A newly-discovered unique habitat By Dragos Micu and Valentina Todorova
advertisement

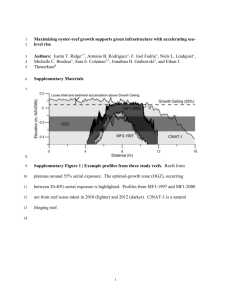
By Dragos Micu and Valentina Todorova During the summer of 2007 a Bulgarian-Romanian diving team of marine biologists carried out a series of expeditions along the Western Black Sea coast with the main goal of identifying sites suitable for designation as Marine Protected Areas. We found more than we expected, including discoveries that went far beyond the initial scope of our project and which we feel deserve a broader audience. A newly-discovered unique habitat © Dragos Micu We were diving along the rocky shores of the Southern Bulgarian coast, searching for live oysters to verify their occurrence. To our surprise, we came across Ostrea edulis biogenic reefs, a new type of habitat and unique to European seas – and probably the world. Unlike other European biogenic reefs, dominated by tube-building polychaetes such as Sabellaria (Porras et al., 1996; Gruet, 1985; Moore, 1996), Serpula vermicularis (Earll, 1992; Bosence, 1973), Ficopomatus enigmaticus (Micu & Micu, 2004) or mussels like Mytilus (Gosling, 1992) and Modiolus (Magorrian et al., 1995), the ostrak is built almost exclusively by the flat oyster, Ostrea edulis. Small serpulid polychaetes do occur on the ostrak also, but, if we imagine ostrak as a fortress wall, then the oysters are the stones and the polychaetes are the cement – a very thin one, that is. Unlike the oyster beds commonly known from the intertidal of Western Europe and North America – 7m in height, 30-50m in length and 10m in width for each mound – Black Sea ostrak are massive, towering biogenic structures, dwarfing the human observer. (a). Flat oyster (Ostrea edulis) biogenic reef. “Ostrak” is what these reefs are called by local fishermen. They think the name comes from the Bulgarian word “ostar,” meaning sharp, and associate it with the edges of oyster shells sticking out from the reef, sharp enough to cut the hands or the neoprene suit of a careless diver. However, in the authors’ opinion the name originates from “Ostrakon,” meaning oyster shell, and was probably given to the oyster reefs by the ancient Greeks who first witnessed their existence thousands of years ago. 26 MarBEF Newsletter Autumn 2007 Unlike the small, estuarine oyster reefs built by Crassostrea virginica on the south-eastern US coast (Posey et al., 2003; Grizzle et al., 2002), ostrak reefs occur in clear marine waters with no freshwater input, forming a belt between 7 and 23m in depth, parallel to rocky coasts. Smaller ostrak formations may also occur on rocky offshore reefs or as a sponge-like structure adhering to rocky vertical drop-off faces. Local people have pointed out many locations where ostrak is present but, due to the limited time and resources, we were only able to dive briefly at three sites. These reefs were overgrown by sponges, mussels (Mytilus galloprovincialis) and algae (Delesseria ruscifolia and Zanardinia prototypus) and harboured a very diverse marine life. While fresh oyster shells were visible on the reefs, no live oysters were recorded at these sites (see photo b). Only a few years ago, local people collected live oysters from these very reefs and there are many more sites at which we could not dive, so we are hopeful that other oyster reefs in the © Dragos Micu Biodiversity of the Western Black Sea (b). Ostrea edulis reef with no live oysters. area may still be alive. Our discoveries have raised some questions: 1. Do live reefs still exist? We are currently trying to find funding to carry out more extensive field research which will allow us to map all the sites where ostrak habitat occurs. During this search, we hope to discover some live oyster reefs. 2. Why did the oysters perish at the sites investigated by us? The oysters were never commercially fished, and recreational harvest was very limited, so overfishing can be ruled out as a cause of extinction. Were oysters killed by eutrophication and diseases brought with cultured Crassostrea gigas in the Russian part of the Black Sea? Were they decimated by the alien predatory whelk Rapana venosa? More research is needed to find out. 3. Is oyster reef restoration possible? The idea of restoring the amazing Ostrak reef ecosystem came to us as soon as we discovered the reefs, but it is very clear that any attempt at reintroduction is futile while we don’t understand the reasons/causes behind the oyster reefs die-out in the first place. Rare species We encountered a number of rare species during our dives – among them, the PontoCaspian relicts Neogobius kessleri and large, older whelks attacking one of the many juveniles around them, but also with several juveniles attacking together a larger whelk, weakened by starvation. © Dragos Micu Syngnathus abaster, both found in the Veleka River estuary. This is a new distribution record for Neogobius kessleri, previously known only from the Danube, Danube Delta, Dniester and adjacent lakes (Banarescu, 1964; Jivkov & Karapetkova, 2007). This Rapana superinvasion changes the reef ecosystem drastically and is bound to interfere with the lives of other species inhabiting the reef. The onset of starvation affects not only Rapanas but all of the other species of invertebrates and demersal fishes which depend on mussels as their staple food. Those which are mobile enough, like the fishes, will leave the reef for better pastures, while those which are less mobile, like crabs, will starve and eventually die, only to be consumed by the whelks. This leads to a massive loss of biodiversity and impaired reef ecosystem functioning. Other Mediterranean fishes, rare in the Black Sea, were Chromis chromis, Diplodus annularis, Gobius bucchichi, Gobius paganellus, Zosterisessor ophiocephalus, Arnoglossus kessleri, Uranoscopus scaber, Gobius cobitis, Salaria pavo, Blennius ocellatus, Coryphoblennius galerita, Aphia minuta and Nerophis ophidion. We found several bivalve molluscs which are rare for the Black Sea (Dumont, 1999; Micu, 2004): Loripes lacteus in fine sands, Petricola lithophaga in sandstone, Teredo navalis in wooden debris and Pholas dactylus in marl, the latter species listed in the Bern and Barcelona Conventions (Micu, 2007; SoHelME, 2005). Few species can cope with the new conditions, but some do. Until now, there have been very few natural enemies of Rapana known from the areas it has invaded around the globe. In the Chesapeakes, blue crabs Callinectes sapidus consumed Rapana in laboratory experiments, but it is not known if they do so in nature (Harding, 2003). Seastars eat Rapana in and around the Bosphorus (Zaitsev & Ozturk, 2001), but they do not live in the Black Sea proper. Rare crustaceans (Dumont, 1999; Micu & Micu, 2006) which we found included the barnacle Chthamalus stellatus, the endemic mysids Hemimysis sp, the thalassinid Pestarella candida, the crab Polybius navigator and the hermit crab Clibanarius erythropus. Climate change-induced phenomena in the Western Black Sea The Black Sea has, by and large, two climate types: submediterranean on the eastern and southern coasts, and Crimean and temperate on the western and northern coasts. The temperate climate on the western coasts has provided, until now, suboptimal conditions for the development of the alien predatory gastropod Rapana venosa, which originates from the warmer Asian seas (Sea of Japan to Hong Kong). Although a common and well adapted species, Rapana has never caused local extinction or generalised stock depletion of its bivalve prey species on the western coast. We could say that its invasiveness has proven to be only mild to medium here, and with the onset of commercial fisheries for it in the 1980s, Rapana became more a resource than a threat. We have heard through personal communications, as well as reading in Russian scientific papers, about the occurrence of massive settlements of the alien predatory (c). Rapana settlement. gastropod Rapana venosa in the Eastern Black Sea (Chikina & Kucheruk, 2005). These cyclic events lead to high densities of young Rapana, which wipe out all prey items available in the area before dying off of starvation, which in turn leads to the onset of impoverished and dysfunctional ecosystems. For us who, for decades, lived “peacefully” on the western coast, with Rapana well-integrated in our marine ecosystems and a major cash crop for our commercial fisheries, these accounts, even when properly illustrated, were hard to believe. Well, not anymore! In 2006 and 2007, massive settlement of Rapana juveniles occurred at many sites along the Bulgarian coast. Shallow rocky shores and offshore reefs seem to be the preferred sites for such events. We have witnessed and documented incredible densities, with the substrate completely covered with a compact blanket of Rapana juveniles of the 0+ to 1+ year-classes. The bivalve populations of the area are completely obliterated, even the smallest species, such as Mytilaster lineatus. The whelks then resort to scavenging, consuming all corpses available in the area: fishes, crabs and even seabirds (see photo c). As a last resort, we have observed for the first time cannibalism among Rapa whelks, with On the Bulgarian coast, we have documented for the first time the consumption of Rapana by a native species in the wild. The robust crab Eriphia verrucosa is strong enough to crush the shells of juvenile Rapana and eat them. This is probably why densities of the crab seem unchanged on the reefs affected by the Rapana superinvasion (see photo d). © Dragos Micu Phyllophora nervosa, Delesseria ruscifolia, Laurencia sp and Zanardinia prototypus should be pointed out as rare algae (Dumont, 1999; Dimitrova-Konaklieva S, 2000) that we found along the Bulgarian coast. (d). Robust crab (Eriphia verrucosa). Autumn 2007 MarBEF Newsletter 27 © Dragos Micu sites. Its only available record from the Black Sea until now refers to the Bosphorus Strait inside Istanbul city (Hureau, 1991). The red mullet’s spread to the north is another clear indication of the Black Sea’s accelerated mediterranisation, associated with global climate change (see photo e). Further evidence was provided by the supralittoral association of the barnacle Chthamalus stellatus and the snail Melaraphe neritoides, whose northern limit used to be at cape Kaliakra but which has extended around 30km northwards to Tyulenovo village. Acknowledgments These studies were carried out within the framework of the project “Marine Protected Areas in Bulgaria & Romania,” supported by the BBI-Matra Action Plan Support Scheme 2005-’08 of the Dutch Ministry of Agriculture, Nature and Food Quality and SIBEMA project, Flanders-Bulgarian Cooperation. (e). Red mullet (Mullus surmuletus). Eriphia verrucosa is not a dedicated predator of Rapana, normally preferring easier prey such as mussels and smaller crabs. In the event of a superinvasion, however, it is able to switch to a diet of young Rapanas and survive. But this is all we can expect from this crab; it is very References Banarescu, P (1964). Fauna Republicii Populare Romîne, Vol XIII. Pisces - Osteichthyes (pesti ganoizi si ososi). Editura Academiei Republicii Populare Romîne, Bucuresti, 962p. Bosence, DWJ (1973). Recent serpulid reefs, Connemara, Ireland. Nature 242, 40-41. Chikina, MV and Kucheruk, NV (2005). Longterm changes in the structure of coastal benthic communities in the northeastern part of the Black Sea: Influence of alien species. Oceanology 45(1), S176-S182. Dimitrova-Konaklieva, S (2000). Flora of the Marine Algae of Bulgaria (Rhodophyta, Phaeophyta, Chlorophyta). Pensoft, Sofia-Moscow, 304pp. Dumont HJ (ed) (1999). Black Sea Red Data Book. Published by the United Nations Office for Project Services, 413pp. Earll, RC (1992). The SeaSearch Habitat Guide: an identification guide to the main habitats found in the shallow seas around the British Isles. Gosling, E (ed) (1992). The mussel Mytilus: ecology, physiology, genetics and culture. Developments in Aquaculture and Fisheries Science, Vol 25. Elsevier, 589pp. Grizzle, RE, Adams, JR and Walters, LJ (2002). Historical changes in intertidal oyster (Crassostrea virginica) reefs in a Florida lagoon, potentially related to boating activities. Journal of Shellfish Research 21(2), 749-756. Gruet, Y (1985). Ecology of sabellarian reefs built 28 MarBEF Newsletter Autumn 2007 unlikely that Eriphia verrucosa will ever be able to control Rapana venosa effectively in the wild. For the first time, we documented the occurrence of Mullus surmuletus all over the Bulgarian coast and at southern Romanian by the Annelida Polychaeta Sabellaria alveolata (Linne). J. Rech. Oceanogr. 10, 32-35. Harding, JM (2003). Predation by blue crabs, Callinectes sapidus, on Rapa whelks, Rapana venosa: possible natural controls for an invasive species? J. Exp. Mar. Biol. and Ecol. 297, 161–177. Hureau, J-C (1991). La base de données GICIM: Gestion informatisée des collections ichthyologiques du Muséum, pp225-227. In: Atlas Préliminaire des Poissons d'Eau Douce de France. Conseil Supérieur de la Pêche, Ministère de l'Environnement, CEMAGREF et Muséum national d'Histoire naturelle, Paris. Jivkov, M and Karapetkova, M (2007). Ribite v Bulgaria, 2nd edition. Geia Libris, Sofia, 216pp. Magorrian, BH, Service, M and Clarke, W (1995). An acoustic bottom classification survey of Strangford Lough, Northern Ireland. Journal of the Marine Biological Association of the United Kingdom 75, 987-992. Micu, D (2004). Annotated Checklist of the Marine Mollusca from the Romanian Black Sea. In: Ozturk, B, Mokievsky, VO and Topaloglu, B (eds): International Workshop on Black Sea Benthos, pp89152. Published by Turkish Marine Research Foundation, Turkey 2004, 244pp. Micu, D (2007). Recent records of Pholas dactylus L. 1758 (Bivalvia: Myoida: Pholadidae) from the Romanian Black Sea, with considerations on its habitat and proposed IUCN regional status. Acta Zoologica Bulgarica (in press). Micu, D and Micu, S (2004). A new type of Dragos Micu National Institute of Marine Research and Development, “Grigore Antipa,” Email: ddrraaggoossmm@yahoo.com macrozoobenthic community from the rocky bottoms of the Black Sea. In: Ozturk, B, Mokievsky, VO and Topaloglu, B (eds): International Workshop on Black Sea Benthos, pp75-88. Published by Turkish Marine Research Foundation, Turkey 2004, 244pp. Micu, S and Micu, D (2006). Proposed IUCN regional status of all Crustacea: Decapoda from the Romanian Black Sea. Ann. Sci. Univ. “Al.I.Cuza” Iasi, sect. Biologie Animala Tom LII, 7-38. Moore, CG (1996). The distribution of serpulid reefs in Loch Creran, Argyll. Scottish Natural Heritage Research, Survey and Monitoring Report, No 53. Papathanassiou, E and Zenetos, A (eds), 2005. SoHelME 2005: State of the Hellenic Marine Environment. HCMR Publ., 360pp. Porras, R, Bataller, JV, Murgui, E and Torregrosa, MT (1996). Reef building worms in Iberian Mediterranean coasts. In: Proceedings of the 2nd International Conference on the Mediterranean Coastal Environment – Medcoast 95, October 24-27 1995, Tarragona, Spain. Ozhan, E (ed). Posey, MH, Cressman, KA, Mallin, MA, Alphin, TD and Leonard, LA (2003). Effects of oyster reefs on water quality in a tidal creek estuary. Journal of Shellfish Research 22(3), 753-762. Zaitsev, Yu. and Ozturk, B (eds) 2001. Exotic species in the Aegean, Marmara, Black, Azov and Caspian Seas. Published by Turkish Marine Research Foundation, Istanbul, Turkey, 267pp. SESAME: Southern European Seas: Assessing and Modelling Ecosystem Changes Participants on the first training course in Plankton Production, held at the Hellenic Centre for Marine Research, Greece, in July 2007. The Integrated Project SESAME IP, launched in November 2006, is a four-year project funded by the EC’s 6th Framework Programme. It is designed to study the past, present and future environmental changes in the Mediterranean and Black Sea ecosystems, and how these affect their abilities to provide goods and services with fundamental societal importance, such as tourism, fisheries, mitigation of climate through carbon sequestration, and ecosystem stability through conservation of biodiversity. SESAME unites scientists from more than 20 countries to investigate the current status of the Mediterranean and the Black Seas. With the help of historical data, in combination with newly collected data, SESAME aims to identify the environmental changes these regions have experienced over the last 50 years. For the first time, the two seas will be approached as a coupled climatic/ecosystem entity. The goal is, based on past and current status, to predict possible changes within the next 50 years. The project will thus provide the basis for developing and implementing sustainable development policies and strategies within these regions, by involving and assisting the relevant stakeholders, especially in a time of strong climate change debates. SESAME’s Figure 1. Study area for the SESAME project. multidisciplinary approach will bring together natural scientists and socio-economists. Exploring the Mediterranean and the Black Sea Although regional data are already available, they were, until now, hardly accessible to the international scientific community due to language barriers. During the first year of the project, previously collected historical and current multidisciplinary datasets of physicochemical and biological parameters will be analysed for signals of environmental changes and trends. During the second year, two multidisciplinary and multiship seasonal research cruises will cover the entire Mediterranean and Black Sea basins (see coloured map lines for transects, Fig. 1) in order to collect new information about the actual status of the two ecosystems. Ten oceanographic research vessels will simultaneously conduct these cruises, which are planned for March-April 2008 and for August-September 2008. The collected highquality in situ data from all the cruises will be used to tune and validate the basin-scale and sub-regional models, and feed the SESAME databases. Data collection for model validation began at the beginning of SESAME in the seven subregional areas, shown as shaded rectangles on the map. Ten 3D coupled biogeochemical-hydrodynamical models, three at basin scale (Mediterranean, Black sea and the whole SES area) and seven at regional scale (five subbasins and the straits – TSS and Gibraltar), as well as a 1D model referring to carbon export, have already been described in terms of their structure, the basic hypotheses on which they rely (e.g. functional/sized classes organisation of the ecosystem) and their resolution. These models will be used for the assessment of the ecosystem functioning modifications that have taken place in the last 50 years. The same models will be used for the prediction of future changes in the ecosystems. Particular focus has been placed on identifying the limitations of the models and the extension/improvement needed to be able to address SESAME’s objectives. The semi-quantitative fields of economics and social sciences will be fully addressed over five study areas (see map). Study of these areas will explore the possibility of transferring and/or adapting state-of-the-art analytical Autumn 2007 MarBEF Newsletter 29