99 TOF / h-1 N.D. [b] 63.9 ± 0.34 N.D. [b] 77.5 ± 0.99 22.5 ± 1.42 [a] Conversion and ee determined by chiral gas chromatography. [b] -1 Maximum turnover frequency (h ) not determined for catalysts 4 and 6. BENEFITS OF OUR NOVEL OSMIUM CATALYSTS The new catalysts no longer require: o an inert atmosphere during synthesis. o further purification after synthesis (which may significantly reduce yield and increase synthetic time). o preparation near to the time of use. Direct synthesis and isolation of the active catalyst as well as a novel pre-catalyst. Catalysts have advantageous properties of o higher catalytic rates than Ru analogues o high reproducibility o higher stability than Ru analogues o high enantiomeric excess o ease of storage REFERENCES 1. a) A. Fujii, S. Hashiguchi, N. Uematsu, T. Ikariya, R. Noyori, J. Am. Chem. Soc. 1996, 118, 2521-2522; b) T. Ikariya, S. Hashiguchi, K. Murata, R. Noyori, in Org. Synth., Vol. 82, John Wiley & Sons, Inc., 2005, pp. 10-17; c) T. Ikariya, K. Murata, R. Noyori, Org. Biomol. Chem. 2006, 4, 393-406; d) N. Uematsu, A. Fujii, S. Hashiguchi, T. Ikariya, R. Noyori, J. Am. Chem. Soc. 1996, 118, 4916-4917. e) S. Hashiguchi, A. Fujii, J. Takehara, T. Ikariya, R. Noyori, J. Am. Chem. Soc. 1995, 117, 7562-7563; f) M. Yamakawa, H. Ito, R. Noyori, R. J. Am. Chem. Soc. 2000, 122, 1466-1478; g) R. Noyori, M. Yamakawa, S. Hashiguchi, J. Org. Chem. 2001, 66, 7931-7944; h) M. Yamakawa, I. Yamada, R. Noyori, Angew. Chem. Int. Ed. 2001, 40, 2818-2821. 2. a) K.-J. Haack, S. Hashiguchi, A. Fujii, T. Ikariya, R. Noyori, Angew. Chem., Int. Ed.. 1997, 36, 285-288 3. a) X. Wu, X. Li, A. Zanotti Gerosa, A. Pettman, J. Liu, A. J. Mills, J. Xiao, Chem. Eur. J. 2008, 14, 2209-2222; b) X. Wu, J. Xiao, Chem. Commun. 2007, 2449-2466; c) T. Thorpe, J. Blacker, S. M. Brown, C. Bubert, J. Crosby, S. Fitzjohn, J. P. Muxworthy, J. M. J. Williams, Tetrahedron Lett. 2001, 42, 4041-4043. d) K. Murata, T. Ikariya, R. Noyori, J. Org. Chem. 1999, 64, 2186-2187; e) X. Sun, G. Manos, J. Blacker, J. Martin, A. Gavriilidis, Org. Proc. Res. Dev. 2004, 8, 909914; f) T. Ohkuma, N. Utsumi, M. Watanabe, K. Tsutsumi, N. Arai; K. Murata, Org. Lett. 2007, 9, 2565-2567; g) Z. M. Heiden; T. B. Rauchfuss, J. Am. Chem. Soc. 2009, 131, 3593-3600. Figure 3: Examples of other alcohols formed in ATH (asymmetric transfer hydrogenation) reductions; 0.5 mol% catalyst, 310K, 24h. Turnover number (TON) 200 160 120 7 (Os) 80 5 (Os) 40 1 (Ru) 0 0 2 4 6 8 Time / h 10 12 14 Figure 4: Reaction monitoring for the reduction of acetophenone by Os and Ru complexes (0.5 mol% catalyst, 310K) TARGET PARTNERS The technology is expected to be of interest to companies who are manufacturing and selling hydrogenation catalysts for asymmetric reduction reactions. The patent application also contains details of the synthesis of a number of catalyst derivatives and their applications to reductions. We can send you a small sample of approx. 100 mg of catalyst 5 to evaluate under a Material Transfer Agreement. PATENT & PUBLICATION ‘Easy to synthesize, robust, organo-osmium asymmetric transfer hydrogenation catalysts’, J. P. C. Coverdale, C. Sanchez Cano, R. Soni, G. J. Clarkson, M. Wills and P. J. Sadler, Chem. A The novel osmium complexes reported herein offer a stable, alternative catalyst system for asymmetric hydrogenation of ketones. The catalysts may be prepared in advance of their use, and retain activity for a period of several months without the need for an inert atmosphere during synthesis or storage. They are rapidly synthesised in under an hour. The osmium catalysts have been found to out-perform existing ruthenium technologies under identical reaction conditions, and do not suffer from an induction phase, unlike analogous Ru catalysts. Eur. J. 2015, 21, 8043–8046. ‘Osmium Catalysts and Method of Synthesis’. UK Patent Application No. GB1504281.5 filed 13 March 2015. CONTACT Further information is available on request from: Dr Shum Prakash, Warwick Ventures Ltd, Tel: +44 (0) 24 7657 4145, or via email: s.prakash@warwick.ac.uk Warwick Ventures Ltd is the commercial arm of the University of Warwick.