Spleen Macroscopic reporting dictation template
advertisement

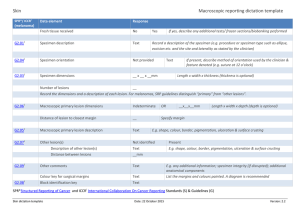
Spleen SPR*/ ICCR¡ Macroscopic reporting dictation template Data element Response Fresh tissue received No Procedure Text Specimen type Text Specimen integrity Intact S2.04 Specimen size __x__x__mm S2.04 Spleen weight __g S2.03 Separate blood clot weight, if present Hilar lymph node Yes If yes, describe any additional tests flow cytometry/microbiology/ cytogenetics/biobanking performed As stated by the clinician Disrupted, describe ___________ __g Absent Present Normal Abnormal If present, maximum dimension __mm Specimen description Outer surface Irregular Nodules Plaques Laceration(s) Describe size ______mm and location__________ Parenchymal lesions Absent Present Number __ For each lesion: (if >1 designate accordingly) G2.03 Lesion size __x__mm Or if >2 range of sizes __x__mm to __x__mm Lesion site Subcapsular Lesion description Solid nodule Other relevant macroscopic information Text E.g. any additional orientation; specimen integrity (if disrupted) etc. Block identification key Text Describe nature and site of blocks Parenchyma Multinodular Cystic Miliary nodules, describe variation in sizes SPR*Structured Reporting of Cancer and ICCR¡ International Collaboration On Cancer Reporting Standards (S) & Guidelines (G) Spleen dictation template Date: 22 October 2015 Version: 1.1