Abiotic and biotic influences on Bromus tectorum invasion
advertisement

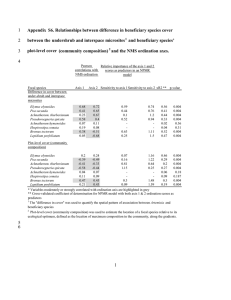
CSIRO PUBLISHING www.publish.csiro.au/journals/ijwf International Journal of Wildland Fire 2011, 20, 597–604 Abiotic and biotic influences on Bromus tectorum invasion and Artemisia tridentata recovery after fire Lea CondonA, Peter J. WeisbergA and Jeanne C. ChambersB A Department of Natural Resources and Environmental Science, University of Nevada – Reno, 1000 Valley Road, Reno, NV 89512, USA. B US Forest Service, Rocky Mountain Research Station, 920 Valley Road, Reno, NV 89512, USA. C Corresponding author. Email: leacondon@yahoo.com Abstract. Native sagebrush ecosystems in the Great Basin (western USA) are often invaded following fire by exotic Bromus tectorum (cheatgrass), a highly flammable annual grass. Once B. tectorum is established, higher fire frequencies can lead to local extirpation of Artemisia tridentata ssp. vaseyana (mountain big sagebrush) and have cascading effects on sagebrush ecosystems and the species that depend on them. We conducted a landscape-scale observational study to examine the distribution and cover of B. tectorum and A. tridentata 6 years after a large wildland fire. We used structural equation models to quantify the interacting influences of pre-fire tree canopy cover, perennial species cover, distance from potential seed source, and site environment on post-fire cover of B. tectorum and A. tridentata. Results confirmed a hypothesised negative effect of pre-fire tree canopy cover on post-fire cover of A. tridentata. Site- and landscape-level abiotic factors influenced pre-fire tree canopy cover, which, in turn, influenced the probability of rapid recovery to A. tridentata. However, B. tectorum cover was primarily influenced by a positive effect of incident solar radiation and a negative effect of perennial herbaceous species cover. Restoration efforts to reduce tree canopy cover should be limited to productive sites with sufficient cover of perennial herbaceous species to facilitate site recovery. Additional keywords: fire effects, Great Basin, landscape-scale, structural equation modelling, succession. Introduction Ecological resilience following wildfire is influenced by local environmental variation and the relative abundances and competitive abilities of both native and exotic species (Keeley et al. 2005; D’Antonio et al. 2009). Higher availability of resources after fire (Badia and Marti 2003; Certini 2005) can increase the probability of establishment and spread of exotic species as described by the fluctuating resource hypothesis (Davis et al. 2000). However, many native species are highly competitive with exotics and can dominate post-fire sites if residual seed banks, surviving meristems capable of resprouting, or viable seed sources are present in sufficient abundance (D’Antonio et al. 2009). In semiarid regions of the western United States, expansion of pinyon and juniper trees into Artemisia tridentata (sagebrush)-dominated ecosystems has cascading effects. Expansion of the highly competitive trees results in progressive decreases in understorey species including A. tridentata and native perennial herbaceous species (Miller et al. 2011). At the same time, infilling and growth of the trees result in increased fuel loads and causes larger and more severe fires (Miller et al. 2008, 2011). High-severity fires in pinyon and juniper woodlands result in almost complete mortality of trees and Artemisia species (Baker and Shinneman 2004; Bauer and Weisberg 2009) and increase the potential for mortality of the residual perennial herbaceous component. The net effect is increased Ó IAWF 2011 susceptibility of these ecosystems to invasion and spread of Bromus tectorum L. (cheatgrass), a Eurasian annual grass, after fire. In the worst-case scenario, native A. tridentata ecosystems are converted to near monocultures of B. tectorum with significantly decreased resource values and ecosystem services. Bromus tectorum is a highly flammable, fire-adapted species that increases the continuity of fine fuels and causes more frequent and often larger wildfires (D’Antonio and Vitousek 1992; Link et al. 2006). Increased fire frequencies favour B. tectorum over fire-intolerant native shrubs such as Artemisia tridentata, which are killed by fire and require much longer firefree intervals for establishment and reproduction (Young and Evans 1978). Sufficient seeds of B. tectorum typically survive after fire to permit reestablishment and high resource availability can result in rapid population growth (West and Young 2000). Seeds of A. tridentata vaseyana can survive after fire (Mueggler 1956), but are short-lived (i.e. no persistent seed bank) and seedling establishment is typically low after fire (Young and Evans 1989). Seed sources of A. tridentata from outside the burn perimeter are often important for establishment in the first few years after fire (Ziegenhagen 2003). Thus propagule limitation is a significant factor reducing seedling establishment of A. tridentata in competition with B. tectorum (Mazzola et al. 2010). Recruiting seedlings of native perennial species are, in general, poor competitors against B. tectorum seedlings because this annual grass can germinate and exhibit 10.1071/WF09082 1049-8001/11/040597 37.7–423.2 0–89 0–22.3 0–1.2 0–0.4 m2 m % % % Area covered by pre-fire tree canopy in a 0.1-ha plot Edge density of unburned patch edge within a 300-m radius of each plot Ocular estimate of cover to closest 1% and averaged over each plot Measured in belt transects by calculating the area of each shrub using an equation for an ellipse Ocular estimate of cover to closest 1% and averaged over each plot 229.9 (71.5) 17.9 (22.6) 8.8 (6.3) 0.2 (0.3) 0.1 (0.1) 17 895–28 253 0–77 23–85 20.2–71.3 2.2–14.92 26 418.4 (1996.6) 38.4 (16.3) 53.3 (19.5) 42.5 (10.6) 5.5 (2.8) kJ m2 day1 8 cm % Wetness index Mean (s.d.) Units Description Incident, cloud-free solar radiation estimated for 15 May Field-measured slope steepness Maximum soil depth Percentage of coarse fragment in surface soils (to 10-cm depth) Topographic Convergence Index: (flow accumulation area)/slope Abiotic variables Solar radiation Slope Maximum soil depth % coarse fragment TCI Biotic variables Pre-fire tree canopy cover Edge density Bromus tectorum cover Artemisia tridentata ssp. vaseyana cover Perennial herbaceous cover Materials and methods Study design and field measurements The 2800-ha Wall Canyon study area lies within the Toiyabe Range of central Nevada, USA, ranges in elevation from 2145 to 2455 m, and encompasses an area burned by a wildland fire in July 2000. The study area is generally xeric, with steep slopes and high levels of solar radiation (Table 1). Pre-fire vegetation was dominated by singleleaf pinyon (Pinus monophylla Torr. Variable greater root elongation earlier in the fall and under colder winter temperatures (Harris 1967; Aguirre and Johnson 1991). Also, B. tectorum typically has higher nutrient uptake rates (Monaco et al. 2003) and higher growth rates (Arredondo et al. 1998) than either native shrubs or grasses. In semiarid Artemisia tridentata shrublands, abundance of native perennial herbaceous species is a major determinant of invasibility by annual grasses. Long-term observational datasets from sagebrush-steppe recovering from livestock grazing (Anderson and Inouye 2001), and from sagebrush semi-desert responding to wildfire and livestock grazing (West and York 2002), show an inverse relationship between Bromus tectorum and total perennial cover. Experimental research shows that the effects of fire and removal of perennial herbaceous vegetation on invasion of B. tectorum in sagebrush ecosystems are additive, with B. tectorum biomass and seed production increasing two to three-fold following removal of perennial herbaceous species, three to six-fold after fire, and 10–30-fold after both removal and fire (Chambers et al. 2007). The negative effects of native perennial herbaceous species on growth and reproduction of B. tectorum may translate to a positive effect on A. tridentata establishment. Conversion of diverse Artemisia tridentata ecosystems to Bromus tectorum dominance results in habitat loss and fragmentation, has placed several species, including sage-grouse (Centrocercus spp.), at risk for federal listing. This has resulted in a degree of urgency in developing management solutions (Knick et al. 2003). Prescribed fire is increasingly used as a management tool to maintain and restore A. tridentata communities threatened by pinyon and juniper expansion (Forbis et al. 2006; Rau et al. 2008). Effective use of prescribed fire requires knowledge of the likelihood of recovery of A. tridentata and the potential for invasion by B. tectorum. We conducted a landscape-scale observational study of the distribution of these two species 6 years following a single large wildfire in central Nevada, USA. We hypothesised that B. tectorum cover following fire is positively associated with pre-fire tree canopy cover and negatively associated with cover of herbaceous perennial species, and that the converse is true for post-fire recovery of A. tridentata. We also hypothesised that there would be a direct negative effect of B. tectorum cover on A. tridentata because of the ability of B. tectorum to out-compete seedlings of native species. We used structural equation models (Grace and Pugesek 1997) to quantify the interacting influences of pre-fire tree canopy cover, perennial herbaceous cover, distance from potential seed source, and site environment on post-fire cover of B. tectorum and A. tridentata. The implications of our results for restoring and maintaining A. tridentata in ecosystems susceptible to B. tectorum invasion are discussed. L. Condon et al. Range Int. J. Wildland Fire Table 1. Environmental variables considered for inclusion in structural equation models 598 Effects of the pre-fire plant community on post-fire recovery Int. J. Wildland Fire 599 Legend Wall Canyon plot locations Oregon Idaho Burn perimeter Wyoming dNBR Nevada Value Utah High: 185 California Low: ⫺199 Arizona 0 250 500 1000 1500 N 2000 Metres Fig. 1. The location of the 2800-ha Wall Canyon fire in central Nevada, USA, and the sampling design within and outside the burn area. Low values of differenced normalised burn ratio (dNBR) (darker areas) represent portions of the landscape that did not burn. Horizontal lines delineate equal-area sections used for stratified random sampling of survey plots along gradsects. and Frém.) and Utah juniper (Juniperus osteosperma (Torr.) Little), with small pockets of sagebrush shrubland (mountain big sagebrush (Artemisia tridentata ssp. vaseyana (Rydb.) Beetle), Sandberg bluegrass (Poa secunda J. Presl) and Wheeler bluegrass (Poa nervosa (Hook.) Vasey) at the lower elevations, and curlleaf mountain mahogany (Cercocarpus ledifolius Nutt.) on north-east-facing slopes). In this semiarid ecosystem, annual precipitation is highly variable but the long-term mean is 170 mm and most precipitation arrives during the winter as snow. Mean precipitation after the fire in 2000 was below average for 2000–05. The mean for this period was 119 mm. In 2005, precipitation totalled only 83.3 mm and immediately before sampling (October 2005 to May 2006) was 79 mm (Western Regional Climate Center; see http://www.wrcc.dri. edu/summary/Climsmnv.html, accessed 29 June 2008). Soils are lithic, deep Arborolls–Haplargids–Torriorthents and the terrain is rugged (USDA NRCS 2006). The study area is managed by the US Forest Service and has been subject to a range of anthropogenic disturbances including cattle grazing, salvage logging, recreational off-highway vehicle use and mining. Although these disturbances occur throughout the study area, they are more frequent near the canyon mouth. Survey plots, constrained to be at least 100 m apart, were selected randomly along east–west transects that spanned the canyon from ridgeline to ridgeline. Transects (i.e. gradsects as described in Gillison and Brewer 1985) provided representative sampling across gradients of elevation and distance from burn perimeter, given that the burn perimeter closely followed the major ridgelines. The east–west transects were randomly located within three equal-area sections to adequately sample the geographic extent of the Wall Canyon burn. A total of one hundred and two 20 50-m (0.1-ha) plots were surveyed, including 71 within the burn, 16 outside the burn and 15 in unburned patches within the burn area (Fig. 1). Aerial cover, topographic and soils data were collected at each plot. Aerial cover of herbaceous species including Bromus tectorum was ocularly estimated to the closest 1% within twenty-five 0.5-m2 quadrats. Quadrats were positioned along transects beginning at 1, 10 and 17 m along the 20-m side of the 0.1-ha rectangular plot. Aerial cover and frequency of all 600 Int. J. Wildland Fire L. Condon et al. Artemisia tridentata individuals were surveyed along three 2 50-m belt transects. Shrub canopy dimensions along the longest axis and the axis perpendicular to the longest were measured for each shrub to estimate aerial cover using the equation for the area of an ellipse. Abiotic data were collected from each plot, including topographic position, aspect and slope. Slope and aspect values were used to validate their respective geographic information systems (GIS) layers. Soil depth was measured by pounding a 0.5-cm metal rod into the ground until further pounding was resisted by rock (Harner and Harper 1976). Soil depth measurements were recorded as an average of three readings from each of ten 0.5-m2 quadrats. A 1-L composite soil sample was collected from the same ten 0.5-m2 quadrats to a depth of 10 cm. Soils were analysed for pH and % coarse fragments. Soil pH was measured with a Corning 320 pH meter (Columbus, OH, USA) using ,10 g of soil, 19 mL of deionised water and 1 mL of CaCl2. Coarse fragments were sieved and weighed to determine their percentage in each soil sample. Soil texture was assessed using a ribbon test according to the classification of Thien (1979). Geographic information systems (GIS)-derived data GIS was used to construct several map layers including solar radiation, pre-fire tree canopy cover, topographic convergence index, unburned patches and burn perimeter. The intensity of incident solar radiation assuming clear-sky conditions (Kumar et al. 1997) was modelled using a 30-m-resolution digital elevation model (DEM) for 15 May, to correspond with the spring seed germination period. Tree canopy cover was delineated from 1996 panchromatic digital orthophotography (1-m resolution). Panchromatic digital orthophotoquads (DOQs) were corrected for topographic shadowing with IDRISI Kilimanjaro version 14.02 software (Clark Labs, Worcester, MA, USA) and used to create a polygon layer of tree canopies that existed before the fire (Greenwood and Weisberg 2009). An automated, object-oriented classification method, implemented in eCognition Professional version 4.0 software (Trimble, Westminster, CO, USA), used brightness, patch shape, patch area, distance, textural homogeneity and local neighbourhood relationships to segment images into homogeneous patches and delineate polygons dominated by tree crowns (Weisberg et al. 2007). Topographic convergence index (TCI) was calculated as: TCI ¼ lnða=tan bÞ ð1Þ where a is the upslope contributing area of water drainage to the centre of the plot and b the local slope angle. High values indicate sites that collect and retain water in runoff events (e.g. depressions, low in the watershed), and low values indicate sites with steep slopes that are high in the watershed. GIS layers for unburned patches and burn perimeter were developed with a classification of Landsat Thematic Mapper (TM) imagery from 2 June 2000 and 20 July 2000, both before the 22 July 2000 fire, and from 8 October 2000, after the fire. All image processing procedures were implemented in IDRISI Kilimanjaro version 14.02 software. Normalised burn ratio (NBR) was calculated to highlight areas of differing burn severity using a ratio of short-wave infrared bands, Band 4 (0.76–0.90 mm) and Band 7 (2.08–2.35 mm) in the equation (Cocke et al. 2005): NBR ¼ ðBand 4 Band 7Þ=ðBand 4 þ Band 7Þ ð2Þ Unburned patches throughout Wall Canyon were identified using differenced NBR (dNBR) values, subtracting the postfire NBR from the pre-fire value for each pixel, such that positive dNBR values indicate vegetation damage or burned areas. To develop an unburned patch layer, dNBR values were generalised to two classes, burned and unburned, using an iterative, unsupervised classification (ISOCLUST algorithm, using IDRISI Kilimanjaro version 14.02) that is a variation of the commonly used ISODATA technique (Ball and Hall 1965). The resulting classification was ground-truthed at the 94 vegetation sampling locations within the burn perimeter. Twenty of twenty-three (87%) unburned patches and 71 of 71 (100%) burned patches were correctly classified. Edge density (m km2) of unburned patches was then calculated for a 300-m neighbourhood surrounding each surveyed plot. Values of environmental variables were extracted by overlaying boundaries of 0.1-ha (20 50-m) plots on GIS layers. Plot boundaries were reconstructed from global positioning system (GPS) points recorded with a Trimble GeoExplorer 3 unit (Sunnyvale, CA, USA) at sub-metre precision and differentially corrected. Reconstructed plots were used to sample prefire tree canopy cover. Plot centroids were sampled in GIS for elevation, slope, aspect, estimated solar radiation and topographic convergence index. Data analysis Structural equation modelling (SEM) allows the testing of complex dependency relationships and partitions direct and indirect effects of explanatory variables such as pre-fire tree canopy cover (Grace and Pugesek 1997). One strength of SEM is that it accounts for correlations between variables that may be masking a relationship of interest (Grace 2006). SEM requires formulation of explicit conceptual models (i.e. path diagrams) representing causal and correlational relationships among measured variables. The resulting relationships of SEM are equivalent to standardised partial regression coefficients (Grace 2006). In the present study, SEM was used to describe the hypothesised interacting effects of pre-fire tree canopy cover, distance from potential seed sources (i.e. proximity to unburned patches), perennial herbaceous species cover and site environment on post-fire cover of Bromus tectorum and Artemisia tridentata (Table 1). The hypothesised network of causal relationships tested using SEM (Fig. 2a) predicts that Artemisia tridentata cover following fire will be higher on mesic sites, as indicated by gradual, shaded slopes, higher position in the watershed (i.e. lower TCI), and deeper soils with a lower proportion of coarse fragments. Pre-fire tree canopy cover was predicted to have similar associations with site environmental variables. Greenwood and Weisberg (2009) observed that sites with deeper, more clayey soils in central Nevada generally supported greater tree cover, associated with high levels of tree establishment in recent decades. Greater pre-fire tree canopy cover was expected Effects of the pre-fire plant community on post-fire recovery (a) Int. J. Wildland Fire 601 (b) Slope Slope Topographic convergence index % coarse fragment Proximity to unburned patches Perennial herbaceous cover Perennial herbaceous cover ⫺0.320 Topographic convergence index ⫺0.284 0.250 ⫺0.223 A. tridentata ssp. vaseyana cover A. tridentata ssp. vaseyana cover % coarse fragment ⫺0.234 ⫺0.225 Maximum soil depth Pre-fire canopy cover Bromus tectorum cover Solar radiation Maximum soil depth ⫺0.362 Pre-fire canopy cover ⫺0.400 ⫺0.485 ⫺0.316 Solar radiation Bromus tectorum cover 0.381 Fig. 2. Path diagrams of the (a) hypothetical and (b) most parsimonious structural equation modelling (SEM) model explaining field-measured cover values of Artemisia tridentata ssp. vaseyana, Bromus tectorum and perennial herbaceous species following fire. Correlations among abiotic variables are shown with dotted double-headed arrows. Negative path coefficients are shown with dashed lines and positive path coefficients are shown with solid lines. Standardised path coefficients show the strength and the direction of the relationship between variables after accounting for the influence of variables that correlate with those variables. to negatively influence the post-fire abundance of A. tridentata and perennial herbaceous vegetation owing to the well-known inverse relationship between overstorey and understorey cover in pinyon–juniper ecosystems (Miller et al. 2005, 2011). Postfire establishment of A. tridentata was predicted to be positively correlated with increased proximity of unburned patches that serve as propagule sources, because seed dispersal of this species occurs over short distances (30 m; Meyer 1994). Proximity to unburned patches was hypothesised to be positively influenced by slope as extremely steep slopes tend to be quite rocky with limited fuel loading and continuity. Bromus tectorum cover was predicted to negatively influence A. tridentata cover through direct competition, given that shrub seedlings are thought to use water from the same soil depth as B. tectorum (Booth et al. 2003). We hypothesised that Bromus tectorum cover would be greatest, and perennial herbaceous cover least, on xeric sites as indicated by high levels of solar radiation (Fig. 2a); cover of native plant species in the Great Basin following wildfire is often greater in more mesic sites (Reilly et al. 2006). The cover of perennial herbaceous species was predicted to exert a negative influence on B. tectorum cover through direct competition effects, consistent with our hypothesis that the presence of perennial herbaceous species increases site resistance to invasion by B. tectorum (Anderson and Inouye 2001; Chambers et al. 2007). Perennial herbaceous cover was predicted to positively influence Artemisia tridentata cover through facilitation effects or as an indicator of improved site conditions not captured by the modelled environmental variables. We further hypothesised that increased cover of perennial herbaceous species would have an indirect, positive effect on the establishment of A. tridentata through competitive effects on B. tectorum (Fig. 2a). To make variable effect sizes comparable despite disparate units, path coefficients were standardised by dividing each variable by its standard deviation. The path coefficient indicates the magnitude and direction of influence of the predictor variable on the response, accounting for other causal and correlational relationships in the model (Grace and Pugesek 1997). SEM analyses were implemented in AMOS (Analysis of Moment Structures) version 7.0 software (SPSS, IBM, Armonk, NY). Models were assessed using fit indices, referring to the correspondence between the hypothesised model and the observed covariance matrix. Chi-square statistics, their associated P values and the root mean square error of approximation (RMSEA) provide complementary measures of model fit (Kaplan 2000). Individual pathways were evaluated using critical ratios, defined as the covariance estimate divided by the standard error. Critical ratios were evaluated for statistical significance assuming a standard normal distribution (Arbuckle 2006). The most parsimonious model (Fig. 2b), representing a subset of the full, hypothesised path model (Fig. 2a), was selected for interpretation. Results Several of the abiotic predictor variables were significantly correlated with one another within the SEM framework (Fig. 2b). Positive correlations existed between % coarse fragment and slope. Negative correlations occurred between slope and both maximum soil depth and solar radiation, between solar radiation and TCI, and between % coarse fragment and TCI. These relationships indicate that sites lower in the watershed were more likely to be shaded and to have finer soil textures and more gradual slopes owing to their topographic position. Significant correlations among abiotic variables were accounted for when modelling their influences on plant cover within the SEM framework. The most parsimonious model (RMSEA , 0.001, x2 ¼ 18.477, P ¼ 0.779) for post-fire recovery of Artemisia tridentata, perennial herbaceous species and invasion and spread of Bromus tectorum, given the influences of site environment, proximity to unburned patches and pre-fire tree canopy cover, represents only a subset of the originally hypothesised network of causal relationships (compare Fig. 2a,b). Site environment 602 Int. J. Wildland Fire factors did not influence A. tridentata directly and had no effect on perennial herbaceous cover. However, there were indirect effects of site environment on A. tridentata mediated through negative influences of TCI and % coarse fragment on the inhibitory variable, pre-fire tree canopy cover. Overall, tree cover was greater on higher positions in the watershed and sparser on rockier sites (Fig. 2b). Solar radiation was the only site environment factor to influence B. tectorum cover, exhibiting a strongly positive path coefficient. Solar radiation did not directly influence A. tridentata or perennial herbaceous cover. The proximity to unburned patches and cover of B. tectorum did not have significant effects on A. tridentata cover and are not included in the final model. The biotic variables exhibited many of the predicted relationships (Fig. 2b). Pre-fire tree canopy cover values were spatially variable, but intermediate overall (mean ¼ 24.91%, 95% CI ¼ 1.80%). Aerial perennial herbaceous cover values were generally low (mean ¼ 0.09%, 95% CI ¼ 0.02%), and Bromus tectorum cover values were comparatively high (mean ¼ 8.81%, 95% CI ¼ 1.50%) (Table 1). These values indicate that the study site as a whole was likely in an intermediate to late stage of tree expansion before the fire. Pre-fire canopy cover of trees was negatively correlated with Artemisia tridentata cover, but was not correlated with B. tectorum cover. Also, perennial herbaceous cover was positively correlated with A. tridentata cover, but was negatively correlated with B. tectorum cover. Discussion The effects of both the abiotic and biotic variables on Artemisia tridentata and Bromus tectorum were complex. The measured abiotic variables had no apparent effect on the post-fire cover of A. tridentata, likely because of its widespread distribution within the watershed and low cover values. As hypothesised, B. tectorum cover was greatest on xeric sites. The apparent preference of B. tectorum for post-burn xeric conditions is largely attributable to the ecological amplitude of the species (Chambers et al. 2007). In the absence of perennial herbaceous species, B. tectorum establishment, growth and reproduction is highest on warmer and more xeric sites and lowest on cold and mesic sites in these upland watersheds (Chambers et al. 2007). Ecophysiological constraints severely limit B. tectorum establishment, growth and reproduction on higher-elevation sites with cold soil temperatures (Evans and Young 1972; Mack and Pyke 1983). Local environmental conditions and the composition and abundance of perennial herbaceous species determine the relative abundance and persistence of B. tectorum over time. As predicted, pre-fire tree canopy cover had a significant negative effect on post-fire cover of Artemisia tridentata, even after accounting for the effects of relevant environmental variables (Fig. 2b). However, pre-fire tree cover did not exhibit the expected positive effect on Bromus tectorum cover or negative effect on perennial herbaceous cover. Low cover values of perennial herbaceous species 6 years after fire likely indicate that the perennial herbaceous species had been depleted before the fire by inappropriate livestock grazing, as shown for multiple post-fire sites in the Great Basin (Koniak 1985). In our study, grazing was reinitiated 2 years following the fire. Thus, lack of an effect of pre-fire tree cover on perennial herbaceous species likely resulted from low initial perennial herbaceous cover that L. Condon et al. was maintained by grazing after the fire. Establishment and persistence of grass species like Festuca idahoensis and Poa secunda under pinyon and juniper on more mesic sites (Miller et al. 2005) also may have contributed to the non-significant relationship between pre-fire tree cover and perennial cover. The lack of a direct effect of pre-fire tree cover on B. tectorum may be due to relatively high abundance of B. tectorum in the watershed, and patterns of B. tectorum spread following the fire that were reinforced by grazing and anthropogenic disturbance. Perennial herbaceous cover exhibited the predicted positive relationship with Artemisia tridentata and negative relationship with Bromus tectorum. These results reflect a growing body of evidence from long-term observational studies (Anderson and Inouye 2001; West and York 2002) and experimental studies showing an inverse relationship between abundance of B. tectorum and cover of native perennial herbaceous species (Chambers et al. 2007). They also show for the first time that reestablishment of A. tridentata following fire is positively related to the cover of native perennial herbaceous species. These results indicate that management aimed at maintaining or increasing the abundance of perennial herbaceous species has the potential to increase both resistance to B. tectorum invasion and recruitment of A. tridentata following fire. Cover of Bromus tectorum did not exhibit the hypothesised negative association with cover of Artemisia tridentata. During the first year after a fire, B. tectorum populations are typically small (Young and Evans 1978). Two to three years are often required for B. tectorum densities to increase sufficiently for the species to be highly competitive. This lag-time in population increase can provide a window of opportunity for native species establishment. It is likely that most mature A. tridentata plants observed in our study established from seed bank sources in the growing season immediately following the burn and before widespread B. tectorum dominance. This was the wettest year following the fire and over the 6 years preceding the study (http://www.wrcc.dri.edu/summary/Climsmnv.html, accessed 10 May 2011). Below-average precipitation before and during the year of the study may have resulted in lower overall establishment of both A. tridentata and B. tectorum. Successful establishment and relative cover of annual grass species can vary dramatically among years (Bradford and Lauenroth 2006; Keeley and McGinnis 2007), and low precipitation may have influenced both B. tectorum abundance and study results. We predicted that post-fire cover of Artemisia tridentata would be positively correlated with closer proximity of unburned patches that serve as seed sources owing to dispersal limitations (Meyer 1994). In sagebrush ecosystems exhibiting pinyon and juniper expansion, unburned patches typically have greater densities of A. tridentata seed than burned areas following fire (Allen et al. 2008). We did not find the predicted relationship, possibly because the residual seed bank can mask the importance of unburned seed sources in post-fire establishment. In an experiment that used covered plots to prevent establishment from wind-borne seed, the residual seed bank contributed substantially to A. tridentata establishment following fire (Mueggler 1956). Also, A. tridentata may establish in phases following fire, and the relative importance of seed sources from outside the burn perimeter or from the seed bank may diminish with time since fire (Ziegenhagen 2003). Effects of the pre-fire plant community on post-fire recovery As A. tridentata individuals that established immediately following fire mature, they contribute their own seed to subsequent A. tridentata establishment. This phased establishment further confounds any influence of distance from unburned patches on A. tridentata distribution. Management implications Our study was conducted across a 2800-ha landscape and examined a single fire. Although our data captured a range of environmental and biotic conditions such as pre-fire canopy cover, cover of perennial herbaceous plants, proximity to unburned patches and cover of Artemisia tridentata and Bromus tectorum, these data are from a single landscape and therefore generalisations of our results are limited. Yet, considering the range of conditions examined, it seems likely that our results would be confirmed at other sites. Landscape-scale preventive management for maintaining and restoring resistance to Bromus tectorum invasion and site resilience following fire is ongoing throughout much of the Great Basin region. Management treatments include allowing or introducing disturbance in the form of fire or mechanical tree removal. Our results suggest that these management activities will be most effective if they target productive areas with high covers of perennial herbaceous species. Site selection should be based on the stage of tree dominance and on the abundance of shrubs and herbaceous species in the understorey that are capable of establishing or resprouting following fire (Chambers 2005; D’Antonio et al. 2009). High-priority areas for preventive management should be those at the early to intermediate stages of tree increase where native herbaceous perennials are still a significant component of the community. In the absence or near-absence of residual native shrubs and herbaceous perennials, these ecosystems are at high risk of invasion and spread of Bromus tectorum. Active restoration in the form of revegetation immediately after fire is often necessary to prevent B. tectorum dominance. This research indicates that restoring productive and more mesic sites could increase overall resilience and resistance by increasing competition between native perennial shrubs and herbs and B. tectorum. Introducing disturbance to more xeric sites will likely lead to increased cheatgrass dominance and should be avoided. Because of the strong affinity of B. tectorum for xeric sites, and the difficulty of restoring these types of sites (Humphrey and Schupp 2004), this exotic species likely will remain a component of these drier ecosystems. Acknowledgements This manuscript benefited from the comments of David Board, Erin Goergen, Steve Jenkins, Dongwook Ko, Ben Rau and Ashley Sparrow. Michael Clark, Teresa Olson, Jon Propp and Chelsea Robison assisted with field work. Bob Blank contributed the use of the USDA Agricultural Research Service Soils Laboratory. This project was funded by the Joint Fire Sciences Program (05-JFSP-2-1-94), USDA Forest Service, Rocky Mountain Research Station and the Nevada Agricultural Experimental Station. References Aguirre L, Johnson DA (1991) Influence of temperature and cheatgrass competition on seedling development of two bunchgrasses. Journal of Range Management 44, 347–354. doi:10.2307/4002397 Int. J. Wildland Fire 603 Allen EA, Chambers JC, Nowak RS (2008) Effects of a spring prescribed burn on the soil seed bank in sagebrush steppe exhibiting pinyon–juniper expansion. Western North American Naturalist 68, 265–277. doi:10.3398/ 1527-0904(2008)68[265:EOASPB]2.0.CO;2 Anderson JE, Inouye RS (2001) Landscape-scale changes in plant species abundance and biodiversity of a sagebrush steppe over 45 years. Ecological Monographs 71, 531–556. doi:10.1890/0012-9615(2001) 071[0531:LSCIPS]2.0.CO;2 Arbuckle JL (2006) ‘Amos 7.0 User’s Guide.’ (Amos Development Corporation: Chicago, IL) Arredondo JT, Jones TA, Johnson DA (1998) Seedling growth of Intermountain perennial and weedy annual grasses. Journal of Range Management 51, 584–589. doi:10.2307/4003380 Badia D, Marti C (2003) Plant ash and heat intensity effects on chemical and physical properties of two contrasting soils. Arid Land Research and Management 17, 23–41. doi:10.1080/15324980301595 Baker WL, Shinneman DJ (2004) Fire and restoration of pinyon–juniper woodlands in the western United States: a review. Forest Ecology and Management 189, 1–21. doi:10.1016/J.FORECO.2003.09.006 Ball GH, Hall DJ (1965) ‘A Novel Method of Data Analysis and Pattern Classification.’ (Stanford Research Institute: Menlo Park, CA) Bauer JM, Weisberg PJ (2009) Fire history of a central Nevada pinyon– juniper woodland. Canadian Journal of Forest Research 39, 1589–1599. doi:10.1139/X09-078 Booth MS, Caldwell MM, Stark JM (2003) Overlapping resource use in three Great Basin species: implications for community invasibility and vegetation dynamics. Journal of Ecology 91, 36–48. doi:10.1046/ J.1365-2745.2003.00739.X Bradford JB, Lauenroth WK (2006) Controls over invasion of Bromus tectorum: the importance of climate, soil, disturbance, and seed availability. Journal of Vegetation Science 17, 693–704. Certini G (2005) Effects of fire on properties of forest soils: a review. Oecologia 143, 1–10. doi:10.1007/S00442-004-1788-8 Chambers JC (2005) Fire-related restoration issues in woodland and rangeland ecosystems. In ‘Mixed Fire Regimes: Ecology and Management. Symposium Proceedings’, 17–19 November, 2004, Spokane, WA. (Eds L Taylor, J Zelnik, S Cadwallader, B Hughes) AFE MIXC03, pp. 149–160. (Association of Fire Ecologists: Redlands, CA) Chambers JC, Roundy BA, Blank RR, Meyer SE, Whittaker A (2007) What makes Great Basin sagebrush ecosystems invasible by Bromus tectorum? Ecological Monographs 77, 117–145. doi:10.1890/ 05-1991 Cocke AE, Fule PZ, Crouse JE (2005) Comparison of burn severity assessments using Differenced Normalized Burn Ratio and ground data. International Journal of Wildland Fire 14, 189–198. doi:10.1071/ WF04010 D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annual Review of Ecology and Systematics 23, 63–87. D’Antonio CM, Chambers JC, Loh R, Tunison JT (2009) Applying ecological concepts to the management of widespread grass invasions. In ‘Ecological Invasions and Restoration’. (Ed. RL Inderjit) pp. 123–149. (Springer Publishing: New York) Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. Journal of Ecology 88, 528–534. doi:10.1046/J.1365-2745.2000.00473.X Evans RA, Young JA (1972) Microsite requirements for establishment of annual rangeland weeds. Weed Science 20, 350–356. Forbis TA, Provencher L, Frid L, Medlyn G (2006) Great Basin land management planning using ecological modeling. Environmental Management 38, 62–83. doi:10.1007/S00267-005-0089-2 Gillison AN, Brewer KRW (1985) The use of gradient directed transects or gradsects in natural resource surveys. Journal of Environmental Management 20, 103–127. 604 Int. J. Wildland Fire L. Condon et al. Grace JB (2006) ‘Structural Equation Modeling and Natural Systems.’ (Cambridge University Press: New York) Grace JB, Pugesek BH (1997) A structural equation model of plant species richness and its application to a coastal wetland. American Naturalist 149, 436–460. doi:10.1086/285999 Greenwood DL, Weisberg PJ (2009) GIS-based modeling of pinyon–juniper woodland structure in the Great Basin. Forest Science 55, 1–12. Harner RF, Harper KT (1976) The role of area, heterogeneity, and favorability in plant species diversity of pinyon–juniper ecosystems. Ecology 57, 1254–1263. doi:10.2307/1935049 Harris GA (1967) Some competitive relationships between Agropyron spicatum and Bromus tectorum. Ecological Monographs 37, 89–111. doi:10.2307/2937337 Humphrey LD, Schupp EW (2004) Competition as a barrier to establishment of a native perennial grass (Elymus elymoides) in alien annual grass (Bromus tectorum) communities. Journal of Arid Environments 58, 405–422. doi:10.1016/J.JARIDENV.2003.11.008 Kaplan D (2000) ‘Structural Equation Modeling: Foundations and Extensions.’ (Sage Publications Inc.: Thousand Oaks, CA) Keeley JE, McGinnis TW (2007) Impact of prescribed fire and other factors on cheatgrass persistence in a Sierra Nevada ponderosa pine forest. International Journal of Wildland Fire 16, 96–106. doi:10.1071/ WF06052 Keeley JE, Fotheringham CJ, Baer-Keeley M (2005) Determinants of postfire recovery and succession in Mediterranean-climate shrublands of California. Ecological Applications 15, 1515–1534. doi:10.1890/041005 Knick ST, Dobkin DS, Rotenberry JT, Schroeder MA, Vander Haegen WM, van Riper C (2003) Teetering on the edge or too late? Conservation and research issues for avifauna of sagebrush habitats. The Condor 105, 611–634. doi:10.1650/7329 Koniak S (1985) Succession in pinyon–juniper woodlands following wildfire in the Great Basin. The Great Basin Naturalist 45, 556–566. Kumar L, Skidmore AK, Knowles E (1997) Modeling topographic variation in solar radiation in a GIS Environment. International Journal of Geographical Information Science 11, 475–497. doi:10.1080/ 136588197242266 Link SO, Keeler SO, Hill RW (2006) Bromus tectorum cover mapping and fire risk. International Journal of Wildland Fire 15, 113–119. doi:10.1071/WF05001 Mack RN, Pyke DA (1983) The demography of Bromus tectorum: variation in time and space. Journal of Ecology 71, 69–93. doi:10.2307/2259964 Mazzola MB, Chambers JC, Pyke D, Schupp EW, Blank RR, Allcock KG, Nowak RS (2010) Effects of resource availability and propagule supply on native species recruitment in sagebrush ecosystems invaded by Bromus tectorum. Biological Invasions 13(2), 513–526. Meyer SE (1994) Germination and establishment ecology of big sagebrush: implications for community restoration. In ‘Proceedings on Ecology and Management of Annual Rangelands’. (Eds SB Monsen, SG Kitchen) USDA Forest Service, Rocky Mountain Research Station, General Technical Report RMRS-313, pp. 244–251. (Ogden, UT) Miller RF, Bates JD, Svejcar AJ, Pierson FB, Jr, Eddleman LE (2005) Biology, ecology, and management of western juniper (Juniperus occidentalis). Oregon State University, Agricultural Experiment Station, Technical Bulletin 152. (Corvallis, OR) Miller RF, Tausch RJ, MacArthur D, Johnson DD, Sanderson SC (2008) Development of post-settlement piñon–juniper woodlands in the Intermountain West: a regional perspective. USDA Forest Service, Rocky Mountain Research Station, Research Paper RMRS-RP-69. (Fort Collins, CO) Miller RF, Knick ST, Pyke DA, Meinke CW, Hanser SE, Wisdom MJ, Hild AL (2011) Characteristics of sagebrush habitats and limitations to long-term conservation. In ‘Greater Sage-Grouse: Ecology and Conservation of a Landscape Species and its Habitats’. Studies in Avian Biology, 38. (Eds ST Knick, JW Connelly) pp. 145–184. (University of California Press: Berkeley, CA) Monaco TA, Johnson DA, Norton JM, Jones TA, Connors KJ, Norton JB, Redinbaugh MB (2003) Contrasting responses of Intermountain West grasses to soil nitrogen. Journal of Range Management 56, 282–290. doi:10.2307/4003820 Mueggler WF (1956) Is sagebrush seed residual in the soil of burns or is it wind-borne? USDA Forest Service, Intermountain Forest Service and Range Experimental Station, Research Note 35. (Ogden, UT) Rau BM, Chambers JC, Blank RR, Johnson DW (2008) Prescribed fire, soil, and plants: burn effects and interactions in the central Great Basin. Rangeland Ecology and Management 61, 169–181. doi:10.2111/ 07-037.1 Reilly MJ, Wimberly MC, Newell CL (2006) Wildfire effects on beta diversity and species turnover in a forested landscape. Journal of Vegetation Science 17, 447–454. Thien SJ (1979) A flow diagram for teaching texture by feel analysis. Journal of Agronomic Education 8, 54–55. USDA NRCS (2006) US General Soil Map (STATSGO) for Nevada. (United States Department of Agriculture, Natural Resources Conservation Service) Available at http://soildatamart.nrcs.usda.gov [Verified 11 February 2007] Weisberg PJ, Lingua E, Pillai RB (2007) Spatial patterns of pinyon–juniper woodland expansion in central Nevada. Rangeland Ecology and Management 60, 115–124. doi:10.2111/05-224R2.1 West NE, York TP (2002) Vegetation responses to wildfire on grazed and ungrazed sagebrush semi-desert. Journal of Range Management 55, 171–181. doi:10.2307/4003353 West NE, Young JA (2000) Intermountain valleys and lower mountain slopes. In ‘North American Terrestrial Vegetation’. (Eds MG Barbour, WD Billings) pp. 256–284. (Cambridge University Press: Cambridge, UK) Young JA, Evans RA (1978) Population dynamics after wildfires in sagebrush grasslands. Journal of Range Management 31, 283–289. doi:10.2307/3897603 Young JA, Evans RA (1989) Dispersal and germination of big sagebrush (Artemisia tridentata) seeds. Weed Science 37, 201–206. Ziegenhagen LL (2003) Shrub reestablishment following fire in the mountain big sagebrush (Artemisia tridentata Nutt. ssp. vaseyana (Rydb.) Beetle) alliance. MSc thesis, Oregon State University, Corvallis, OR. Manuscript received 22 July 2009, accepted 1 November 2010 http://www.publish.csiro.au/journals/ijwf