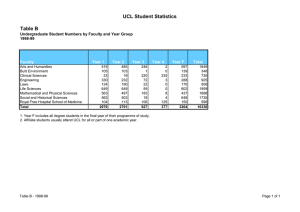
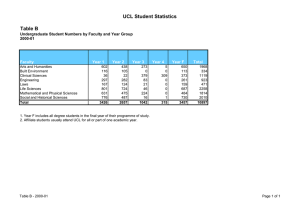
Comprehensive Clinical Trials Unit Collaboration Request Proforma
advertisement

Comprehensive Clinical Trials Unit Collaboration Request Proforma On receipt of this form you will receive notification of a meeting date at which you will be asked to make a brief presentation which should be provided 48 hours before the meeting in PowerPoint format Trial Title CCTU Reference Investigator: Position: Organisation: Email: Telephone: 1. Brief summary of research question: 2a. Brief summary of importance/relevance to NHS priorities: Comprehensive Clinical Trials Unit at UCL. Collaboration Request Proforma v6 27 March 2014 2b. Brief summary of importance/relevance to UCL or UCLH priorities: 3. Is Patient and Public Involvement planned? YES/NO If yes, describe level of Patient and Public involvement thus far: 4. Have you performed a literature Review? YES/NO Please provide a maximum of 5 key references (ideally as pdfs) for the proposed area of research: 5. Background to clinical problem (including demographic/frequency of condition/problem/resource implications): Comprehensive Clinical Trials Unit at UCL. Collaboration Request Proforma v6 27 March 2014 6. Please describe your project in terms of PICOS (Patient, Intervention, Control, Outcomes and Study/Statistical design) P - Patient Group: I- Intervention(s): C - Control: O - Outcomes and follow up period: S - Study/Statistical design (e.g. randomised controlled trial, case control study, pilot study): 7. For trial development and sample size calculations, you will need to provide: An estimate of your primary study outcome measure for the control group (for example response rate expected without the new intervention) Also think about a clinically significant difference you would want to observe between groups for the study to be convincing (e.g be worthwhile for funders and patients, change practise) Please provide sources/justification for these estimates from pilot work/literature: Comprehensive Clinical Trials Unit at UCL. Collaboration Request Proforma v6 27 March 2014 8. Sample size (not required, but helpful if already performed or estimated): 9. Anticipated number of sites: 10. Number of sites already identified as willing to participate: a. UK: b. International (if applicable): 11. Funding plans and status (if known): Name of proposed funder: Deadline for submission: Type of application: Estimated potential grant total (if known): If your proposed trial is in response to a specific call, please include a pdf copy of the call when you submit this form 12. Have you made contact with a research network or national speciality group(s)? YES/NO If yes, name of research network or national speciality group: 13. Have you made contact with a Comprehensive Local Research Network (CLRN)? YES/NO Comprehensive Clinical Trials Unit at UCL. Collaboration Request Proforma v6 27 March 2014 If yes, name of CLRN: 14. Have you engaged with a Research Design Service (RDS)? YES/NO If yes, name of RDS: 15. Have you previously approached a CTU regarding this study? YES/NO If yes, what was the outcome? 16. Briefly describe the clinical trials experience of the Chief Investigator and the current trial team 17. For CCTU Linked Sites (if you are unsure please contact the CCTU for clarification) Please confirm the name of the Clinical Trial Lead that has been contacted prior to submission of this form to the CCTU For CCTU only CCTU Reference Date application received Date of TAG meeting to review this trial Date Investigator informed Investigator informed by (name) Comments Comprehensive Clinical Trials Unit at UCL. Collaboration Request Proforma v6 27 March 2014 Please email your completed form to CCTU-enquiries@ucl.ac.uk Comprehensive Clinical Trials Unit at UCL. Collaboration Request Proforma v6 27 March 2014