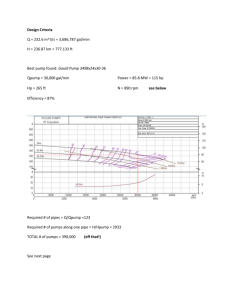
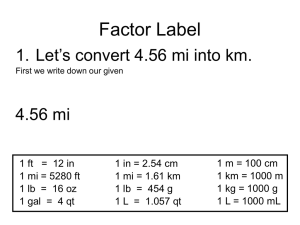
Georgia Vegetable Extension-Research Report 2001
advertisement

Georgia Vegetable Extension-Research Report 2001 Edited by William Terry Kelley and David B. Langston, Jr. The University of Georgia College of Agricultural & Environmental Sciences Cooperative Extension Service Agricultural Experiment Station U.S. Department of Agriculture Notice to Users Use of products mentioned in this publication must be consistent with the manufacturer’s current label as registered with the appropriate agencies. Mention of a product does not constitute an endorsement or guarantee by the University of Georgia or any other agencies and personnel nor discrimination of similar products not mentioned. Trade names and brand names are used only for informational purposes. Authors are responsible for statements made and for the accuracy of the data presented. The editors, affiliated associations, typists, etc., assume no responsibility for typographical or other errors found in text or tables. Copies of this publication or parts of it should not be made without the consent of authors. Reprints of reports may be obtained from the authors. The Georgia Agricultural Experiment Stations, The Cooperative Extension Service and the University of Georgia College of Agricultural and Environmental Sciences offer educational programs, assistance and materials to all people without regard to race, color or national origin. An Equal Opportunity Employer UGA/CPES Research - Extension Publication No. 5-2001 December 2002 Issued in furtherance of Georgia Agricultural Experiment Stations research work, Cooperative Extension work, Acts of May 8 and June 30, 1914, The University of Georgia College of Agricultural and Environmental Sciences and the U.S. Department of Agriculture cooperating. Gale A. Buchanan Dean and Director i ii Table of Contents Cultural Practices - Bed Press Incorporation Study.............................................................................................3 Paul E Sumner - Effect of BM86 on Fall Snap Bean Production..................................................................11 William Terry Kelley - Effect of BM86 and MZ63 on Fall Peppers........................................................................13 William Terry Kelley - Effect of K-tionic on Fall Bell Pepper Production.............................................................15 William Terry Kelley Variety Evaluation - Cantaloupe Variety Trial, 2001...........................................................................................19 George E. Boyhan, Darbie M. Granberry, William Terry Kelley, C. Randell Hill - Watermelon Variety Trial, 2001..........................................................................................21 George E. Boyhan, Darbie M. Granberry, William Terry Kelley, C. Randell Hill - Georgia Commercial Pumpkin Variety Trials - 2001........................................................24 William Terry Kelley - Commercial Snap Bean Fall Cultivar Evaluation - 2001...................................................28 William Terry Kelley - Summer Squash Variety Trial - 2001..................................................................................32 William Terry Kelley, David Curry, Gregory Hardison - Sweet Corn Variety Trial - 2001...........................................................................................45 William Terry Kelley, David Curry, Gregory Hardison Weed Control - Weed Management in Watermelon and Cantaloupe Transplanted on Polyethylene Covered Seedbeds............................................................................................51 W. Carroll Johnson, III and Theodore M. Webster - Sandea Has a Variable Affect on Squash Growth and Yield...........................................65 T.M. Webster Disease Control - Application Methods of Soil Chemical Treatments on Nematodes, Disease and Yield of Cucumber............................................................................................71 A.S. Csinos, K. W. Seebold, P. Timper, R.F.Davis, J.E. Laska - Soil Chemical Treatment Alternatives to Methyl Bromide in Cucumber......................82 iii A.S. Csinos, K.W. Seebold, P. Timper, R.F. Davis, J.E. Laska - Effects of Vydate and Telone Treatments for Management of Nematodes, Diseases and Yield of Cucumber......................................................................93 A.S. Csinos, K.W. Seebold, P.Timper, J.E. Laska - Evaluation of Chisel Placement of Telone Products and Drip Irrigation for Efficacy and Yield in Bell Pepper................................................................103 A.S. Csinos, K.W.Seebold, P.Timper, J.E. Laska - Telone C-17, Metham Sodium Combinations for Efficacy and Evaluation of Phytotxicity in Squash .................................................................................114 A.S. Csinos, K.W. Seebold, P.Timper, J.E. Laska - Effects of Tomato Spotted Wilt Virus on Plant Growth, Yield and Fruit Quality of Drip-Irrigated Tomato Plants...........................................................................125 Juan C.Diaz-Perez, Dean Batal, Denne Bertrand, David Giddings -Evaluation of Fungicides and Biological Control Materials for Control of Cercospora Lea Spot of Turnip......................................................................................129 D.B. Langston, Jr, K.W. Seebold -Evaluation of Fungicides and Spray Programs for Control of Gummy Stem Blight of Watermelon..................................................................................132 D.B. Langston, Jr., R.T. Boland, Jr., J.G. Price -Evaluation of Actigard, Messenger, and Vacciplant for Suppression of Bacterial Spot of Tomato.....................................................................................................136 D.B. Langston, Jr., J.T. Flanders -Evaluation of Fungicides and Biological Control Materials for Control of Powdery Mildew in Transgenic Yellow Crookneck Squash............................................138 D.B. Langston, Jr., W.T. Kelley -Evaluation of Fungicides and Biological Materials for Control of Downy Mildew and Plectosporium Blight of Pumpkin.................................................................141 D.B. Langston, Jr., K.W.Seebold -Evaluation of Fungicides and Protection Intervals for Control of Alternaria Leaf Spot of Carrot Using Two Nitrogen Fertility Regimes.............................................144 D.B. Langston, Jr., J.E. Hudgins - Screening Fungicides for Control of Phytophthora capsici in Summer Squash..........146 Kenneth W. Seebold, David B. Langston, Jr., T. Bryan Horton - Evaluation of Experimental Fungicide for Control of Phytophthora Crown Rot of Summer Squash...........................................................................................148 Kenneth W. Seebold, David B. Langston, Jr., T. Bryan Horton Insect Control iv - Insect Pest Control Trials in Vegetables in 2000-2001..................................................153 David G. Riley Economics & Marketing - Commercial Vegetable Price Outlook ...........................................................................169 Greg E. Fonsah iv Cultural Practices -1- -2- Bed Press Incorporation Study Paul E. Sumner Extension Engineer Biological and Agricultural Engineering Department University of Georgia Introduction The phasing out of methyl bromide as a method of sterilizing the soil within the vegetable bed has made the industry rethink methods for weed, disease and nematode control. Weed control has become a major concern. Some chemicals are labeled but have had varying results and damage to vegetable crops. To ensure maximum weed control, uniform mixing in the recommended depth of the soil is essential. Growers and University scientist using herbicides for weed control under plastic have had mixed results for weed control. The objective of herbicide incorporation is to uniformly place the pesticide as close to the target pest as possible. Most labels specify the proper depth of incorporation. Herbicides that are not uniformly placed may result in hot spots of high concentration or untreated spots. The objective of this study was to simulate the use of herbicides with flourescent dyes in vegetable plastic culture and determine where the herbicide goes once the final bed has been formed. Methods and Materials To study the chemical placement and distribution by different soil incorporation equipment two different types of bed presses(Figure 1 and 2) were evaluated. A superbedder and conventional bed press was used to make the final beds. Prior to bedding, the land was prepared by turning the soil, next fluorescent dye sprayed and then incorporated with one of the following pieces of equipment: disk-harrow, field cultivator, vertical tiller (rototiller) and combination rototiller and bed shaper. The orange fluorescent dye was sprayed on a 6 foot by 9 foot area. (Shown in Figure 3) The dye was then incorporated by an implement using either a single pass or two passes. After the tillage operations were performed on each plot, a cross-section was made by first digging a trench perpendicular to the tillage direction. After the trench was dug, enough soil was removed to place a four foot black light in front of the cross-section (Shown in Figure 4). The fluorescent dye glowed wherever it was deposited, giving an example of where the herbicide would have been placed with the tillage implement. Results The following figures show where the fluorescent dye was incorporated. The lighter spots in the cross-section indicate the location of the dye. Figures 5-8 are illustrations of using a bed press as the method of making the final bed before applying plastic. In all cases the operation of this type of bed press added a layer of untreated soil to the top of the bed making it undesirable for herbicide application. -3- Figure 5 shows the using a rototiller to incorporate the dye. Using a rototiller is the best method of completely mixing the soil with in the set depth. Figure 6 shows the cross-section resulting from a single pass incorporation using a diskharrow. The cross-section shows that a one pass incorporation leaves streaks of soil not moved or mixed. It further indicates that the dye was placed at a depth of 6 inches in some locations, which maybe too deep to be effective depending on the chemical used. The incorporation obtained by a single pass-field cultivator is shown in Figure 7. The horizontal distribution of dye is not uniform after one pass. The distribution of dye improved with the second pass. Figure 8 illustrates the effect of bedding two times. Some growers do this practice in order to make a good firm bed. Shown are field cultivator and rototiller. When the second pass was made more untreated soil was placed on top of the profile. The use of two pass disk-harrow followed by a superbedder is shown in Figure 9. Mixing of the dye was fairly uniform from top to bottom of the bed. Normally depth of incorporation for a disk-harrow is half the depth of operation. Figure 10 shows the incorporating with a rototiller to the depth of 4 inches. Dye distribution was uniform from top to bottom of the incorporated area. Figures 11 and 12 show the combination rototiller and bed shaper followed by the superbedder. Incorporation shows streaks of concentrated dye, that may have been caused by rototiller knives not being close enough together. Conclusions The study with the bed press showed that there was significant difference between the different types of soil incorporation tillage equipment. The only problem being untreated soil was place on top of bed. This may have been due to the fact of having to operate the bed press to a depth that would have pulled untreated soil up. The supperbedder was better than the bed press in the conditions tested. The combination of tillage methods and the superbedder resulted in acceptable distribution of the dye throughout the bed. If growers incorporate herbicides prior to making the final bed and laying plastic covering should be cautious of herbicide damage. -4- Figure 1. Superbedder bed press. Figure 2. Bed Press. -5- Figure 3. Fluorescent dye sprayed on plot. Figure 4. Daylight View of Black Lights & Soil Profile. Figure 5. Rototiller incorporation followed by bed press. Figure 6. Disk-harrow incorporation followed by bed press. -6- Figure 7. Field cultivator incorporation followed by bed press. Figure 8. Double bed pressing of field cultivator(left) and rototiller(right). -7- Figure 9. Disk-harrow followed by super bedder. Figure 10. Rototiller followed by super bedder. -8- Figure 11. Combination rototiller and bed shaper. Figure 12. Combination rototiller and bed shaper followed by super bedder. -9- Figure 13. Dye applied prior to turning and again before disk-harrow followed by super bedder. -10- Effect of BM 86 on Fall Snap Bean Production William Terry Kelley Extension Horticulturist, Rural Development Center P.O. Box 1209, Tifton, Georgia 31793 wtkelley@uga.edu Introduction BM 86 is a foliar nutritional product manufactured by Goemar. It contains approximately 5% nitrogen, 2.4% magnesium, 3.2% sulfur, 2.0% boron, 0.02% molybdenum and 0.6% sodium. Since it is marketed to Georgia growers, BM 86 was tested on snap beans during fall production to determine its effects on plant growth, pod characteristics and marketable yield. Methods “Bronco” variety (Seminis Seed Co.) snap beans were planted at the Coastal Plain Experiment Station (elev. 382 feet) in Tifton, Georgia. Snap beans were direct-seeded on August 27, 2001 into a Tifton sandy loam (fine-loamy siliceous thermic Plinthic Kandiudults) soil. Plots consisted of two side-by-side rows with three seed planted per foot of row. Plots were eight feet in length with three feet between rows. The planting was arranged in a Randomized Complete Block Design with four replications. Normal cultural practices were used for bare ground snap bean culture in Georgia. Base fertilizer consisted of 400 pounds/A of 10-10-10 incorporated prior to planting followed by two sidedress applications of 15-0-14 (150 pounds/A each). Weed control was accomplished with a pre-emergence application of s-metolachlor (0.94 lb a.i./A). Fungicide and insecticide applications were made according to current University of Georgia recommendations. Irrigation was applied as needed. BM 86 was applied with a solo backpack sprayer at two quarts per acre at first bloom. An additional two quarts was applied to the same plots at the pod stage. Treated plots were compared to an untreated check. Snap beans were harvested at maturity on October 17, 2001. Data were collected on marketable yield, percent marketability, plant height, canopy width, and pod characteristics including color, width, length, shape, straightness and smoothness. Results are summarized in Tables 1 and 2. Results Marketable yield was greater in the untreated plots compared to the treated plots, although not significantly. BM 86 plots produced more immature beans at harvest, but this also was not a significant difference. Percent marketability, plant height, canopy width and all pod characteristics were unaffected by treatments. There appears to be no significant effect of using BM 86 for snap bean production. -11- Table 1. Treatment Yield, percent marketability and plant characteristics of snap beans treated with BM 86 (two quarts /A at first bloom and two quarts/A at pod stage) and untreated snap beans at Tifton, Georgia in 2001. Marketable Immature Percent Plant Height Canopy Yield (lb/A) Yield (lb/A) Marketable (cm) Width (%) (cm) Untreated 9178 565 93.6 12.8 17.5 BM86 6735 653 90.9 12.6 17.5 Mean of Test 7957 609 92.2 12.7 17.5 L.S.D. (0.05) 3804 445 7.6 1.5 2.2 C.V. (%) 32.21 49.21 5.53 8.17 8.66 Table 2. Treatment Fruit characteristics of snap beans treated with BM 86 (two quarts /A at first bloom and two quarts/A at pod stage) and untreated snap beans at Tifton, Georgia in 2001. Pod Color1 Pod Width Pod Pod Shape2 Pod Pod (mm) Length Straightnes Smoothnes (cm) s3 s4 Untreated 2.4 10.0 14.6 2.8 1.4 2.3 BM86 2.5 9.8 12.8 2.6 1.7 2.1 Mean of Test 2.5 1.0 13.7 2.7 1.5 2.2 L.S.D. (0.05) 0.5 1.4 3.1 0.4 0.3 0.7 C.V. (%) 12.39 9.63 15.30 9.16 13.73 20.73 Based on a scale of 1=dark green; 2=medium green; 3=light green; 4=yellow. 2Based on a scale of 1=flat; 2=oval; 3=round. 3Based on a scale of 1=straight; 2=slightly curved; 3=curved. 4 Based on a scale of 1=smooth, 2=moderately smooth, 3=rough. 1 -12- Effect of BM 86 and MZ 63 on Fall Pepper Production William Terry Kelley Extension Horticulturist, Rural Development Center P.O. Box 1209, Tifton, Georgia 31793 wtkelley@uga.edu Introduction BM 86 and MZ 63 are foliar nutritional products manufactured by Goemar. BM 86 contains approximately 5% nitrogen, 2.4% magnesium, 3.2% sulfur, 2.0% boron, 0.02% molybdenum and 0.6% sodium. MX 63 contains 5% nitrogen, 1.89% magnesium, 5.72% combined sulfur, 1.57% water soluble manganese and 2.36% soluble zinc. Since these products are marketed to Georgia growers, BM 86 and MZ 63 were tested on pepper during fall production to determine its effects on plant growth, pod characteristics and marketable yield. Methods “Camelot” variety (Seminis Seed Co.) bell pepper transplants were commercially produced in 200-cell polystyrene trays at a local greenhouse. Peppers were planted into the field at the Coastal Plain Experiment Station (elev. 382 feet) in Tifton, Georgia on August 27, 2001 into a Tifton sandy loam (fine-loamy siliceous thermic Plinthic Kandiudults) soil. Plots consisted of two side-by-side rows on a plastic mulch-covered bed with eight plants in each row and 12 inches between plants. Beds were spaced six feet apart from center to center. Plots were eight feet in length with three feet between plots. The planting was arranged in a Randomized Complete Block Design with eight replications. Normal cultural practices were used for bell pepper grown with plasticulture in Georgia. Second crop plastic was used, therefore all fertilization was applied through the drip irrigation system. Application rates ranged from 1.0 pounds nitrogen and potassium per acre per day for the first two weeks up to 2.5 pounds/acre/day at the peak requirement times of fruit set and enlargement. Total nutrients applied included approximately 165 pounds of N and K applied as a 7-0-7 analysis soluble fertilizer. Fungicide and insecticide applications were made according to current University of Georgia recommendations. Irrigation was applied daily. MZ 63 was applied as a foliar spray with a solo backpack sprayer at three pints per acre two weeks after transplanting. BM 86 was applied as a foliar spray with a solo backpack sprayer at three pints per acre at first bloom and again in the same manner and at the same rate14 days later. Treated plots were compared to an untreated check. Pepper were harvested three times at maturity from November 7 through November 27, 2001. Data were collected on marketable yield, percent marketability, plant height, plant width, and pod characteristics including width, length, wall thickness, size, number of locules and smoothness. Results are summarized in Tables 1 and 2. Results Jumbo and U.S. No. 1 grade pepper yield was greater in the treated plots, although not significantly. Total marketable yield was almost identical in both treatments. Jumbo size peppers were only slightly larger in individual fruit weight in the untreated plots, while the reverse was true for U.S. No. 1 grade individual fruit weight. Percent marketability was not different. Fruit length and width also tended to be greater in untreated pepper, but not significantly so. Other parameters were virtually equal, although plant height and width varied some between treatments. BM 86 and MZ 63 did not show any significant improvement in yield and fruit quality, however, additional testing on this product is needed. -13- Table 1. Treatment Untreated BM86/MZ Mean of L.S.D. C.V. (%) Yield by grade, total marketable yield, average fruit size and percent marketability of bell peppers treated with BM 86 and MZ 63 and untreated peppers at Tifton, Georgia in 2001. Yield (28# cartons)/Acre Average Fruit Size (g) Percent Marketable Total (%) Jumbo U.S. No. 1 Jumbo U.S. No. 1 Marketabl Grade e1 163 141 152 66 36.96 81 106 93 48 45.53 281 284 282 106 31.80 196 190 193 24 10.65 133 140 136 17 10.37 98.0 96.0 97.0 4.1 3.61 1 Total of Jumbo, U.S. No. 1 and U.S. No. 2 peppers. ZMZ-63 applied at 3 pints/A at 4-leaf stage; BM-86 applied at 3 pints/A at first flower and 7-14 days later. Table 2. Fruit and plant characteristics of bell peppers treated with BM 86 and MZ 63 and untreated peppers at Tifton, Georgia in 2001. Fruit Characteristics Plant Characteristics Treatment Fruit Length (cm) Untreated BM86/MZ Mean of L.S.D. C.V. (%) 8.8 8.1 8.4 1.6 16.51 Fruit Width (cm) 8.2 8.0 8.1 2.0 21.20 Fruit Smoothn ess2 Fruit Wall Thicknes s 2.2 2.1 2.1 0.3 13.83 1.1 0.8 0.9 0.9 86.28 2 No. of Locules 3.2 3.1 3.1 0.5 14.76 Plant Height (cm) 13.8 13.1 13.5 2.8 17.37 Plant Width (cm) 14.4 15.1 14.8 3.3 18.74 Based on a scale of 1=smooth, 2=moderately smooth, 3=rough. ZMZ-63 applied at 3 pints/A at 4-leaf stage; BM-86 applied at 3 pints/A at first flower and 7-14 days later. -14- Effect of K-tionic on Fall Bell Pepper Production William Terry Kelley Extension Horticulturist, Rural Development Center P.O. Box 1209, Tifton, Georgia 31793 wtkelley@uga.edu Introduction K-tionic is a nutrient uptake promoter manufactured by Grupo Bioquimico Mexicano (GBM) that consists of 25% fulvic organic complex. It is recommended on all crops for use in conjunction with a balanced fertilization program to promote and optimize nutrient uptake. It is supposed to increase soil cation exchange capacity and buffering capability to enhance nutrient availability. Since it is marketed to Georgia growers, K-tionic was tested on bell pepper during fall production to determine its effects on plant growth, pod characteristics and marketable yield. Methods “Camelot” variety (Seminis Seed Co.) bell pepper transplants were commercially produced in 200-cell polystyrene trays at a local greenhouse. Peppers were planted into the field at the Coastal Plain Experiment Station (elev. 382 feet) in Tifton, Georgia on August 27, 2001 into a Tifton sandy loam (fine-loamy siliceous thermic Plinthic Kandiudults) soil. Plots consisted of two side-by-side rows on a plastic mulch-covered bed with eight plants in each row and 12 inches between plants. Beds were spaced six feet apart from center to center. Plots were eight feet in length with three feet between plots. The planting was arranged in a Randomized Complete Block Design with eight replications. Normal cultural practices were used for bell pepper grown with plasticulture in Georgia. Second crop plastic was used, therefore all fertilization was applied through the drip irrigation system. Application rates ranged from 1.0 pounds nitrogen and potassium per acre per day for the first two weeks up to 2.5 pounds/acre/day at the peak requirement times of fruit set and enlargement. Total nutrients applied included approximately 165 pounds of N and K applied as a 7-0-7 analysis soluble fertilizer. Fungicide and insecticide applications were made according to current University of Georgia recommendations. Irrigation was applied daily. K-tionic was applied through the drip irrigation system at a rate of two quarts/acre at planting with an additional two quarts/acre applied at the beginning of fruiting. Treated plots were compared to an untreated check. Pepper were harvested three times at maturity from November 7 through November 27, 2001. Data were collected on marketable yield, percent marketability, plant height, plant width, and pod characteristics including width, length, wall thickness, size, number of locules and smoothness. Results are summarized in Tables 1 and 2. Results Total marketable yield and jumbo yield was greater in the pepper treated with K-tionic, although not significantly. Yield of U.S. No. 1 grade was almost identical in both treatments. Jumbo size peppers were only slightly larger in individual fruit weight in the treated plots. Percent marketability was not different. Fruit length and width also tended to be greater in treated pepper, but not significantly so. Other parameters were virtually equal. K-tionic showed a tendency to improve fruit size and yield, however, since the data were not significant, there should be additional testing of this product. -15- Table 1. Treatment Yield by grade, total marketable yield, average fruit size and percent marketability of bell peppers treated with K-tionic and untreated peppers at Tifton, Georgia in 2001. Yield (28# cartons)/Acre Average Fruit Size (g) Percent Marketable Total (%) Jumbo U.S. No. 1 Marketable1 Jumbo Grade U.S. No. 1 Untreated 163 87 281 196 133 98.0 K-tionicZ 223 81 351 206 146 95.8 Mean of Test 193 84 316 201 140 96.9 L.S.D. (0.1) 71 50 103 22 21 2.6 62.79 34.47 11.50 18.87 2.85 C.V. (%) 38.83 1 Z Total of Jumbo, U.S. No. 1 and U.S. No. 2 peppers. K-tionic applied at two quarts/A at planting and two quarts/A at fruiting. Table 2. Fruit and plant characteristics of bell peppers untreated and treated with K-tionic at Tifton, Georgia in 2001. Fruit Characteristics Plant Characteristics Treatment Untreated Fruit Width (cm) Fruit Smoothness2 Fruit Wall Thickness No. of Locules Plant Height (cm) Plant Width (cm) 8.1 8.2 2.2 1.0 3.2 13.9 14.4 10.1 8.8 1.9 0.9 3.9 14.0 14.2 Mean of Test 9.1 8.5 2.0 1.0 3.5 13.9 14.3 L.S.D. (0.1) 1.7 1.3 0.3 0.8 0.7 1.2 1.6 C.V. (%) 19.75 15.78 13.51 83.63 22.26 9.07 11.91 K-tionic 2 Fruit Length (cm) Z Z Based on a scale of 1=smooth, 2=moderately smooth, 3=rough. K-tionic applied at two quarts/A at planting and two quarts/A at fruiting. -16- Variety Evaluation -17- CANTALOUPE VARIETY TRIAL, 2001 -18- *George E. Boyhan, Darbie M. Granberry, W. Terry, Kelley, C. Randell Hill Extension Horticulturist, Horticulture - CES, P.O. Box 8112 Statesboro, GA 30460 A cantaloupe variety trial was held at the Vidalia Onion and Vegetable Research Center in Lyons, Georgia with nine varieties. Seed were stared in the greenhouse at the Bamboo Farm and Coastal Garden in Savannah, Georgia in March and April. The transplants were set on April 25, 2001. The experiment was arranged in a randomized complete block design with four replications. Each experimental unit consisted of ten hills with an in-row spacing of three feet and a between-row spacing of six feet. The fertility program consisted of 750 pounds per acre of 10-10-10 applied preplant and incorporated. This was followed by 750 pounds per acre of 15-0-14 applied on May 11, 2001. This level of fertility is considered too high for cantaloupe in Georgia and was the result of a miscommunication with the farm superintendent. Weed control consisted of two quarts per acre of Cucurbit herbicide applied on April 27, 2001 over the top of the transplants. In addition, Poast Plus was applied at one quart per acre on May 7, 2001 for control of post emergent grasses. Finally, Permit herbicide was spot sprayed on May 7-8, 2001 to control nutsedge. Hand weeding was also used as needed. Neither the use of Curbit over the top of transplants or the use of Permit on cucurbits is labeled. We did not apply any fungicides or insecticides to see how the varieties perform under natural disease and insect pressure. Cantaloupe harvest began on June 29 and continued on July 5, 6, and 9, 2001. The Bonferroni adjusted LSD divides the probability (0.05) by 5 before calculating to control the experiment wise error rate. This allows up to five mean comparisons between varieties. There were nine cantaloupe varieties in the trial with ‘Odyssey’ from Sunseeds having the highest yields (Table 1). Both Eastern and Western types were in the trial with the Eastern types yielding higher overall as well as having larger average fruit size. The Georgia market continues to be dominated by Athena type cantaloupes. These are referred to as Eastern shipping types. They are larger than their Western cousins with less netting overall. The can be harvested at full-slip (full maturity) and still be shipped compared to Western types which are harvested at half-slip and mature after harvest. Table 2. Cantaloupe Variety Trial, Vidalia Onion and Vegetable Research Center, Lyons, GA, 2001 -19- Variety Source Yield (lbs/acr e) Fruit Weight (lbs) Length (in.) Width Flesh (in.) Thickness (in.) Soluble Solids (%) Flesh Color Melon Type Comments Odyssey (7119) Sunseeds 16,970 5.6 7.5 6.8 1.9 7.8 Orange Eastern Athena Syngenta 13,891 4.0 6.9 6.2 1.8 9.8 Orange Eastern Vienna Asgrow 10,557 5.5 6.8 6.8 2.0 8.1 Orange Eastern Eclipse Petoseed 10,037 4.9 6.6 6.6 2.0 9.0 Orange Eastern EX 04204099 Asgrow 9,378 4.1 6.8 6.3 2.0 8.2 Orange Eastern AC-75-1A Auburn Univ 7,575 2.2 5.0 5.1 1.5 7.1 Orange Western Some Eastern type Super 45 Willhite 6,044 2.9 6.2 5.2 1.5 7.5 Orange Western Some Eastern type AC-89-55MI Auburn Univ 6,032 2.6 5.2 5.0 1.4 7.2 Orange Western Some Eastern type AC-82-37RNL Auburn Univ 2,311 2.4 5.8 4.9 1.6 4.9 Orange Western R2 0,574 CV 60% Adjusted LSD (p<0.05) 7,015 0.597 21% WATERMELON VARIETY TRIAL, 2001 -20- George E. Boyhan, Darbie M. Granberry, W. Terry Kelley, C. Randell Hill Extension Horticulturist, Horticulture - CES, P.O. Box 8112 Statesboro, GA 30460 A watermelon variety trial was held at the Vidalia Onion and Vegetable Research Center in Lyons, Georgia consisting of 29 varieties. Seed were stared in the greenhouse at the Bamboo Farm and Coastal Garden in Savannah, Georgia in March and April. The transplants were set on April 19, 2001. The experiment was arranged in a randomized complete block design with four replications. Each experimental unit consisted of ten hills with an in-row spacing of five feet and a between-row spacing of 12 feet. Although there was a 12 foot between-row spacing the yield per acre was calculated based on a six-foot between-row spacing. The fertility program consisted of 750 pounds per acre of 10-10-10 applied preplant and incorporated. This was followed by 750 pounds per acre of 15-0-14 applied on May 11, 2001. This level of fertility is considered a little too high for watermelon in Georgia and was the result of a miscommunication with the farm superintendent. Weed control consisted of two quarts per acre of Cucurbit herbicide applied on April 27, 2001 over the top of the transplants. In addition, Poast Plus was applied at one quart per acre on May 7, 2001 for control of post emergent grasses. Finally, Permit herbicide was spot sprayed on May 7-8, 2001 to control nutsedge. Hand weeding was also used as needed. Neither the use of Curbit over the top of transplants or the use of Permit on cucurbits is labeled. We did not apply any fungicides or insecticides to see how the varieties perform under natural disease and insect pressure. The harvest began on July 2, 2001 and continued on July 5, 6 and 9, 2001. The Bonferroni adjusted LSD divides the probability (0.05) by 5 before calculating to control the experiment wise error rate. This allows up to five mean comparisons between varieties. ‘Royal Star’ watermelon from Petoseed was the highest yielding variety followed by ‘Big Stripe’, “WX8', and ‘WX22' from Willhite (Table 1). ‘Montreal’ from Sunseeds rounds out the top five performers. Surprisingly ‘Moon & Stars’ also had good yields. This is an old heirloom variety with an unusual rind pattern (dark green with yellow spots of varying sizes from pencil point to 2-3 inches across). The quality of this old variety was very poor, however, with white streaking in the flesh. Yields overall were poor in this trial due to the fact the vines were turned into the plots after the fruit had set and was about 1-2 lbs in size. This resulted in damage to the vines were turned into the plots after the fruit had set and was about 1-2 lbs in size. This resulted in damage to the vines particularly where thy attach to the fruit. Seedless watermelons continue to be tested and grow in popularity. Nine of the varieties or almost a third were triploid varieties. We are beginning to see triploid varieties outside the Crimson Sweet type. This year ‘WX55' from Willhite and ‘Revolution’ from Sunseeds were Allsweet type and ‘Freedom’ was a Jubiliee type from Sunseeds. Table 1. Watermelon Variety Trial, Vidalia Onion and Vegetable Research Center, Lyons, GA, 2001 -21- Variety Source Yield (lbs/acre) Fruit Weight (lbs) Length (in.) Width Flesh (in.) Thickness (in.) Soluble Solids (%) Flesh Color Melon Type Comments Royal Star Petoseed 27,240 16.0 12.5 8.9 0.75 10.2 Red Crimson Sweet Big Stripe Willhite 25,406 14.9 12.7 8.6 0.83 10.4 Red Jubilee Blocky WX8 (large seed) Willhite 23,766 12.6 13.6 8.1 0.78 10.8 Red Allsweet WX22 (small seed) Willhite 21,312 14.0 12.4 8.3 0.66 10.2 Red Jubilee Montreal (5023) Sunseeds 21,225 13.3 12.7 8.2 0.53 10.0 Red Allsweet Moon & Stars G.Hunter 20,045 16.7 11.6 9.6 0.78 8.6 Red Moon & Stars WX55 Triploid Willhite 19,889 12.7 13.7 7.3 0.70 9.0 Red Allsweet Seedless Festival (large seed) Willhite 19,548 12.0 14.4 7.3 0.63 9.5 Pink/ Red Allsweet Revolution (4034) Triploid Sunseeds 19,471 10.9 12.8 7.9 0.81 11.7 Red Allsweet Seedless Variable some jubilee fruit Pinata (large seed) Willhite 19,185 13.9 12.4 8.4 0.83 9.4 Red Allsweet Some jubilee & blocky types XP 4525247 Asgrow 18,999 13.1 13.4 8.0 0.58 9.2 Red Allsweet Tribute (PX59696) Triploid Petoseed 18,999 11.9 10.7 8.7 0.70 11.0 Red Crimson Sweet Seedless Stars n Stripes Asgrow 18,891 12.7 13.8 7.5 0.72 10.2 Red Jubilee Falcon (PS 56395) Petoseed 17,874 14.5 14.7 8.0 0.69 11.1 Red Allsweet Sweet Eat’n Triploid D.Palmer 17,598 10.3 9.6 7.7 0.67 11.4 Red Crimson Sweet Seedless -22- Some blocky & jubilee fruit Old variety, white streaked flesh Variable Fruit, Some icebox size Variety Source Yield (lbs/acre) Fruit Weight (lbs) Length (in.) Width Flesh (in.) Thickness (in.) Soluble Solids (%) Flesh Color Melon Type Sentinel (PS 36694) Petoseed 17,544 11.8 12.0 7.9 0.72 11.3 Red Allsweet Sweetheart (large seed) Willhite 17,105 12.7 11.6 8.8 0.95 10.3 Red Jubilee Legacy (OP) Willhite 16,212 10.6 12.3 7.6 0.72 8.3 Red Allsweet Blocky Vista Fl Hollar Seed 15,043 14.3 12.0 8.4 0.69 11.6 Red Jubilee AU Golden Producer Hollar Seed 14,810 13.2 9.9 8.8 0.67 10.0 Yellow Crimson Sweet Freedom (3022) Triploid Sunseeds 13,957 12.0 12.3 8.2 0.72 11.8 Red Jubilee Seedless AU Producer ZYMV Auburn U. 13,605 13.4 10.1 9.1 0.72 9.6 Red Crimson Sweet Afternoon Delight Triploid D.Palmer 13,511 8.9 9.2 8.4 0.77 11.5 Red Crimson Sweet Seedless Stargazer Asgrow 12,977 11.2 12.7 7.4 0.64 8.5 Red Allsweet WX24 (large seed) Willhite 12,814 13.1 13.5 7.4 0.81 9.3 Red Blocky Crimson Sweet Cooperstown Asgrow 11,576 10.6 9.6 8.2 0.58 10.9 Red Crimson Sweet Seedless Triton Petoseed 11,558 10.0 8.9 8.4 0.75 11.3 Yellow Crimson Sweet Seedless AU Allsweet Auburn U. 11,489 13.8 12.0 7.4 0.61 9.1 Red Allsweet Sapphire Fl Hollar Seed 2,222 10.2 8.8 7.5 0.66 10.9 Red Crimson Sweet Seedless 0.353 R2 CV 50% Adjusted LSD (p<0.05) 14,199 0.563 14% 1.8 -23- Comments Some variability with Crimson Sweet Type Some jubilee type Georgia Commercial Pumpkin Variety Trials-2001 William Terry Kelley Extension Horticulturist, Rural Development Center P.O. Box 1209, Tifton, Georgia 31793 wtkelley@uga.edu Introduction Many new pumpkin varieties have been introduced to the commercial market in recent years. Multi-year data has been collected from annual trials for the last several years. Many nearby states use this information. This project is an annual evaluation to keep growers updated with local information on new pumpkin variety releases. Methods Twenty-seven commercially-available pumpkin varieties and three experimental lines were compared at the Georgia Mountain Branch Experiment Station (elev. 1900 feet) in Blairsville, Georgia. Pumpkins were field-seeded on June 19-20, 2001 into a Transylvania clay loam soil. Plots consisted of single rows which contained an appropriate number of hills for each variety’s plant habit. Vining types were planted with four hills per plot, semi-bush (or semi-vining) types with six hills and bush types with eight hills. Plots were 16 feet in length with eight feet between rows. The planting was arranged in a Randomized Complete Block Design with three replications. Normal cultural practices were used for bare ground pumpkin culture in Georgia. Base fertilizer consisted of 300 pounds/A of 10-10-10 incorporated prior to planting followed by two sidedress applications of 10-10-10 (300 pounds/A each). Ethafluralin (0.75 lb a.i./A) was applied pre-emergence for weed control. Fungicide and insecticide applications were made according to current recommendations. Irrigation was applied as needed. Pumpkins were harvested at maturity on October 2, 2001. Data were collected on yield, fruit number and weight, rind color, rind texture and fruit shape. Results are summarized in Table 1. Results Overall yields and individual pumpkin weights were generally slightly lower than those expected according to commercial variety descriptions. “Prizewinner” produced the greatest yield and largest fruit size among all varieties; it was the only “giant” size variety in the test. Among miniature varieties, “Jack Be Little” produced the most fruit. “Little October” and “Lil’ Ironsides” produced similar fruit numbers with weights in the 1½ to half-pound range. “Wee B Little” only yielded a fraction of the fruit of these varieties. Most large and medium varieties produced yields and fruit numbers within the range for -24- acceptability in north Georgia. Less than average performers included “Gold Strike”, “Harvest Jack”, “HMX 0681", “HMX 6689" and “SVR 9022”. These did not produce yields and fruit number per acre that were competitive with other similarly-sized pumpkins. “Appalachian”, “Early Autumn”, “Gold Bouillon”, “Gold Gem”, “Gold Standard", “Howdy Doody” and “Mother Lode” were all superior performers among the 10-20-pound pumpkins. Among pumpkins in the five to 10-pound range, “Autumn Gold” and “Big Autumn” were the best performers. In the two to five-pound size class, “Peek a Boo” and “Pick a Pie” produced the most fruit and greatest yield. Marketability was acceptable for most varieties, although “Gold Bouillon", “Gold Strike” and “Jumpin’ Jack” were below 80% marketability. There was some variance among varieties for rind color and rind texture. Rind color ranged from deep orange to light orange. “Lumina” was the only pumpkins in the trial with a white rind. Fruit shape was generally in accordance with the type of pumpkin, with smaller pumpkins having a flatter shape. Table 1. Yield, number, marketability and horticultural characteristics of 30 varieties of pumpkins grown at Blairsville, GA in 2001. -25- No. Fruit/A Yield2 (lb/Acre) Fruit Wt (lbs.) Percent Marketable Wt Large Wt Small Rind3 Color Fruit4 Shape Rind5 Texture Seigers 4311 65729 15.3 83.5 21.0 8.9 1.0 3.7 2.0 Autumn Gold Seeds by Design 7487 47347 5.9 80.1 11.6 3.9 2.0 2.8 2.0 Big Autumn Seeds by Design 4311 44613 9.4 81.5 18.9 5.8 2.7 2.7 2.0 Early Autumn Seeds by Design 4084 47512 12.0 83.4 19.3 4.9 2.0 2.7 2.0 Frosty Twilley 3630 38806 10.6 91.1 17.6 6.4 2.3 3.2 2.3 Ghost Rider Rupp 2722 36004 15.0 81.0 19.9 6.6 2.3 3.0 2.0 Gold Bouillon Rupp 2496 44574 17.1 71.1 23.0 7.7 2.0 3.3 2.0 Gold Gem Rupp 3403 51640 14.8 87.0 25.1 7.9 1.3 3.0 1.7 Gold Standard Rupp 4424 54034 12.3 93.9 16.4 5.6 1.7 2.7 1.7 Gold Strike Rupp 1475 24053 14.7 69.5 22.1 8.9 1.7 4.0 2.0 Harvest Jack Seeds by Design 1475 26748 17.5 100.0 25.2 14.3 1.3 3.5 2.0 HMX 0681 Harris Moran 2269 26419 11.8 92.7 16.0 8.3 2.3 3.7 2.3 HMX 6689 Harris Moran 1248 24734 19.8 88.1 27.3 14.6 1.3 3.0 2.0 Howdy Doody Seeds by Design 6466 73726 12.5 100.0 22.7 7.1 2.0 3.0 2.3 Jack Be Little Rupp 18149 8502 0.5 98.3 0.7 0.3 1.7 1.0 1.3 Jack Pot Harris Moran 2042 38046 18.6 82.5 27.0 11.2 1.7 3.7 2.0 Jumpin Jack Seeds by Design 567 6642 11.5 51.0 16.2 6.4 1.3 3.7 2.0 Lil Ironsides Harris Moran 10776 20089 1.9 97.6 2.5 1.1 1.7 2.3 2.7 Little October Willhite 11003 8366 0.8 95.9 1.1 0.5 1.7 2.2 2.7 Lumina Stokes 1134 3834 5.0 85.7 6.1 1.0 4.7 2.0 3.0 Variety Sponsor Appalachain -26- No. Fruit/A Yield2 (lb/Acre) Fruit Wt (lbs.) Percent Marketable Wt Large Wt Small Rind3 Color Harris Moran 2722 38386 14.3 88.8 21.0 6.9 1.3 2.7 1.7 Mother Lode Rupp 2495 40144 15.5 88.4 24.5 10.6 2.0 3.8 2.3 Mystic Plus Harris Moran 1475 6006 4.1 84.7 6.7 2.5 1.0 2.0 2.0 Peek a Boo Seeds by Design 6693 23702 3.5 97.5 5.8 2.1 1.7 2.0 2.0 Pick a Pie Rupp 5672 26095 4.6 98.1 5.7 2.9 1.7 2.0 2.0 Prize Winner Seigers 2609 233673 89.4 100.0 131.3 54.3 3.0 2.2 2.7 Pro Gold #500 Rupp 1361 27303 23.6 85.3 25.3 13.3 1.7 3.0 2.0 SVR 9022 Seminis 1135 12676 10.0 81.5 18.1 4.5 2.5 3.5 2.0 Touch of Autumn Rupp 9529 24501 2.5 95.2 3.2 1.2 1.7 2.0 2.3 Wee B Little Seeds by Design 2723 1463 0.5 88.0 0.8 0.4 2.0 2.0 2.3 13.2 87.4 19.4 7.7 1.9 2.8 2.1 Variety Sponsor Magic Lantern 37512 Fruit4 Shape Rind5 Texture Mean of Test 4329 L.S.D. (0.05) 2933 27039 5.8 25.0 8.5 4.0 1.0 0.7 0.6 C.V. (%) 41.45 44.10 27.0 17.5 26.7 32.3 33.3 15.7 17.3 -27- Commercial Snap Bean Fall Cultivar Evaluation-2001 William Terry Kelley Extension Horticulturist, Rural Development Center P.O. Box 1209, Tifton, Georgia 31793 wtkelley@uga.edu Introduction Although Bronco continues to be the most widely planted snap bean variety in Georgia, several new varieties with similar characteristics have been introduced in the last several years. Darker beans are the choice of the market in most cases. This project was done as an evaluation of how some standard varieties and some newer cultivars perform during the fall growing season. Methods Nine commercially-available and three experimental snap bean varieties were compared at the Coastal Plain Experiment Station (elev. 382 feet) in Tifton, Georgia. Snap beans were directseeded on August 27, 2001 into a Tifton sandy loam (fine-loamy siliceous thermic Plinthic Kandiudults) soil. Plots consisted of two side-by-side rows with three seed planted per foot of row. Plots were eight feet in length with three feet between rows. The planting was arranged in a Randomized Complete Block Design with four replications. Normal cultural practices were used for bare ground snap bean culture in Georgia. Base fertilizer consisted of 400 pounds/A of 10-10-10 incorporated prior to planting followed by two sidedress applications of 15-0-14 (150 pounds/A each). Weed control was accomplished with a pre-emergence application of s-metolachlor (0.94 lb a.i./A). Fungicide and insecticide applications were made according to current University of Georgia recommendations. Irrigation was applied as needed. Snap beans were harvested at maturity on October 15-23, 2001. Data were collected on marketable yield, percent marketability, plant height, canopy width, and pod characteristics including color, width, length, shape, straightness and smoothness. Results are summarized in Tables 2 and 3. Results Marketable yield varied greatly. “Festina” yielded the greatest marketable weight followed by “Platina”, “EX 8184379" and “HMX 0104". The standard, “Bronco”, actually was one of the lowest yielding varieties. “Tasman”, “Casino” and “Bronco” were the poorest yielding beans. All varieties except “Bronco” were greater than 94% marketable. Plant height for all varieties was between 10.7 (“Casino”) to 14.6 (“Festina”) inches. Canopy -28- width ranged from 17.6 (“Casino”) to 24.3 inches (“Festina”). Obviously, “Casino” was the smallest framed plant and “Festina” the largest. Pod characteristics were within normal ranges for most varieties. The darkest pods were produced on “Casino”, “Festina” and “Nelson”. Lightest color was produced by “Grenoble”, “Hialeah” and “WBL 420". Pod width was always in the range of 0.9 to 1.8 cm and length varied from 9.7 to 16.2 cm. “EX 8184379" produced the shortest pods and “Nelson” the longest. Pod shape was consistent in all varieties with the exception of “Nassau” and “Platina” which produced strictly straight pods. All of the other varieties were slightly curved. There was little variation in pod straightness and smoothness. Since there were no great differences in pod characteristics, primary selection of variety should be based on yield and market acceptability. Pod color would have the greatest influence on acceptability. “Festina”, “HMX 0104" and “Nassau” appeared to have the best combinations of yield and a dark pod color. All of these had other pod characteristics that were acceptable. Table 1. Varieties and sponsoring companies of snap beans in a variety trial at the Coastal Plain Experment Station in Tifton, in Fall 2001. Variety Sponsor Bronco Seminis Casino Holland Select EX 8184379 Seminis Festina Seminis Grenoble Seminis Hialeah Harris Moran HMX 0104 Harris Moran Nassau Holland Select Nelson Holland Select Platina Holland Select Tasman Holland Select WBL 420 Seminis Table 2. Marketable yield, percent marketability, plant height, plant width and pod color of -29- snap beans at the Coastal Plain Experment Station in Tifton, Georgia in 2001. Variety Marketable Yield (lbs/A) Bronco 5863 88.0 12.8 19.3 2.8 Casino 5173 95.0 10.7 17.6 1.3 EX8184379 10326 97.0 12.5 23.5 2.8 Festina 12233 97.0 14.6 24.3 1.3 Grenoble 9531 94.0 11.5 19.1 3.0 Hialeah 8902 97.0 12.2 18.3 3.0 10028 97.0 12.2 22.3 2.1 Nassau 9611 96.0 11.5 19.3 2.2 Nelson 7267 94.0 13.7 24.2 1.7 Platina 11417 95.0 13.7 21.3 2.9 Tasman 4794 94.0 12.2 19.3 2.5 WBL 420 8744 95.0 11.3 19.0 3.1 HMX 0104 Percent Marketability (%) Plant Height (inches) Canopy Width (inches) Pod Color1 Mean 8741.0 1.0 12.0 21.0 2.0 CV (%) 35.72 3.12 13.59 15.34 16.78 L.S.D. (0.05) 4491.7 0.04 2.40 4.60 Based on a scale of 1=dark green; 2=medium green; 3=light green; 4=yellow. 0.60 1 Table 3. Pod width, pod length, pod shape, pod straightness and pod smoothness of snap -30- beans at the Coastal Plain Experiment Station in Tifton, Georgia in 2001. Variety Pod Width (cm) Pod Length (cm) Pod Shape1 Pod Straightness2 Pod Smoothness3 Bronco 0.9 11.7 2.5 1.7 1.7 Casino 0.8 11.6 2.6 1.9 1.0 EX8184379 0.9 9.7 2.7 1.9 1.7 Festina 0.9 14.3 2.7 2.0 1.7 Grenoble 0.8 13.1 2.7 1.9 1.4 Hialeah 0.9 15.9 2.5 1.6 1.8 HMX 0104 0.9 14.1 2.6 1.4 2.1 Nassau 1.5 15.9 1.0 2.0 2.8 Nelson 0.9 16.2 2.5 2.1 1.6 Platina 1.8 15.1 1.0 1.3 2.3 Tasman 1.1 12.6 2.6 1.9 2.4 WBL 420 0.9 14.1 2.3 1.9 1.4 Mean 1.0 14.0 2.0 2.0 2.0 CV (%) 9.25 24.85 16.56 22.87 30.57 L.S.D. (0.05) 0.14 4.90 0.55 0.59 0.80 2 Based on a scale of 1=flat; 2=oval; 3=round. Based on a scale of 1=smooth; 2=somewhat smooth; 3=rough; 3Based on a scale of 1=straight; 2=slightly curved; 3=curved; 1 -31- Summer Squash Variety Trial - 2001 William Terry Kelley1, David Curry2 and Gregory Hardison3 1 Extension Horticulturist, Rural Development Center P.O. Box 1209, Tifton, Georgia 31793 wtkelley@uga.edu 2 Toombs County Extension Director Courthouse Square Lyons, Georgia 30436 3 Montgomery County Extension Director P.O. Box 276 Mount Vernon, Georgia 30445 Introduction Summer squash production continues to be an important part of the Georgia vegetable industry. Production occurs throughout the state, but mostly in the southern coastal plain. Both yellow (crookneck and straightneck) and zucchini squash are produced. Introduction of virusresistant squash hybrids in the last few years has drastically changed variety selection in squash. Some of the varieties currently produced have resistance to at least two and in some cases three of the four major squash viruses. The first variety resistant to all four major squash viruses was released this year. Some of the virus resistance has been obtained through genetic modification while other varieties have been developed through traditional means. Varieties with the precocious yellow gene are also used by Georgia growers. Continual evaluation of squash varieties is necessary to determine which of these new varieties perform to an acceptable level of production and quality under Georgia conditions. Methods Nine zucchini, five yellow crookneck and seven yellow straightneck squash varieties were compared at the Vidalia Onion and Vegetable Research Center (elev. 250 feet) near Lyons, Georgia. All varieties were commercially available except for three zucchini lines and three yellow straightneck lines that were still in the experimental stage of development. Squash varieties were field-seeded on 24 April, 2001 into an Irvington loamy sand soil (Fine-loamy, siliceous, thermic Plinthic Fragiudults). Plots consisted of two side-by-side rows with zucchini spaced 24 inches between plants and yellow spaced 18 inches between plants. Plots were 12 feet in length. The planting was arranged in a Randomized Complete Block Design with four replications. Normal cultural practices were used for bare ground squash production in Georgia. Base fertilizer consisted of 400 pounds/A of 10-10-10 incorporated prior to planting. Additional fertilizer was through two side dressed applications of 300 pounds/A of 10-10-10 each. Ethafluralin (0.75 lb a.i./A) was applied pre-emergence for weed control. Fungicide and insecticide applications were made according to current University of Georgia recommendations. Irrigation was applied through an overhead system as needed. -32- Squash were harvested on June 6, 11, 13, 16, 18 and 20, 2001. Data were collected on yield by grade, early yield by grade, percent marketability, average fruit weight and fruit number. Results are summarized in Tables 1-12. Results A higher percentage of squash fell into the medium grade than would be expected under grower conditions since harvests were only conducted three times per week. Among zucchinis, Cashflow, HMX 8714, Bobcat, Spineless Beauty and Tigress produced the highest yields of fancy and medium squash (Table 1). Tigress, Spineless Beauty and Dividend produced the greatest early yield. Tigress, Dividend and Bobcat had the highest percentage of marketability while HMX 9728 had the lowest (Table 3). HMX 8714, Tigress, Dividend and Spineless Beauty produced the greatest number of fancy fruit (Table 4). Among yellow crookneck, Supersett and Dixie produced the highest yields of fancy and medium fruit (Table 5). The same varieties produced the highest early yields (Table 6). All varieties produced similar percentages of marketability. Supersett, Dixie and Sunglo produced the greatest numbers of fancy fruit (Table 8). Among yellow straightneck, Cougar, Enterprise, XPT 46020767 and XPT 1832 produced the highest yield of fancy and medium squash (Table 9) with similar results for early yield (Table 10). All varieties produced marketability levels higher than 95%. XPT 1832, Cougar and Multipik produced the greatest numbers of fancy fruit. Table 1. Season yield by grade and combined fancy and medium yield of zucchini squash -33- at the Vidalia Onion and Vegetable Research Farm in Lyons, Georgia in 2001. Season Yield (21-lb boxes/A) Variety Fancy Bobcat 530 295 25 826 Cashflow 640 318 38 958 Dividend 495 218 0 713 HMX 8714 586 393 58 979 HMX 8715 359 346 66 705 HMX 9728 450 200 149 649 Independence II 232 138 61 370 Spineless Beauty 496 371 102 867 Tigress 577 358 0 935 Mean 485 293 55 778 34.64 46.20 143.40 30.88 245 197 116 351 CV (%) L.S.D. (0.05) Medium Jumbo Total Fan/Med Harvests occurred on June 6, 11, 13, 16, 18 and 20, 2001. Planting date: April 24, 2001. Plot size: 12 plants per plot (two rows of six) on 36" X 24" spacing. -34- Table 2. Early* yield by grade and combined fancy and medium yield of zucchini squash at the Vidalia Onion and Vegetable Research Farm in Lyons, Georgia in 2001. Early Yield (21-lb boxes/A) Variety Fancy Medium Jumbo Total Fan/Med Bobcat 212 283 25 495 Cashflow 245 276 0 521 Dividend 330 207 0 537 HMX 8714 321 279 58 600 HMX 8715 206 303 66 509 HMX 9728 273 169 149 442 Independence II 149 138 61 287 Spineless Beauty 279 257 39 537 Tigress 365 334 0 698 Mean 264 249 44 514 40.57 51.72 152.45 31.46 157 188 98 236 CV (%) L.S.D. (0.05) Early* harvests occured on June 6, 11 and 13, 2001 . Planting date: April 24, 2001. Plot size: 12 plants per plot (two rows of six) on 36" X 24" spacing. -35- Table 3. Percent marketability by weight of early and season harvests and average weight of fancy and medium grade zucchini squash at the Vidalia Onion and Vegetable Research Farm in Lyons, Georgia in 2001. Variety Percent Marketable Season (%) Percent Marketable Early (%) Bobcat 97.0 96.0 169 744 Cashflow 95.8 100.0 175 605 Dividend 99.8 99.5 199 593 HMX 8714 95.5 94.5 177 701 HMX 8715 89.5 87.8 205 679 HMX 9728 81.3 71.0 214 725 Independence II 89.8 89.5 220 745 Spineless Beauty 90.5 94.0 202 709 Tigress 98.5 98.0 236 641 Mean 93.1 92.3 200 682 CV (%) 10.01 11.43 14.45 L.S.D. (0.05) 13.6 15.4 42 -36- Average Fancy Weight (g) Average Medium Weight (g) 17.54 175 Table 4. Early and season fruit number by grade of zucchini squash at the Vidalia Onion and Vegetable Research Farm in Lyons, Georgia in 2001. *Early Harvest Variety Medium Jumbo 9277 3722 202 29572 3923 202 Cashflow 10307 4289 0 34631 4894 259 Dividend 11732 3333 0 23826 3515 0 HMX 8714 14944 3287 303 31863 5203 303 HMX 8715 8635 3933 468 16748 4703 468 HMX 9728 9287 2234 1039 19753 2637 1039 Independence II 5661 1945 605 10458 1945 605 Spineless Beauty 10857 3574 347 23261 5175 693 Tigress 12312 4916 0 23383 5279 0 Bobcat Fancy Season Harvest Fancy Medium Jumbo Mean 10334 3470 329 23655 4141 396 CV (%) 34.62 52.52 169.14 31.41 46.41 156.05 L.S.D. (0.05) 5222 2660 812 10842 2805 903 Harvests occurred on June 6, 11, 13, 16, 18 and 20, 2001. Planting date: April 24, 2001. Plot size: 12 plants per plot (two rows of six) on 36" X 24" spacing. *Early harvest includes the first three harvests. -37- Table 5. Season yield by grade and combined fancy and medium yield of yellow crookneck squash at the Vidalia Onion and Vegetable Research Farm in Lyons, Georgia in 2001. Season Yield (30-lb boxes/A) Variety Fancy Medium Jumbo Total Fan/Med Dixie 512 296 20 808 Gentry 317 241 11 530 Prelude II 281 293 11 574 Sunglo 332 278 25 610 Supersett 496 471 0 967 Mean 388 141 13.0 698 37.97 45.41 214.96 35.43 227 217 44 381 CV (%) L.S.D. (0.05) Harvests occurred on June 6, 11, 13, 16, 18 and 20, 2001. Planting date: April 24, 2001. Plot size: 12 plants per plot (two rows of eight) on 36" X 18" spacing. -38- Table 6. Early* yield by grade and combined fancy and medium yield of yellow crookneck squash at the Vidalia Onion and Vegetable Research Farm in Lyons, Georgia in 2001. Early Yield (30-lb boxes/A) Variety Fancy Medium Jumbo Total Fan/Med Dixie 307 195 20 502 Gentry 167 131 11 298 Prelude II 141 193 11 334 Sunglo 182 159 25 340 Supersett 254 392 0 645 Mean 210 214 13.0 424 57.45 54.14 214.96 44.09 186 178 44 288 CV (%) L.S.D. (0.05) Early* harvests occurred on June 6, 11 and 13, 2001. Planting date: April 24, 2001. Plot size: 12 plants per plot (two rows of eight) on 36" X 18" spacing. -39- Table 7. Percent marketability by weight of early and season harvests and average weight of fancy and medium grade yellow crookneck squash at the Vidalia Onion and Vegetable Research Farm in Lyons, Georgia in 2001. Percent Marketable Season (%) Percent Marketable Early (%) Dixie 98.3 97.0 145 273 Gentry 96.3 93.5 106 271 Prelude II 97.8 96.3 108 299 Sunglo 94.5 90.0 110 288 100.0 100.0 112 289 97.4 95.4 116 284 Variety Supersett Mean Average Fancy Weight (g) Average Medium Weight (g) CV (%) 3.76 6.62 35.6 10.21 L.S.D. (0.05) 5.6 9.7 64 45 Table 8. Early and season fruit number by grade of yellow crookneck squash at the Vidalia Onion and Vegetable Research Farm in Lyons, Georgia in 2001. *Early Harvest Variety Fancy Season Harvest Medium Jumbo Fancy Medium Jumbo Dixie 21131 9795 545 50465 14867 545 Gentry 19899 6386 272 40293 10321 272 Prelude II 16304 8605 227 35514 13194 227 Sunglo 20691 7351 545 40565 13068 545 Supersett 28357 17697 0 60028 21095 0 Mean 21277 9967 318 45373 14509 318 CV (%) 21.71 48.56 222.05 21.92 39.27 222.05 L.S.D. (0.05) 7115 7456 1087 15320 8777 1087 Harvests occurred on June 6, 11, 13, 16, 18 and 20, 2001. Planting date: April 24, 2001. Plot size: 12 plants per plot (two rows of eight) on 36" X 18" spacing. *Early harvest includes the first three harvests. -40- Table 9. Season yield by grade and combined fancy and medium yield of yellow straight neck squash at the Vidalia Onion and Vegetable Research Farm in Lyons, Georgia in 2001. Season Yield (30-lb boxes/A) Variety Fancy Medium Jumbo Total Fan/Med Cougar 346 296 0 642 Enterprise 296 250 8 546 Liberator II 233 101 0 334 Multipik 311 93 25 403 XP 4970297 319 150 11 469 XPT 1832 464 38 0 502 XPT 46020767 311 296 0 513 Mean 326 161 6 487 30.33 89.15 350.83 45.27 147 214 33 327 CV (%) L.S.D. (0.05) Harvests occurred on June 6, 11, 13, 16, 18 and 20, 2001. Planting date: April 24, 2001. Plot size: 12 plants per plot (two rows of eight) on 36" X 18" spacing. -41- Table 10. Early* yield by grade and combined fancy and medium yield of yellow straight neck squash at the Vidalia Onion and Vegetable Research Farm in Lyons, Georgia in 2001. Early Yield (30-lb boxes/A) Variety Fancy Medium Jumbo Total Fan/Med Cougar 224 197 0 421 Enterprise 133 171 8 304 Liberator II 155 70 0 225 Multipik 177 72 25 249 XP 4970297 161 91 11 251 XPT 1832 302 20 0 322 XPT 46020767 170 146 0 316 Mean 189 110 6 298 34.56 97.16 350.83 51.98 97 158 33 230 CV (%) L.S.D. (0.05) Early* harvests occurred on June 6, 11 and 13, 2001. Planting date: April 24, 2001. Plot size: 12 plants per plot (two rows of eight) on 36" X 18" spacing. -42- Table 11. Percent marketability by weight of early and season harvests and average weight of fancy and medium grade yellow straight neck squash at the Vidalia Onion and Vegetable Research Farm in Lyons, Georgia in 2001. Variety Percent Marketable Season (%) Percent Marketable Early (%) Average Fancy Weight (g) Average Medium Weight (g) Cougar 99.5 99.3 112 342 Enterprise 97.8 95.8 122 358 Liberator II 100.0 100.0 127 376 Multipik 95.8 93.8 91 318 XP 4970297 97.8 96.3 125 388 XPT 1832 99.8 99.5 99 385 XPT 46020767 99.8 99.8 136 394 Mean 98.6 97.8 116 366 CV (%) 3.83 6.02 18.74 15.52 L.S.D. (0.05) 5.6 8.7 32 84 -43- Table 12. Early and season fruit number by grade of yellow straight neck squash at the Vidalia Onion and Vegetable Research Farm in Lyons, Georgia in 2001. *Early Harvest Season Harvest Variety Fancy Medium Jumbo Fancy Medium Jumbo Cougar 25297 7563 0 41102 10928 0 Enterprise 11081 6167 195 33367 9078 195 Liberator II 16195 2675 0 24640 3669 0 Multipik 21625 3157 545 45145 3860 545 XP 4970297 14954 2994 227 35694 5066 227 XPT 1832 35285 663 0 63944 1326 0 XPT 46020767 14561 5549 0 31821 6925 0 Mean 19857 4110 138 39388 5836 138 CV (%) 30.53 85.91 344.98 24.63 75.60 344.98 L.S.D. (0.05) 9005 5245 707 14410 6554 707 Harvests occurred on June 6, 11, 13, 16, 18 and 20, 2001. Planting date: April 24, 2001. Plot size: 12 plants per plot (two rows of eight) on 36" X 18" spacing. *Early harvest includes the first three harvests. -44- Sweet Corn Variety Trial - 2001 William Terry Kelley1, David Curry2 and Gregory Hardison3 1 Extension Horticulturist, Rural Development Center P.O. Box 1209, Tifton, Georgia 31793 wtkelley@uga.edu 2 Toombs County Extension Director Courthouse Square Lyons, Georgia 30436 3 Montgomery County Extension Director P.O. Box 276 Mount Vernon, Georgia 30445 Introduction Sweet corn is by acreage one of the top three vegetable commodities in the state of Georgia. In recent years sweet corn production has begun to branch out from the traditional center of production in southwestern Georgia. A considerable amount of sweet corn is now produced in eastern Georgia as well. New sweet corn varieties are numerous each year. Comparing these new varieties to the current standards on the market is essential to growers who want to remain competitive in the sweet corn market. This trial was conducted to compare new and existing varieties of sweet corn in yellow, white and bi-color types. Methods Twelve yellow, five white and ten bi-color sweet corn varieties were compared at the Vidalia Onion and Vegetable Research Center (elev. 250 feet) near Lyons, Georgia. All varieties were commercially available except for two yellow and two bi-color varieties that were still in the experimental stage of development. Corn varieties were field-seeded on 19 April, 2001 into an Irvington loamy sand soil (Fine-loamy, siliceous, thermic Plinthic Fragiudults). Plots consisted of a single row with nine inches between plants and 36 inches between rows. Plots were 20 feet in length. The planting was arranged in a Randomized Complete Block Design with four replications. Normal cultural practices were used for sweet corn production in Georgia. Base fertilizer consisted of 1000 pounds/A of 10-10-10 incorporated prior to planting. Additional fertilizer was through two side dressed applications of 400 pounds/A of 10-10-10 each. Atrazine (2.0 lb a.i./A) was applied pre-emergence for weed control. Insecticide applications were made according to current University of Georgia recommendations. Irrigation was applied through an overhead system as needed. Corn was harvested at maturity from June 22 to July 5, 2001. Data were collected on yield, percent marketability and average ear weight and subjected to analysis of variance and means separated. Results are summarized in Table 1. Results -45- Yields were calculated by two methods. One method was on the basis of a 42-pound crate, which is the standard accepted weight of a crate of corn. The other method was to use 50-ear crates since most corn is packed to a count of 50 ears. Based on the 50-ear method, Brut, CNS 710A, Cronus, GSS 0966, Morningstar, Prime Plus and Variety #6800 all yielded more than 300 boxes per acre among yellow types. Percent marketability varied widely with Cronus having the highest percentage and Brut the lowest. Sunvolt and CNS 710A had the largest ears by weight. Among bi-color types, BSS 0977, HMX 8344, Twin Star and Variety # 8102 all yielded over 300 boxes per acre. HMX 8344 had the greatest marketability and BSS 0977 the lowest, although all varieties were in a fairly narrow range. HMX 8344 had the largest ear based on weight. In the white corn types, Vail was the only variety that produced over 300 boxes per acre. Ice Queen was the only variety above 80% marketable and WSS 1921 had the lowest marketability. Vail also had the greatest ear weight. -46- Table 1. Variety Yield in 50-ear count boxes and 42-pound boxes, percent marketability and average ear weight of sweet corn in 2001 at Lyons, Georgia. Sponsor Type 1 Yield/A Yield/A % Avg Ear Wt. (42-lb boxes) (50-ear box) Marketable (grams) Yellow ACX 844 Abbott & Cobb Ysh2 219 285 75.9 281.9 ACX 945 Abbott & Cobb Ysh2 228 285 77.6 291.2 Brut Seminis Ysh2 270 355 54.8 294.5 CNS 710A Crookham Ysh2 262 306 81.9 329.0 Cronus Syngenta Ysh2 211 317 88.5 252.5 GSS 0966 Syngenta Ysh2 225 322 76.7 268.7 GSS 5771 Syngenta Ysh2 142 226 54.3 230.8 Morningstar Harris Moran Ysh2 222 305 79.3 279.2 Prime Plus Syngenta Ysh2 219 339 73.5 251.7 Sunvolt Harris Moran Ysh2 223 284 80.0 333.5 Variety #6800 Abbott & Cobb Ysh2 240 338 82.2 264.9 Variety #7630 Abbott & Cobb Ysh2 150 211 75.1 269.0 Mean of Test 216.0 294.6 74.3 279.5 L.S.D. (0.05) 133.6 172.4 17.0 79.8 43.1 40.8 15.9 19.9 C.V. (%) Bi-Color ACX 946 BC Abbott & Cobb BCsh2 224 269 80.0 315.0 Bigtime BSS 0977 Syngenta BCsh2 211 267 72.9 297.1 Syngenta BCsh2 287 422 72.6 261.8 BSS 9686 Syngenta BCsh2 257 289 78.1 341.4 HMX 8344 Harris Moran BCsh2 314 325 87.1 374.5 Hollywood Seminis BCsh2 183 243 80.5 283.4 Tango Crookham BCsh2 222 284 81.5 301.4 Tethys Syngenta BCsh2 195 255 84.5 292.9 Twin Star Harris Moran BCsh2 297 366 74.2 319.1 Variety #8102 Abbott & Cobb BCsh2 269 336 86.0 305.0 Mean of Test 245.8 305.5 79.7 309.2 L.S.D. (0.05) 110.8 148.0 14.1 57.2 31.1 33.4 12.2 12.8 C.V. (%) White Ice Queen Harris Moran Wsh2 116 175 80.1 249.1 Vail Syngenta Wsh2 262 332 75.0 304.9 Variety #6801 Abbott & Cobb Wsh2 146 216 74.8 252.6 Variety #8101 Abbott & Cobb Wsh2 222 278 71.2 300.3 WSS 1921 Syngenta Wsh2 200 259 66.6 286.5 Mean of Test 186.4 249.8 75.3 276.7 L.S.D.(0.05) 119.8 134.9 22.6 89.3 C.V. (%) 40.2 33.8 BCsh2 = Bicolor shrunken, Ysh2 = yellow shrunken, Wsh2 = white shrunken. 18.8 20.2 1 -47- -48- Weed Control -49- -50- WEED MANAGEMENT IN WATERMELON AND CANTALOUPE TRANSPLANTED ON POLYETHYLENE COVERED SEEDBEDS W. Carroll Johnson, III and Theodore M. Webster Research Agronomists - Weed Science USDA-ARS Coastal Plain Experiment Station Tifton, GA 31793 Introduction Cucurbit crops are grown on approximately 11,000 A in Georgia, with watermelon and cantaloupe accounting for 62% of the cucurbit acreage. In previous years, much of the watermelon and cantaloupe acreage was direct seeded on freshly prepared seedbeds. Systems using hybrid cultivars seeded in greenhouses and transplanted on polyethylene covered seedbeds have recently become common. Currently, 57 and 35% of the cantaloupe and watermelon acreage, respectively, are being grown as transplants on polyethylene covered seedbeds. Hybrid seed are costly and transplanting reduces the risk of stand loss associated with direct seedings caused by an assortment of early-season production problems. Polyethylene covered seedbeds warm the soil, allowing for earlier planting and harvest during periods of historically premium commodity prices. Polyethylene covered seedbeds are generally fumigated with a broadspectrum fumigant, particularly methyl bromide. Methyl bromide fumigation controls most pests of cantaloupe and watermelon, including annual and perennial weeds, pathogenic fungi, bacteria, plant parasitic nematodes, and soilinhabiting arthropods. Several weeks before seeding or transplanting, methyl bromide is injected approximately 8-in deep and immediately covered with a polyethylene tarp forming a finished seedbed approximately 12 to 60-in wide. Wider seedbeds are used for multiple crops during a growing season with drip irrigation, compared to narrower seedbeds used for one crop during a growing season with overhead irrigation. Approximately 51 and 74% of the transplanted cantaloupe and watermelon acreage, respectively, is on narrow polyethylene covered seedbeds and irrigated with overhead irrigation. Seedlings are transplanted through the polyethylene tarp two to four weeks after fumigation to allow fumigant dissipation. Methyl bromide is thought to contribute to the depletion of stratospheric ozone. In anticipation of these findings, the U. S. Environmental Protection Agency initiated a mandatory phase-out of all methyl bromide containing fumigants by 2005. Acceptable alternatives to methyl bromide have been developed in vegetable crop transplant production and related cropping systems. Metham has been shown to be effective as methyl bromide in controlling many cool- and warm-season weeds. Sequentially applying metham with 1,3-D and/or chloropicrin improved the control of pathogenic fungi, bacteria, plant parasitic nematodes, and soil-inhabiting arthropods. These studies inferred that growers could customize the fumigant combination according to -51- the pests present in the soil. For example, metham alone is an excellent herbicide capable of killing dormant weed seed and fungicide. Furthermore, 1,3-D and/or chloropicrin are poor herbicides but effective nematicides. Coupled with the innate differential selectivity of the fumigants are the different types of application for optimum efficacy. Metham is chisel applied 8-in deep for control of root diseases of peanut, but provides little weed control when applied in this manner. In contrast, metham applied as spray and incorporated to a depth of 3-in gives excellent weed control, but is less effective on root diseases than chiseled applications. There are few herbicides registered for use on watermelon and cantaloupe. In general terms, annual grasses can be effectively controlled with ethalfluralin, bensulide, sethoxydim, or clethodim. Troublesome dicot weeds such as annual morningglories (Ipomoea spp.), smallflower morningglory [Jacquemontia tamnifolia (L.) Griseb.], common cocklebur (Xanthium strumarium L.), sicklepod [Senna obtusifolia (L.) Irwin & Barneby], and Florida beggarweed [Desmodium tortuosum (Sw.) DC] cannot be controlled by any of the herbicides currently registered for use on watermelon or cantaloupe. Similarly, neither yellow nutsedge (Cyperus esculentus L.) nor purple nutsedge (C. rotundus L.) can be adequately controlled in these crops. In 1998, trials were initiated with cantaloupe and watermelon to develop a weed management system for these crops transplanted on polyethylene covered seedbeds. Furthermore, these trials were also designed to study the role of metham fumigation, a proven alternative to methyl bromide, for weed management in these crops. Materials and Methods Irrigated field trials were conducted at the Coastal Plain Experiment Station Ponder Farm from 1998 to 2001. Cantaloupe trials were conducted in 1998, 1999, and 2001. Watermelon trials were conducted 1998 and 2001. All trials in 2000 were terminated due to uncontrollable foliar diseases of cantaloupe and watermelon that confounded all data. Soils were a Tifton loamy sand (fine-loamy, kaolinitic, thermic Plinthic Kandiudults), composed of the following fractions 1998 and 1999 - 86% sand, 8% silt, and 6% clay with 0.2% organic matter; 2001 - 88% sand, 6% silt, and 6% clay with 0.6% organic matter The experimental design was a split-plot with four replications. Main plots were preplant soil fumigation; metham and a nonfumigated control. The entire experimental area was irrigated to field capacity one day prior to fumigation. Metham was sprayed and incorporated in the designated treatments with a modified power tiller using the implement described by Johnson and Webster (2001). Nondiluted metham was sprayed and incorporated at 80 gal./A (broadcast basis) in a 24-in band at a ground speed of 2 mi/h. Black polyethylene tarp (1 mil thick and 24in wide) was spread in a separate operation using a mulch layer1. 1 Pro-Junior Series mulch layer; Buckeye Tractor Company; P. O. Box 123; Columbus Grove,OH 45830. Sub-plots were herbicide systems in watermelon and cantaloupe; ethalfluralin (1.0 pt/A) PRE, ethalfluralin PRE followed by glyphosate (1.0 qt/A) POST-SHIELDED, and a nontreated control. Additional treatments were added in 1999 and 2001 to achieve better control of yellow nutsedge. These additional treatments were ethalfluralin plus halosulfuron (b oz/A) PRE and ethalfluralin plus halosulfuron PRE followed by glyphosate POST-SHIELDED. The PRE treatments were applied immediately after transplanting. All PRE herbicides were applied with a tractor-mounted plot sprayer pressurized with CO2, calibrated to deliver 25 gal/A with off-center nozzle tips2 directing spray onto the edges of the polyethylene covered seedbed and into the row middles. The width of the treated swath was approximately 18-in. The POST-SHIELDED treatments were applied with a tractor mounted PTO-powered sprayer, with hoods3 mounted on a rigid frame that used gauge wheels to stabilize the sprayer. The hoods were 28-in wide and had three overlapping nozzles inside each hood, with a total output of 25 gal/A at 22 lb/in2. The hoods were attached to the rigid frame using articulating mounts that allowed the hood height to adjust according to topography of the field. Nylon brushes4 were added to the bottom edges of the hoods to prevent spray from drifting under the hoods and keep the hoods from tearing the polyethylene tarp covering the seedbeds. Main plots in both crops were 6-ft. wide and 20-ft long, with crops established in one row centered in the middle of the plot. Watermelon plots had a 12-ft. fallow border on either side of the drill for the crop’s aggressive vine growth. The fallow border areas were kept weed free with tillage until vine encroachment. Cantaloupe, a crop with less aggressive vine growth than watermelon, did not require a fallow border between adjacent plots. ‘Cordele®’5 (1998 and 1999) and ‘Vienna®’7 (2001) cantaloupe and ‘Stargazer®’7 watermelon were seeded in Speedling®6 trays in a greenhouse in mid-March, which was concurrent with the time of fumigation and laying the polyethylene tarp in the field. Cantaloupe and watermelon seedlings were transplanted three weeks after fumigation. Seedlings were established in the field using a Kennco®7 transplanter that cut holes in the polyethylene tarp and transplanted in one operation. Cantaloupe seedlings were spaced 22-in apart, while watermelon seedlings were spaced 36-in apart. Plots were irrigated as needed with a solid set sprinkler system. Cultural practices and pest management decisions were based on recommendations 2 TeeJet® OC-03 spray tips; Spraying Systems Co.; P.O. Box 7900; Wheaton, Illinois 60189-7900. 3 RedBall Hooded Sprayer; Custom Ag Products, inc.; Benson, MN 56215. 4 Sealeze Corporation; 8000 Whitepine Rd.; Richmond, VA 23237. 5 Seminis Inc.; 2700 Camino del Sol; Oxnard, CA 93030-7967. 6 Hummert International, Earth City, MO 63045. 7 Kennco Manufacturing Inc., Ruskin, FL 33570. from the Georgia Cooperative Extension Ser. Visual estimates of weed control and crop injury were taken mid-season each year. Yields were measured by harvesting mature fruits from the entire plot at multiple intervals, depending on the continued presence of marketable fruits. The number and weight of cantaloupe and watermelon fruits were recorded by harvest date. Due to differences in growing conditions and weed species among years, data were not pooled. Within each year, data were subjected to analysis of variance to determine sources of variation and significant interactions. Difference in treatment means were determined using the Fisher's Protected Least Significant Difference Test at P<0.05. Results and Discussion Weed control. Florida pusley (Richardia scabra L.) was present in cantaloupe nontreated plots in 1998 and 1999 at approximately 10 and 2 plants/ft2, respectively. Most of the Florida pusley were present in the row middles and some occasionally in the transplant hole. No Florida pusley emerged through the polyethylene tarp. All of the PRE weed control systems effectively controlled Florida pusley, including ethalfluralin alone (Table 1). The addition of halosulfuron PRE or glyphosate POST-SHIELDED was not necessary for adequate Florida pusley control. Regardless of the herbicides used, metham fumigation did not improve Florida pusley control compared to nonfumigated plots. Smallflower morningglory was present in cantaloupe in 1998 and 1999 at approximately 1 plant/ft2 both years. As was the case with Florida pusley, smallflower morningglory was present in row middles or occasionally in the transplant hole. Ethalfluralin alone did not adequately control smallflower morningglory. The sequential application of ethalfluralin PRE followed by glyphosate POST-SHIELDED improved smallflower morningglory control. In 1999, the addition of halosulfuron PRE provided a slight increase in smallflower morningglory control, but not as much as glyphosate POST-SHIELDED. Generally, metham fumigation did not improve smallflower morningglory control compared to nonfumigated plots. Smallflower morningglory was unable to penetrate the polyethylene tarp, without respect to fumigation. Smooth pigweed (Amaranthus hybridus L.) was present in watermelon in 1998 and 2001. All of the PRE weed control systems effectively controlled smooth pigweed, including ethalfluralin alone (Table 2). The addition of halosulfuron PRE or glyphosate POSTSHIELDED was not necessary for adequate smooth pigweed control. Metham fumigation did not improve smooth pigweed control, compared to nonfumigated plots. Crowfootgrass [Dactyloctenium aegyptium (L.) Willd.], southern crabgrass [(Digitaria ciliaris (Retz.) Koel.], and Texas panicum (Panicum texanum Buckl.) were present in some of the trials (Tables 1 and 2). Any of the PRE treatments which included ethalfluralin effectively controlled these annual grasses in cantaloupe and watermelon. Metham fumigation did not improve control of any of the annual grasses compared to nonfumigated plots. As seen with the -54- dicot weeds, the annual grasses did not emerge through the polyethylene tarp and were present primarily in the row middles in the nontreated controls. Yellow nutsedge was present in both crops 1999 and 2001 at approximately 10 and 2 plants/ft2, respectively. The most consistent and effective yellow nutsedge control in cantaloupe and watermelon was a system of metham fumigation, followed by either halosulfuron PRE or glyphosate POST-SHIELDED (Tables 1 and 2). Yellow nutsedge has been successfully controlled in several cucurbit crops with halosulfuron, applied PRE and POST, although preplant fumigation was not evaluated in those trials. In our trials, yellow nutsedge control with either halosulfuron PRE or glyphosate POST-SHIELDED was inconsistent without metham fumigation. In the absence of herbicides, metham fumigation provided only partial control of yellow nutsedge. Clearly, effective control on yellow nutsedge in cantaloupe and watermelon will require an integrated system of metham fumigation and either halosulfuron PRE or glyphosate POST-SHIELDED. Relying strictly on one tactic for yellow nutsedge control will result in escapes. The ratings reflect a visual composite of weed control on the polyethylene covered seedbeds and row middles. Most of the yellow nutsedge present in plots fumigated with metham were in the row middles, which were not fumigated. Very little yellow nutsedge emerged through the polyethylene tarp in plots fumigated with metham, indicating good to excellent control of yellow nutsedge with metham applied using the modified power tiller developed by USDA-ARS at the Coastal Plain Experiment Station in Tifton, GA (Figure 1). Visual injury. Cantaloupe was not significantly injured by metham fumigation or any of the herbicide treatments in 1998, 1999, and 2001 (Table 1). Injury was observed, but was sporadic and nonsignificant. Watermelon was not injured by metham fumigation or any of the herbicide treatments in 1998 (Table 2). However, some of the herbicide treatments stunted watermelon in 2001. The most injurious treatment to watermelon in 2001 was ethalfluralin plus halosulfuron, despite precise application onto the shoulders of the polyethylene covered seedbed and with no contact onto the cucurbit seedlings. Ethalfluralin applied after transplanting is an acceptable time of application, without significant injury. Wells (1999) significantly injured watermelon with halosulfuron PRE, although those applications were to direct seeded watermelon. Our results demonstrate an overall acceptable level of crop safety by applying PRE herbicides to the shoulders of the polyethylene covered seedbeds and avoiding direct contact with cantaloupe and watermelon seedlings. Cantaloupe yield. Cantaloupe yields (number of fruits/A) were not affected by the interactive effects of metham fumigation and herbicide systems for weed control throughout the duration of the trial (Table 3). Similarly, cantaloupe yields expressed as weight of fruits/A were not affected (data not shown). Marketable cantaloupe fruits were harvested multiple times to determine if treatments affected maturity. Data from each of the harvest dates showed no effect of metham fumigation and herbicide systems on cantaloupe maturity (data not shown). Size of cantaloupe fruits (lb/fruit) were not consistently affected by metham fumigation and herbicide treatments -55- throughout the duration of the trial (Table 3). Fruit size was significantly smaller only in 1999 in the nonfumigated, nontreated control compared to any of the herbicide treatments in the nonfumigated plots. Watermelon yield. Watermelon yields (number of fruits/A) were not affected by the interactive effects of metham fumigation and herbicide treatments (Table 4). Similarly, yields expressed as weight of fruits/A were not affected (data not shown). Marketable watermelon fruits were harvested multiple times to determine if treatments affected maturity. Data from each of the harvest dates showed no effect of metham fumigation and herbicide treatments on watermelon maturity (data not shown). Size of watermelon fruits were not affected by metham fumigation and herbicide treatments throughout the duration of the trial (Table 4). The lack of cantaloupe and watermelon yield response to weed control was not expected, considering the extraordinary weed densities encountered each year. These results suggest that transplanted cantaloupe and watermelon grown on polyethylene-covered seedbeds are quite competitive with weeds and the polyethylene tarp itself may be an effective weed control practice. The only weed to penetrate and emerge through the polyethylene tarp was yellow nutsedge. All the dicot weeds and annual grasses in these trials were unable to penetrate the polyethylene tarp. In addition, transplant holes were punched through the polyethylene tarp concurrent with transplanting cantaloupe and watermelon seedlings. This appears to have helped minimize incidence of weeds emerging through the transplant hole, compared to transplant holes present for several days or weeks prior to planting. A rapidly growing cucurbit seedling has a decided competitive advantage with newly emerged weeds in this system. Rapidly growing cucurbit crops have been recognized as being highly competitive with weeds. The rapid crop growth seen in systems of cucurbit transplants on polyethylene covered seedbeds gives growers opportunities to manage many troublesome weed species, including yellow nutsedge, with minimal dependence on herbicides. It was beyond the scope of this trial to compare weed control strategies for direct seeded versus transplanted cucurbits and bare ground versus polyethylene-covered seedbeds. However, inferences can be made that weed control in cucurbits transplanted on polyethylene-covered seedbeds will be assisted by the mechanical barrier provided by the polyethylene tarp. With the weed densities and species diversity encountered in these trials, weed control efforts outside those provided by the polyethylene tarp were generally not needed. It is possible that under different conditions, additional weed control would be needed. Metham fumigation effectively controlled all species under the polyethylene tarp, including yellow nutsedge. Ethalfluralin PRE, halosulfuron PRE, and glyphosate POST-SHIELDED effectively controlled the species encountered in these trials, without excessive crop phytotoxicity and delays in maturity. Halosulfuron PRE and glyphosate POST-SHIELDED have the potential to significantly broaden the weed control spectrum available to cucurbit growers if granted registration. The occasional stunting of cantaloupe and watermelon from halosulfuron PRE was not expressed in yield reduction or delayed maturity. It appears that the weed control -56- benefits of halosulfuron PRE in transplanted cantaloupe and watermelon compensate for the risks of phytotoxicity in these cropping systems. Regardless of weed control options employed by cucurbit growers, the most successful approach is an integration of cultural practices (such as using transplants on polyethylene covered seedbeds) and judicious use of fumigants and herbicides tailored for the pests present. Optimum growing conditions and production practices are necessary for maximizing the substantial crop competition benefits. Properly applying PRE and POST-SHIELDED herbicides on the edges of the polyethylene covered seedbeds will optimize weed control efficacy and minimize crop phytotoxicity. Acknowledgments We acknowledge the technical contributions of Daniel R. Evarts in these trials. Mr. Evarts was solely responsible for the design and construction of the modified equipment used for preplant fumigation and herbicide application in these trials, along with overall field operations. Seminis Inc. graciously provided the cantaloupe and watermelon seeds used in these trials. -57- Literature Cited Anonymous. 1998. Scientific assessment of ozone depletion: 1998 - executive summary. World Meteorological Organization Global Ozone Research and Monitoring Project. Report No. 44. Cline, W. O. and M. K. Beute. 1986. Effect of metam sodium, peanut genotype and inoculum density on incidence of Cylindrocladium black rot. Peanut Sci. 13:41-45. Csinos, A. S., W. C. Johnson, III, A. W. Johnson, D. R. Sumner, R. M. McPherson, and R. D. Gitaitis. 1997. Alternative fumigants for methyl bromide in tobacco and pepper transplant production. Crop Protection 16:585-594. Csinos, A. S., D. R. Sumner, W. C. Johnson, III, A. W. Johnson, R. M. McPherson, and C. C. Dowler. 1999. Methyl bromide alternatives in tobacco, tomato and pepper transplant production. Crop Protection (In press). Doherty, B. A. and W. O. Mizelle, Jr. 2001. 2000 Vegetable Survey. Georgia Fruit and Vegetable Growers News. Summer 2001. Grey. T. L., D. C. Bridges, and D. S. NeSmith. 2000. Tolerance of cucurbits to the herbicides clomazone, ethalfluralin, and pendimethalin. II. watermelon. HortScience. 35:637-641. Johnson, W. C., III and T. M. Webster. 2001. A modified power-tiller for metham application on cucurbit crops transplanted to polyethylene covered seedbeds. Weed Technol. 15:387395. Mitchem, W. E., D. W. Monks, and R. J. Mills. 1997. Response of transplanted watermelon (Citrullus lanatus) to ethalfluralin applied PPI, PRE, and POST. Weed Technol. 11:88-91. Monks, D. W. and J. R. Schultheis. 1998. Critical weed-free period for large crabgrass (Digitaria sanguinalis) in transplanted watermelon (Citrullus lanatus). Weed Sci. 46:530532. Nerson, H. 1989. Weed Competition in muskmelon and its effects on yield and fruit quality. Crop Protection. 8:439-443. Noling, J. W. and J. O. Becker. 1994. The challenge of research and extension to define and implement alternatives to methyl bromide. Suppl. J. Nem. 26:573-586. Teasdale, J. R. and R. B. Taylorson. 1986. Weed seed response to methyl isothiocyanate and metham. Weed Sci. 34:520-524 USDA. 1999. Administration extends deadline on methyl bromide ban to 2005. Methyl Bromide Alternatives 5:1. -58- Webster, T. M., A. S. Csinos, A. W. Johnson, C. C. Dowler, D. R. Sumner, and R. L. Fery. 2001. Methyl bromide alternatives in a bell pepper-squash rotation. Crop Protection. 20:605-614. Wells, J. J. 1999. Yellow nutsedge control in summer vegetables and development of glyphosate-tolerant spinach. PhD Dissertation. University of Arkansas. 127 p. -59- Table 1. Visual estimates of weed control and crop injury in transplanted cantaloupe on polyethylene covered seedbeds at Tifton, GA. 1998 RCHSC1 IAQTA1 1999 Injury ------------ % ------------ RCHSC IAQTA CYPES1 2001 PANTE1 Injury ------------------------- % ------------------------- CYPES DIGSP1 Injury ------------ % ------------ Metham fumigation Ethalfluralin 90 69 0 95 92 78 95 0 76 90 0 Ethalfluralin/glyphosate 94 85 0 95 93 95 94 0 79 90 0 Ethalfluralin + halosulfuron - - - 95 95 95 95 2 88 90 1 Ethalfluralin + halosulfuron/glyphosate - - - 95 94 95 95 7 89 90 0 43 64 0 73 70 75 68 0 73 48 3 Ethalfluralin 93 85 0 90 88 77 94 0 54 90 5 Ethalfluralin/glyphosate 95 93 0 95 95 78 92 2 55 90 3 Ethalfluralin + halosulfuron - - - 93 90 82 88 2 56 90 4 Ethalfluralin + halosulfuron/glyphosate - - - 95 95 92 95 0 60 90 10 Nontreated 0 0 0 0 0 0 0 0 0 0 0 5 14 - 7 7 14 11 ns 16 2 6 Nontreated Nonfumigated LSD (0.05) 1 Abbreviations: CYPES, yellow nutsedge, Cyperus esculentus L.; DIGSP, southern crabgrass, Digitaria ciliaris (Retz.) Koel.; IAQTA, smallflower morningglory, Jacquemontia tamnifolia (L.) Griseb.; PANTE, Texas panicum, Panicum texanum Buckl.; RCHSC, Florida pusley, Richardia scabra L. -60- Table 2. Visual estimates of weed control and crop injury in transplanted watermelon on polyethylene covered seedbeds at Tifton, GA. 1998 AMACH1 DTTAE 2001 Injury CYPES ------------ % ------------ AMACH DIGSP Injury ------------------------- % ------------------------- Metham fumigation Ethalfluralin 95 95 0 76 89 90 5 Ethalfluralin/glyphosate 95 95 0 89 90 90 6 Ethalfluralin + halosulfuron - - - 89 90 90 13 Ethalfluralin + halosulfuron/glyphosate - - - 89 90 90 0 50 50 0 90 70 40 0 Ethalfluralin 91 94 0 80 90 90 11 Ethalfluralin/glyphosate 95 95 0 71 90 90 3 Ethalfluralin + halosulfuron - - - 85 89 90 18 Ethalfluralin + halosulfuron/glyphosate - - - 88 90 90 13 Nontreated 0 0 0 0 0 0 0 8 7 - 12 10 1 12 Nontreated Nonfumigated LSD (0.05) 1 AMACH, smooth pigweed, Amaranthus hybridus L.; CYPES, yellow nutsedge, Cyperus esculentus L.; DIGSP, southern crabgrass, Digitaria ciliaris (Retz.) Koel.; DTTAE, crowfootgrass, Dactyloctenium aegyptium (L.) Willd. -61- Table 3. Effect of weed control with metham fumigation and herbicides on transplanted cantaloupe yield at Tifton, GA. Total Yield1 1998 1999 Fruit Size 2001 1998 1999 2001 ------------------- no/A ------------------- ------------------ lb/fruit ---------------- Ethalfluralin 5900 2420 5900 1.0 1.1 1.9 Ethalfluralin/glyphosate 4810 4960 5450 1.1 1.2 1.9 Ethalfluralin + halosulfuron - 2780 6810 - 1.2 1.8 Ethalfluralin + halosulfuron/glyphosate - 3510 5990 - 1.2 1.7 3900 2300 5450 0.8 1.1 1.8 Ethalfluralin 5170 3510 5630 1.1 1.4 1.7 Ethalfluralin/glyphosate 5720 4360 6170 1.1 1.3 1.7 Ethalfluralin + halosulfuron - 3750 5900 - 1.3 1.4 Ethalfluralin + halosulfuron/glyphosate - 3270 5530 - 1.3 1.8 3810 2780 5530 0.8 1.0 1.7 ns ns ns ns 0.2 ns Metham fumigation Nontreated Nonfumigated Nontreated LSD (0.05) 1 Total yield from multiple harvest dates; 1998, four harvests; 1999, two harvests; 2001, three harvests. -62- Table 4. Effect of weed control with metham fumigation and herbicides on transplanted watermelon yield at Tifton, GA. Total Yield1 1998 2001 ------- no/A ------- Fruit Size 1998 2001 ----- lb/fruit ----- Metham fumigation Ethalfluralin 2810 3000 5.2 5.8 Ethalfluralin/glyphosate 4170 3810 5.4 5.9 - 2720 - 6.0 - 3090 - 6.0 2450 1810 4.9 5.0 Ethalfluralin 3090 3000 5.3 6.0 Ethalfluralin/glyphosate 3180 3270 5.4 6.5 - 2180 - 5.8 - 2630 - 5.9 2720 910 5.2 4.2 ns ns ns 1.5 Ethalfluralin + halosulfuron Ethalfluralin + halosulfuron/glyphosate Nontreated Nonfumigated Ethalfluralin + halosulfuron Ethalfluralin + halosulfuron/glyphosate Nontreated LSD (0.05) 1 Total yield from three harvest dates. -63- LIST OF FIGURES Figure 1. Overall view of Ferguson Tillervator® modified for banded applications of metham in transplanted cantaloupe and watermelon on polyethylene covered seedbeds. Notable features include: gang of C-shaped tines set to till a band width of 24-in, gauge wheels to stabilize depth of tillage, a single flood-jet spray tip spraying a band 24-in wide, metal shield protecting the spray pattern from disruption by tilled soil, fluted coulter, a single in-row subsoil shank with mounting bracket allowing clearance beneath PTO shaft, hiller disks to shape seedbeds after tillage, and steel plate to seal the tilled seedbed with a light crust. A complete description of this tiller is published in Weed Technol. 15:387-395 (2001). This tiller was designed and constructed by Mr. Dan Evarts, USDA-ARS. -64- SANDEA® HAS A VARIABLE AFFECT ON SQUASH GROWTH AND YIELD T. M. Webster Crop Protection and Management Research Unit USDA-Agricultural Research Service, Tifton, GA 31793 Introduction Yellow and purple nutsedge are among the most troublesome weeds in Georgia vegetables (Webster and MacDonald 2001). The impending elimination of methyl bromide in 2005 will soon leave vegetable growers without a valuable tool for pest management, with limited options for suppression of nutsedge growth. One potential herbicide that is being evaluated in several vegetable crops by the IR-4 (Inter-Regional Project #4) program is Sandea® (common name: halosulfuron). This herbicide controls yellow and purple nutsedge, stops tuber production, and reduces tuber viability. The objective of this study was to evaluate the effect of nutsedge management treatments (including Sandea®) on squash growth and yield and nutsedge control. Materials and Methods Spring crop field studies were conducted in 2000 at the Blackshank Farm and in 2001 at the Jones Farm in Tifton, GA. Black polyethylene mulch was laid in 40-foot plots, leaving a bed top 30 inches wide. Zucchini squash (‘Spineless Beauty’) was transplanted into the first half of each plot and direct-seeded into the other half. Prior to planting, the experimental area was treated with Inline® (combination of 1,3-dichloropropene and chloropicrin) injected through the drip tape irrigation system1. The test was managed uniformly for fertility and pests following University of Georgia Extension recommendations. Nutsedge management treatments included 1) Vapam® (common name: metham) applied through the drip tape irrigation at 75 gal/A of product (320 lbs ai/A)1, 2) Sandea® applied PRE to the soil surface prior to plastic laying at 0.75 oz/A of product (0.035 lbs ai/A), 3) Sandea® applied PRE through the drip tape following the laying of plastic at 0.75 oz/A of product, 4) nontreated control with black plastic mulch, and 5) nontreated control without black plastic mulch (bare-ground). Treatments were arranged as a randomized complete block design with four replications. Crop growth (measured in terms of plant diameter) and nutsedge control were evaluated throughout the season. Squash yields were measured two to three times a week, for a total of 5 and 11 harvests in 2000 and 2001, respectively. Results and Discussion Sandea® applied PRE under the plastic or injected through the drip tape reduced squash growth 19 to 23% relative to the Vapam® treatment, for both the transplanted and direct-seeded squash (Table 1). Most of the visual injury observed from Sandea® was a reduction in plant size -65- (i.e. smaller plant diameters). In the early season, squash plants were a shade of yellow lighter than the dark green plants in the Vapam® and nontreated plots, but often grew out of this condition by the conclusion of the season. Early-season nutsedge control was equivalent in all plots that had black plastic mulch, including the nontreated control, and had fewer nutsedge shoots than the bareground-nontreated control (Table 1). By the conclusion of the season,crowfootgrass, pink purslane, smallflower morningglory, and Florida pusley had outcompeted the purple nutsedge in the bareground nontreated control, virtually eliminating it from the plot. Therefore, late-season weed ratings were expressed as a percent of the number of purple nutsedge shoots in the plastic-mulch nontreated control. The Vapam® and Sandea® systems reduced late-season purple nutsedge shoot populations 66 to 76% relative to the plastic mulch-nontreated control. Highest squash yields occurred in plots treated with Vapam® and were lowest in the bareground-nontreated control plots in both years and with both transplanted and direct-seeded crops (Table 2). Squash yields did not respond to Sandea® in a consistent manner. In 2000, all halosulfuron treatments were similar to Vapam® treatments, with the exception of Sandea® applied PRE to transplanted squash. In 2001, only halosulfuron applied through the drip tape to transplant squash had total yields similar to the highest yielding Vapam® treatment. Sandea® yields of direct-seeded squash were at least 23% lower than the direct-seeded Vapam® treatment in 2001. Squash yields in the plastic mulch-nontreated controls were lower than yields in the Vapam® treatments. The cause of these differences is unknown. Squash yields from all Sandea® treatments were not different than their respective plastic mulch-nontreated controls. While nutsedge populations were present in each plot, shoot densities coming through the plastic mulch-nontreated control were relatively low (1.13 shoots/ft2). Other researchers have found that 1.4 yellow nutsedge shoots/ft2 reduced cucumber yields 5% (Johnson and Mullinix 1999), 7.4 purple nutsedge shoots/ft2 reduced tomato yields 14% (Kadir et al. 1999), and 18.6 purple nutsedge shoots/ft2 reduced bell pepper yields 32% (Morales-Payan et al. 1997). Therefore, the effect of Sandea® on squash yields was at most, equivalent to the effect of competition from low populations of purple nutsedge. Conclusions 1. Sandea® reduced squash growth relative to Vapam®, but squash growth was similar to the plastic mulch-nontreated control. 2. Nutsedge control was similar among Sandea®-PRE, Sandea®-drip, and Vapam®-drip treatments. 3. Squash yields were highest in Vapam® plots and lowest in bareground-nontreated control plots. Squash yields in Sandea® treatments were equivalent to the plastic mulch-nontreated controls. -66- 4. Both direct-seeded and transplant squash responded similarly to Sandea®, in terms of growth reduction and crop yield. Future Studies The future of pesticide (especially herbicide) applications through drip tape irrigation systems is promising, though there has been little research on this subject. Crop safety is one of key issues with application of Sandea®. The current study on zucchini squash will be repeated and concluded in 2002. Additional studies will be initiated in 2002 to compare cucumber and eggplant response to Sandea® when applied through the drip tape irrigation following crop planting. Future studies will also need to compare the efficacy of POST over-the-top applications of Sandea® to Sandea® applied through the drip-tape. Acknowledgements The author recognizes the technical support of James E. Davis. His expertise and technical skills were vital in conducting this study. Citations Johnson, W. C. and B. G. Mullinix 1999. Cyperus esculentus interference in Cucumis sativus. Weed Sci. 47: 327-331. Kadir, J. B., R. Charudattan, W. M. Stall and T. A. Bewick 1999. Effect of Dactylaria higginsii on interference of Cyperus rotundus with L. esculentum. Weed Sci. 47: 682-686. Morales-Payan, J. P., B. M. Santos, W. M. Stall and T. A. Bewick 1997. Effects of purple nutsedge (Cyperus rotundus) on tomato (Lycopersicon esculentum) and bell pepper (Capsicum annuum) vegetative growth and fruit yield. Weed Technol. 11: 672-676. Webster, T. M. and G. E. MacDonald 2001. A survey of weeds in various crops in Georgia. Weed Technol. 15: 771-790. -67- Table 1. The effect of weed management treatments on squash growth and nutsedge control.a Squash growth Purple nutsedge controlc Treatment Reductionb Early season Late season _________ ______ _________ % % control _________ Sandea®: drip tape 19 bc 97 a 75 a Sandea®: PRE 23 bc 94 a 66 a Vapam®: drip tape 0a 99 a 76 a Nontreated control: plastic mulch 15 b 79 a 0b Nontreated control: bareground 28 c 0b N/Ad a Treatment means separated using Fisher’s Protected LSD0.05. Means with the same letter were not different from one another. b Squash growth reduction evaluated in terms of measured plant diameter, expressed as a percent reduction relative to the Vapam® treatment. c Control ratings made using counts of nutsedge shoot populations in each treatment, expressed as a percent reduction relative to the bare-ground nontreated control (early season) and plastic-mulch nontreated control (late season). d While nutsedge populations dominated the bare ground nontreated control early in the season, by the conclusion of the season other species outcompeted purple nutsedge for resources, virtually eliminating it from the plot. Table 2. The effect of weed management treatment on squash yield.a _________ _________ Treatment 2000 _________ 2001 _________ Transplan Direct Seed Transplan Direct t t Seed _________ b _________ Squash Yield (% of highest yield) Sandea®: drip tape 75 a-c 82 a-c 84 ab 65 cd Sandea®: PRE 60 c 78 a-c 70 bc 57 c-e Vapam®: drip tape 89 ab 100 a 100 a 88 ab Nontreated control: plastic mulch 66 bc 66 bc 76 bc 62 cd Nontreated control: bare ground 25 d 1d 51 de 42 e a Treatment means separated using Fisher’s Protected LSD0.05. Squash yields can be compared within a crop year. Treatment means with the same letters were not different from one another. b Squash yields are expressed as a percent of the highest yielding treatment in each of the years (42 lbs/plot and 89 lbs/plot in 2000 and 2001, respectively). -68- Disease Control -69- -70- APPLICATION METHODS OF SOIL CHEMICAL TREATMENTS ON NEMATODES, DISEASE AND YIELD OF CUCUMBER A. S. Csinos, Phytopathologist, Department of Plant Pathology, University of Georgia, Tifton Campus K. W. Seebold, Plant Pathologist, Department of Plant Pathology, University of Georgia, Tifton Campus P. Timper, Crop protection and Management Research Unit, USDA-ARS Tifton, GA R. F. Davis, Crop protection and Management Research Unit, USDA-ARS Tifton, GA J. E. Laska, Agricultural Research Coordinator, Plant Pathology University of Georgia, Tifton Campus Introduction Methyl bromide is a broad-spectrum biocide and is an important management tool for controlling nematodes, soilborne diseases and other pests in a wide range of crops including vegetable crops. The developing vegetable industry in Georgia is valued at over $635 million. By the year 2005 all uses of methyl bromide in the United states are scheduled to be terminated. Methyl bromide production and importation will be reduced from 1991 levels as follows: 25% in 1999, 50% in 2001, 70% in 2003, and 100% in 2005. Therefore, alternative pest management strategies must be developed before 2005. The objective of this study was to determine the effects of soil chemical treatments on a susceptible cultivar of cucumber on yield, nematodes, and residual effects on cucumber, using two different soil fumigant injection rigs. Materials and Methods Cucumber Crop Field plots were established 8 August 2001 at the Blackshank Research Farm in Tifton Georgia, and maintained through November 2001 on a Tifton loamy sand (fine-loamy, siliceous thermic Plinthic Kandiudults: 85% sand, 10% silt, 5% clay; 0.5% organic matter; pH 5.4). The plots were naturally infested with Meloidogyne incognita, Patatrichodorus, Helicotylenchus, Pythium spp. The experiment was a randomized block design with drip tape irrigation and chemical treatments injected with chemical injection rigs and replicated five times. Initially, the soil was disc-harrowed, plowed 10 to 12 inches deep with a moldboard plow and shaped into beds 30 inch wide and 8 inches high. Five hundred pounds per acre of 5-10-15 fertilizer was applied broadcast to all plots and incorporated approximately four inches deep with a tractor-powered rototiller. Whole-plots were 30 inches wide and 25 feet long. All treatments, except number 6, were injected on 8 August. The final treatment, number 6, was injected 23 August. Non-treated plots served as controls. The irrigation drip tubing, “Aquatraxx” brand, with emitters spaced 12 inches apart, each emitter delivering 0.30 gallons per hour at 10 psi of pressure, was placed in the center of all beds as they were covered with 3 mil white polyethylene. Thirteen days after the last treatment, 5 September, holes (2 inches diam.) were cut 18 -71- inches apart in single rows using a mechanical-hand transplanter and a single greenhouse-grown cucumber seedling, cultivar “General Lee”, was planted in each hole per bed. The total number of living plants per plot was recorded on 17 September. A vigor rating was done on 27 September, with 1 to10 scale, 10 representing live and healthy plants and 1 representing dead plants. All cucumbers were hand-harvested, each harvest was separated into marketable and cull, counted, and weighed. There were a total of four harvests, they were as follows; 15, 24, and 31 October, and a final on 7 November. All plots were sprayed with Manex + Zinc (1.8 qts./A) and Kocide LF (5 pts./A) on a 7to 10- day schedule for foliage disease control and Ambush (10 oz./A), alternating with Pounce 3.2 (6 oz./A) for insect control on a 7 to 10 day interval. As per the recommendation of the University Of Georgia Extension service, all plots received liquid fertilizer (Miracle-Gro 15-3015) injected through the irrigation tubing, at a rate of 12 pounds of nitrogen per acre, per week through the growing season. Twenty cores of soil, 2.5-cm-diam. × 25-cm-deep, were collected from each row of each subplot on 5 September and 15 November. Soil cores were mixed, and nematodes were extracted from a 150-cm3 sub-sample with centrifugal flotation (Jenkins, 1964). Following final harvest on 14 November, ten plants were dug from each row and rated for root galling by M. incognita on a 1 to 5 scale: 1 = 0%, 2 = 1% to 25%, 3 = 26% to 50%, 4 = 51% to 75%, 5 = 76% to 100% roots galled (Barker et al., 1986). All data collected was analyzed with an analysis of variance (P = 0.05) and means were separated using Duncan’s Multiple range test. Two different injection rigs were used in this study. The first injector, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable products. The second injection rig, referred to as the Yetter rig, it is an injection unit designed for deep chemical injection 10-14 inches deep. It also is equipped with large 36 in. round disks which slice through field debris and trash such as old plastic and drip tape left behind after previous film mulch plantings. Results and Discussion Methyl bromide and all treatment including Telone II or Telone C-35 suppressed rootknot nematode populations (Table 1) and root galling (Table 2) better than the control (treatment 8) or treatments receiving only metam sodium (treatment 5). Vigor ratings (Table 2), cumulative number of marketable cucumbers (Table 3), and cumulative weight of marketable cucumbers (Table 4) were lower with treatments that had higher root-knot nematode populations and gall ratings. The Yetter fumigant injection rig and the chisel fumigant injection rig were equally effective in applying fumigant nematicides. All Telone C-35 application alone or with metam sodium performed as well as the methyl bromide standard. Telone C-35 applied at 35 gallons per acre, performed batter than the methyl bromide standard to increase yield of cucumber. Populations of Fusarium spp. three weeks after fumigation were significantly reduced in comparison to the untreated control by all fumigation treatments except Vapam injected alone at -72- 37.5 GPA. Three months after fumigation, populations of Fusarium spp. in plots treated with Telone II in combination with chloropicrin, Telone C-35 (shallow and deep injection), and Telone C-35 plus Vapam (applied 3 weeks prior to transplanting) were significantly lower than the untreated check. Fusarium populations were higher where Telone C-35 was injected at a depth of 8 inches as compared to 12 inches. All treatments significantly reduced populations of Pythium spp. at three weeks after fumigation; however, Vapam injected alone was the least effective material. Telone C-35, injected at 8 or 12 inches, and Telone C-35 plus Vapam (applied 3 weeks prior to transplanting) significantly reduced total populations of Pythium spp. three months after fumigation. No differences in Rhizoctonia populations were seen between any fumigation treatment and the untreated check at either three weeks or three months after fumigation. Survival of Fusarium solani, was reduced only by fumigation with Telone C-35, injected at either 8 or 12 inches. Telone C-35, injected at 8 or 12 inches, and Telone C-35 plus Vapam (applied 3 weeks prior to transplanting) significantly reduced the survival of yellow nutsedge nutlets as compared to the untreated check. Treatment effects on Phytophthora capsici and Rhizoctonia solani could not be determined due to microbial contamination of the inoculum recovered from test plots. Acknowledgments The authors wish to thank Unessee Hargett, Don Hickey, Lewis Mullis, Tonya Jo Cravens, Brandi Hogan, Bryan Horten, Eduvina McDonald, Donnie Starling, Matt McKinnon, Thomas H. Hilton, David L. Clements, Billy Wilson, and A. Kyle Monfort for technical support, Cindy H. LaHue for typing. Also, Dow Agrosciences for financial support. -73- Table 1. Effects of soil chemical treatments on nematode population densities in cucumbers 2001a Treatment Rate 5th September Method of Application M.i.b 1. Telone II 18 gal/A Yetter Rig (deep application at 12") 100-125lbs/A Chisel Rig (shallow application at 8") (3 weeks) 2. Telone C-35 35 gal/A 3. Telone C-35 P.m. Nematodes per 150-cm3 soil 15th November C.o. M.i. P.m. C.o. 276 0 8 16 c 60 a 0b Chisel Rig (shallow application at 8") (3 weeks) 200 0 0 0c 0b 0b 35 gal/A Yetter Rig (deep application at 12") (3 weeks) 232 0 4 0c 0b 0b 4. Telone C-35 35 gal/A Chisel Rig (shallow application at 8") 984 0 0 2c 20 b 0b + Metam Sodium 37.5 gal/A Rototill (3 weeks) 5. Metam Sodium 37.5 gal/A Chisel Rig Rototill (shallow application at 8") (3 weeks) 216 0 4 174 b 0b 0b 6. Telone C-35 10 gal/A Chisel Rig (shallow application at 8") 48 0 0 2c 0b 0b + Metam Sodium 37.5 gal/A Rototill (1 week) 7. Methyl Bromide 200 lbs ai/A Chisel Rig (shallow application at 8") (3 weeks) 168 0 0 6c 0b 0b 8. Control N/A ***************** 120 0 0 646 a 0b 100 a + Chloropicrin a Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant. b M.i. = Meloidogyne incognita, P.m. = Paratrichodorus, C.o. = Criconemoides. -74- Table 2. Effects of soil chemical treatments on root-gall indices, Vigor and stand counts of cucumbers - 2001a Treatment 1. Telone II Rate Method of Application Vigor Rating September 27 Stand count d September 17 1.6 b 9.2 a 17 Root-gall index 14 November b c 18 gal/A Yetter Rig (deep application at 12") 100125lbs/A Chisel Rig (shallow application at 8") (3 weeks) 2. Telone C-35 35 gal/A Chisel Rig (shallow application at 8") (3 weeks) 1.2 b 9.2 a 17 3. Telone C-35 35 gal/A Yetter Rig (deep application at 12") (3 weeks) 1.3 b 8.4 a 17 4. Telone C-35 35 gal/A Chisel Rig (shallow application at 8") 1.3 b 8.8 a 16 + Metam Sodium 37.5 gal/A Rototill (3 weeks) 5. Metam Sodium 37.5 gal/A Chisel Rig Rototill (shallow application at 8") (3 weeks) 4.8 a 6.0 b 17 6. Telone C-35 10 gal/A Chisel Rig (shallow application at 8") + Metam Sodium 37.5 gal/A Rototill (1 week) 1.5 b 8.6 a 17 7. Methyl Bromide 200 lbs ai/A Chisel Rig (shallow application at 8") (3 weeks) 1.3 b 8.6 a 17 8. Control N/A ***************** 5.0 a 5.2 b 17 + Chloropicrin a Data are means of five replications. Means in the same column followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant difference. b Root Gall Index 1-5 scale: 1 = no galls, 2 = 1% to 25%, 3 = 26% to 50%, 4 = 51% to 75%, and 5 = 76% to 100% of roots galled. c Vigor was done a 1-10 scale with 10= live and healthy plants and 1=dead plants. d Maximum number of plants per plot = 17. Table 3. Effect of soil chemical treatments on marketable yield of cucumbers -2001a -75- Number Treatment 1. Telone II Method of Application 15 18 gal/A Yetter Rig (deep application at 12") 100125lbs/A Chisel Rig (shallow application at 8") (3 weeks) 2. Telone C-35 35 gal/A 3. Telone C-35 of marketable cucumbers per plot (35 lin. ft. row) October November Total 24 31 7 Mean 31.6 a 78.8 a 59.8 ab 18.8 a 189.0 ab Chisel Rig (shallow application at 8") (3 weeks) 31.6 a 75.6 a 68.4 a 20.0 a 195.6 a 35 gal/A Yetter Rig (deep application at 12") (3 weeks) 31.2 a 94.6 a 47.0 bc 23.8 a 196.6 a 4. Telone C-35 35 gal/A Chisel Rig (shallow application at 8") 30.4 a 84.8 a 61.2 ab 17.6 ab 194.0 a + Metam Sodium 37.5 gal/A Rototill (3 weeks) 5. Metam Sodium 37.5 gal/A Chisel Rig Rototill (shallow application at 8") (3 weeks) 8.2 b 48.6 a 21.6 e 11.0 bc 89.4 c 6. Telone C-35 10 gal/A Chisel Rig (shallow application at 8") 30.6 a 79.8 a 43.2 bcd 22.4 a 176.0 ab + Metam Sodium 37.5 gal/A Rototill (1 week) 7. Methyl Bromide 200 lbs ai/A Chisel Rig (shallow application at 8") (3 weeks) 27.0 a 84.4 a 35.4 cde 23.2 a 170.0 b 8. Control N/A ***************** 4.0 b 38.6 b 25.6 de 7.6 c 75.8 c + Chloropicrin a Rate Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. Table 4. Effect of soil chemical treatments on marketable yield of cucumbers - 2001a -76- Treatment 1. Telone II Weight (lbs.) of marketable cucumbers per plot (35 lin. ft. row) October November Total 15 24 31 7 Mean Method of Application 18 gal/A Yetter Rig (deep application at 12") 100-125lbs/A Chisel Rig (shallow application at 8") (3 weeks) 2. Telone C-35 35 gal/A 3. Telone C-35 15.2 a 59.7 a 32.1 ab 9.8 a 116.9 ab Chisel Rig (shallow application at 8") (3 weeks) 14.1 a 54.8 a 36.7 a 10.6 a 116.3 ab 35 gal/A Yetter Rig (deep application at 12") (3 weeks) 12.5 a 65.1 a 24.8 bc 12.5 a 115.0 ab 4. Telone C-35 35 gal/A Chisel Rig (shallow application at 8") 15.4 a 62.4 a 32.2 ab 9.0 a 119.2 a + Metam Sodium 37.5 gal/A Rototill (3 weeks) 5. Metam Sodium 37.5 gal/A Chisel Rig Rototill (shallow application at 8") (3 weeks) 3.1 b 34.1 b 11.3 d 4.2 b 52.8 c 6. Telone C-35 10 gal/A Chisel Rig (shallow application at 8") 12.9 a 53.8 a 25.8 bc 11.1 a 103.7 b + Metam Sodium 37.5 gal/A Rototill (1 week) 7. Methyl Bromide 200 lbs ai/A Chisel Rig (shallow application at 8") (3 weeks) 12.9 a 62.7 a 20.5 cd 10.7 a 106.9 ab 8. Control N/A ***************** 1.5 b 27.3 b 13.4 d 3.4 b 45.8 c + Chloropicrin a Rate Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. -77- Table 5. Effect of soil chemical treatment on cull yield of cucumbers - 2001a Number Treatment 1. Telone II Rate Method of Application 15 18 gal/A Yetter Rig (deep application at 12") 100-125lbs/A Chisel Rig (shallow application at 8") (3 weeks) 2. Telone C-35 35 gal/A 3. Telone C-35 of cull cucumbers per plot (35 lin. ft. row) October November Total 24 31 7 Mean 0.0 2.2 a 1.4 ab 3.2 ab 6.8 bc Chisel Rig (shallow application at 8") (3 weeks) 0.4 1.2 ab 2.6 ab 5.2 a 9.4 ab 35 gal/A Yetter Rig (deep application at 12") (3 weeks) 0.0 1.6 ab 3.6 a 5.8 a 11.0 a 4. Telone C-35 35 gal/A Chisel Rig (shallow application at 8") 0.4 2.6 a 2.2 ab 1.8 b 7.0 bc + Metam Sodium 37.5 gal/A Rototill (3 weeks) 5. Metam Sodium 37.5 gal/A Chisel Rig Rototill (shallow application at 8") (3 weeks) 0.0 0.4 b 1.0 ab 5.6 a 7.0 bc 6. Telone C-35 10 gal/A Chisel Rig (shallow application at 8") 0.2 1.4 ab 2.6 ab 3.8 ab 8.0 abc + Metam Sodium 37.5 gal/A Rototill (1 week) 7. Methyl Bromide 200 lbs ai/A Chisel Rig (shallow application at 8") (3 weeks) 0.8 2.6 a 2.0 ab 3.2 ab 8.6 ab 8. Control N/A ***************** 0.2 0.6 b 0.4 b 3.4 ab 4.6 c + Chloropicrin a Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non- significant difference. Table 6. Effect of soil chemical treatment on cull yield of cucumbers - 2001a -78- Treatment 1. Telone II Rate Method of Application 18 gal/A Yetter Rig (deep application at 12") 100-125lbs/A Chisel Rig (shallow application at 8") (3 weeks) 2. Telone C-35 35 gal/A 3. Telone C-35 Weight (lbs.) of cull cucumbers per plot (35 lin. ft. row) October November Total 15 24 31 7 Mean 0.0 1.6 a 0.4 ab 1.0 ab 3.1 abc Chisel Rig (shallow application at 8") (3 weeks) 0.1 0.1 ab 0.7 ab 1.5 ab 3.2 ab 35 gal/A Yetter Rig (deep application at 12") (3 weeks) 0.0 1.2 ab 0.9 a 2.0 a 4.1 a 4. Telone C-35 35 gal/A Chisel Rig (shallow application at 8") 0.1 1.6 a 0.6 ab 0.6 b 2.9 abc + Metam Sodium 37.5 gal/A Rototill (3 weeks) 5. Metam Sodium 37.5 gal/A Chisel Rig Rototill (shallow application at 8") (3 weeks) 0.0 0.3 b 0.3 ab 1.7 a 2.4 bc 6. Telone C-35 10 gal/A Chisel Rig (shallow application at 8") 0.1 1.1 ab 0.6 ab 1.5 ab 3.3 ab + Metam Sodium 37.5 gal/A Rototill (1 week) 7. Methyl Bromide 200 lbs ai/A Chisel Rig (shallow application at 8") (3 weeks) 0.4 1.7 a 0.5 ab 1.0 ab 3.6 ab 8. Control N/A ***************** 0.1 0.4 ab 0.1 b 1.0 ab 1.6 c + Chloropicrin a Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant difference. Table 7. Population densities of Pythium spp., Rhizoctonia spp., and Fusarium spp., and viability of Phytophthora capsici, Fusarium solani, -79- Rhizoctonia solani, and yellow nutsedge in soil after treatment with alternatives to methyl bromide in August, 2001 (cucumber test). Fungal populations a Treatment 1. Telone II Rate Method of Application Fusarium YN 18 gal/A Yetter Rig (deep application at 12") 100-125lbs/A Chisel Rig (shallow application at 8") (3 weeks) 2. Telone C-35 35 gal/A 3. Telone C-35 Pathogen and weed viability b Pythium Rhizoctonia PC FS RS 1344 c 0d 0.08 0.0 6.6 abc 0.0 b 5.4 abc Chisel Rig (shallow application at 8") (3 weeks) 1168 c 0.8 cd 0.0 0.0 6.4 bc 0.0 b 3.8 cd 35 gal/A Yetter Rig (deep application at 12") (3 weeks) 688 c 4.8 cd 0.03 1.6 5.0 c 0.0 b 1.0 de 4. Telone C-35 35 gal/A Chisel Rig (shallow application at 8") 1072 c 0d 0.0 1.8 7.2 abc 0.0 b 0.0 e + Metam Sodium 37.5 gal/A Rototill (3 weeks) 5. Metam Sodium 37.5 gal/A Chisel Rig Rototill (shallow application at 8") (3 weeks) 8752 a 25.6 b 0.0 0.0 10.0 a 0.0 b 7.8 ab 6. Telone C-35 10 gal/A Chisel Rig (shallow application at 8") 2928 bc 8.8 c 0.0 0.6 7.6 abc 5.6 a 8.8 ab + Metam Sodium 37.5 gal/A Rototill (1 week) 7. Methyl Bromide 200 lbs ai/A Chisel Rig (shallow application at 8") (3 weeks) 1312 c 0d 0.0 2.0 9.6 ab 0.0 b 4.8 bc 8. Control N/A ***************** + Chloropicrin 5184 b 39.2 a 0.03 0.0 10.0 a 0.0 b 8.0 ab Total populations of Fusarium spp., Pythium spp., and Rhizoctonia spp. expressed as the number of colony forming units per gram of soil. b Number of pathogen-infested grains or yellow nutsedge nutlets (out of 10 per mesh bag) that were viable after removal from treated soil prior to transplanting of cucumbers on 9 September. PC=Phytophthora capsici, FS=Fusarium solani, RS=Rhizoctonia solani (AG-4), and YN=yellow nutsedge. Means followed by the same letter do not differ significantly as determined by Fisher’s protected least significant difference test (P#0.05). No letters indicate non-significant difference. a -80- Table 8. Population densities of Pythium spp., Rhizoctonia spp., and Fusarium spp., and viability of Phytophthora capsici, Fusarium solani, Rhizoctonia solani, and yellow nutsedge in soil on November 20th after treatment with alternatives to methyl bromide in August, 2001 (cucumber test). Treatment 1. Telone II Rate Fungal populations a Method of Application 18 gal/A Yetter Rig (deep application at 12") 100125lbs/A Chisel Rig (shallow application at 8") (3 weeks) 2. Telone C-35 35 gal/A 3. Telone C-35 Fusarium Pythium Rhizoctonia 4288 cd 14.4 b 0.0 b Chisel Rig (shallow application at 8") (3 weeks) 9408 ab 3.2 c 0.0 b 35 gal/A Yetter Rig (deep application at 12") (3 weeks) 3328 cd 0.8 c 0.0 b 4. Telone C-35 35 gal/A Chisel Rig (shallow application at 8") 3712 cd 0.0 c 0.0 b + Metam Sodium 37.5 gal/A Rototill (3 weeks) 5. Metam Sodium 37.5 gal/A Chisel Rig Rototill (shallow application at 8") (3 weeks) 6944 bc 33.6 a 0.05 b 6. Telone C-35 10 gal/A Chisel Rig (shallow application at 8") 1104 d 13.6 b 0.0 b + Metam Sodium 37.5 gal/A Rototill (1 week) 7.Methyl Bromide 200 lbs ai/A Chisel Rig (shallow application at 8") (3 weeks) 12288 a 6.4 bc 0.0 b 8. Control N/A ***************** 11024 ab 14.4 b 0.13 a + Chloropicrin a Total populations of Fusarium spp., Pythium spp., and Rhizoctonia spp. expressed as the number of colony forming units per gram of soil. Means followed by the same letter do not differ significantly as determined by Fisher’s protected least significant difference test (P#0.05). -81- SOIL CHEMICAL TREATMENT ALTERNATIVES TO METHYL BROMIDE IN CUCUMBER A. S. Csinos, Phytopathologist, Department of Plant Pathology, University of Georgia, Tifton Campus K. W. Seebold, Plant Pathologist, Department of Plant Pathology, University of Georgia, Tifton Campus P. Timper, Crop protection and Management Research Unit, USDA-ARS Tifton, GA R. F. Davis, Crop protection and Management Research Unit, USDA-ARS Tifton, GA J. E. Laska, Agricultural Research Coordinator, Plant Pathology University of Georgia, Tifton Campus Introduction Methyl bromide is a broad-spectrum biocide and is an important management tool for controlling nematodes, soilborne diseases and other pests in a wide range of crops including vegetable crops. The developing vegetable industry in Georgia is valued at over $635 million. By the year 2005 all uses of methyl bromide in the United states are scheduled to be terminated. Methyl bromide production and importation will be reduced from 1991 levels as follows: 25% in 1999, 50% in 2001, 70% in 2003, and 100% in 2005. Therefore, alternative pest management strategies must be developed before year 2005. The objective of this second Methyl Bromide Alternative study was to determine the effects of soil chemical treatments on a susceptible cultivar of cucumber on yield, nematodes, and residual effects on cucumber. Materials and Methods Cucumber Crop Field plots were established 8 August 2001 at the Blackshank Research Farm in Tifton Georgia, and maintained through November 2001 on a Tifton loamy sand (fine-loamy, siliceous thermic Plinthic Kandiudults: 85% sand, 10% silt, 5% clay; 0.5% organic matter; pH 5.4). The plots were naturally infested with Meloidogyne incognita, Patatrichodorus, Helicotylenchus, and Pythium spp. The experiment was a randomized block design with drip tape irrigation and chemical treatments injected with chemical injection rigs and replicated five times. Initially, the soil was disc-harrowed, plowed 10 to 12 inches deep with a moldboard plow and shaped into beds 30 inches wide and 8 inches high. Five hundred pounds per acre of 5-10-15 fertilizer was applied by broadcast to all plots and incorporated approximately four inches deep with a tractor-powered rototiller. Whole-plots were 30 inches wide and 25 feet long. Two treatments, number 4 and 7, were chisel injected on 9 August. All other treatments were injected through the drip tape over a 6 hour delivery period. Treatments 2, 3, and 5a were injected on 14 August. Treatments 1 and 6 were injected on 15 August. The final treatment 5b was injected on 17 August. Non-treated plots served as controls. -82- The irrigation drip tubing, Toro “Aquatraxx” brand, with emitters spaced 12 inches apart, each emitter delivering 0.30 gallons per hour at 10 psi of pressure, was placed in the center of all beds as they were covered with 3 mil white polyethylene. Nineteen days after the last treatment, 5 September, holes (2 inches diam.) were cut 18 inches apart in single rows using a mechanical-hand transplanter and a single greenhouse-grown cucumber seedling, cultivar “General Lee”, was planted in each hole per bed. The total number of living plants per plot was recorded on 17 September. A vigor rating was done on 27 September, with 1 to10 scale, 10 representing live and healthy plants and 1 representing dead plants. All cucumbers were hand-harvested, each harvest was separated into marketable and cull, counted, and weighed. There were a total of four harvests, they were as follows; 15, 24, and 31 October, and a final on 7 November. All plots were sprayed with Manex + Zinc (1.8 qts./A) and Kocide LF (5 pts./A) on a 7to 10- day schedule for foliage disease control and Ambush (10 oz./A), alternating with Pounce 3.2 (6 oz./A) for insect control on a 7 to 10 day interval. As per the recommendation of the University Of Georgia Extension service, all plots received liquid fertilizer (Miracle-Gro 15-3015) injected through the irrigation tubing, at a rate of 12 pounds of nitrogen per acre, per week through the growing season. Twenty cores of soil, 2.5-cm-diam. × 25-cm-deep, were collected from each row of each subplot on 5 September and 15 November. Soil cores were mixed, and nematodes were extracted from a 150-cm3 sub-sample with centrifugal flotation (Jenkins, 1964). Following final harvest on 14 November, ten plants were dug from each row and rated for root galling by M. incognita on a 1 to 5 scale: 1 = 0%, 2 = 1% to 25%, 3 = 26% to 50%, 4 = 51% to 75%, 5 = 76% to 100% roots galled (Barker et al., 1986). All data collected was analyzed with an analysis of variance (P = 0.05) and means were separated using Duncan’s Multiple range test. The injector used in this study, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable products. Results and Discussion All nematicide treatments were equally effective in reducing root-knot nematode populations (Table 1) and root galling (Table 2) compared to the non-treated control (treatment 8). All nematicide treatments improved vigor ratings (Table 2) compared to the non-treated control. All nematicide treatments significantly increased cumulative weight of marketable cucumbers (Table 3) and cumulative number of marketable cucumbers (Table 4). In-Line 35 EC injected through drip tape was an effective means of nematode suppression. All of the chemical treatments whether applied through tractor powered equipment or through drip tape performed as well as methyl bromide for maintaining Vigor increasing yield of cucumber. All fumigation treatments significantly reduced the soil populations of Fusarium spp. and Pythium spp. as compared to the untreated check in samples taken prior to transplanting. Inline -83- 35 EC, when injected at 35 GPA, reduced populations of Fusarium spp. by greater than 90% of those seen in plots treated with Telone II EC (18 GPA) or Inline 35 EC (20 GPA), and did not differ from Telone C-35 chisel-injected at 35 GPA or methyl bromide chisel-injected at 200 lb a.i./A. The combination of Inline 35 EC (20.5 GPA) and Vapam (37.5 GPA) injected simultaneously or 3 days apart also reduced Fusarium populations as well as methyl bromide, and performed better than Telone II at 18 GPA or Inline 35 EC at 20 GPA. Rhizoctonia solani was not detected in soil samples taken prior to transplanting. When soil samples were taken in late November, populations of Fusarium spp. were lowest where Inline 35 EC (20.5 GPA), Telone C-35 (chisel injected at 35 GPA), or Inline 35 EC (20.5 GPA) injected simultaneously with Vapam (37.5 GPA) had been applied. A similar pattern was observed with populations of Pythium spp. All drip-injected materials reduced the survival of inoculum of Fusarium solani and Rhizoctonia solani, placed in the soil at the time of injection, as compared to the untreated check or methyl bromide. A similar pattern was seen with the survival of yellow nutsedge nutlets from plots treated with drip-applied materials. Inline 35 EC (35 GPA) or Inline 35 EC (20.5 GPA) plus Vapam (37.5 GPA), injected simultaneously or 3 days apart, killed all nutlets that were placed in plots at the time of injection. Acknowledgments The authors wish to thank Unessee Hargett, Don Hickey, Lewis Mullis, Tonya Jo Cravens, Brandi Hogan, Bryan Horten, Eduvina McDonald, Donnie Starling, Matt McKinnon, Thomas H. Hilton, David L. Clements, Billy Wilson, and A. Kyle Monfort for technical support. Also, Dow Agrosciences for financial support. -84- Table 1. Effects of soil chemical treatments on nematode population densities in cucumbers 2001a Treatment Rate Method of Application c M.i.b Nematodes per 150-cm3 soil 5 September 15 November P.m. C.o. M.i. P.m. C.o. 1. Telone II EC 18 gal/A Inject through drip tape 156 0 0 4b 0 0 2. In-Line 35 EC 20.5 gal/A Inject through drip tape 64 0 0 10 b 0 0 3. In-Line 35 EC 35 gal/A Inject through drip tape 44 0 0 0b 0 0 4. Telone C-35 35 gal/A Chisel Injection 36 0 0 0b 0 0 5. In-Line 35 EC 20.5 gal/A Inject through drip tape 496 0 0 0b 0 0 37.5 gal/A Inject through drip tape (3 days later) 20.5 gal/A Inject through drip tape simultaneously (mix together in tank) 28 0 0 36 b 0 0 Chisel Injection 48 0 0 2b 0 0 + Metam Sodium 6. In-Line 35 EC + Metam Sodium 7. Methyl Bromide 37.5 gal/A 200lbs ai/A 8. Non-Treated Control N/A ------------288 0 0 582 a 0 0 Data are means of five replications. b M.i. = Meloidogyne incognita, P.m. = Paratrichodorus, C.o. = Criconemoides c Treatments number 4 and 7, were chisel injected on 9 August. All other treatments were injected through the drip tape over a 6 hour delivery period. Treatments 2, 3, and 5a were injected on 14 August. Treatments 1, and 6 were injected on 15 August. The final treatment, 5b was injected on 17 August. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant. a -85- Table 2. Effects of soil chemical treatments on root-gall indices, Vigor and stand counts of cucumbers - 2001a Vigor Rating 27 September Stand count d 17 September 1.7 b 9.6 a 17 Inject through drip tape 1.6 b 9.4 a 17 35 gal/A Inject through drip tape 1.5 b 8.6 a 17 4. Telone C-35 35 gal/A Chisel Injection 1.7 b 9.6 a 16 5. In-Line 35 EC 20.5 gal/A Inject through drip tape 1.4 b 9.4 a 16 37.5 gal/A Inject through drip tape (3 days later) 20.5 gal/A 1.9 b 9.0 a 16 37.5 gal/A Inject through drip tape simultaneously (mix together in tank) 200 lbs ai/A Chisel Injection 1.7 b 9.0 a 17 Treatment Rate Method of Application 1. Telone II EC 18 gal/A Inject through drip tape 2. In-Line 35 EC 20.5 gal/A 3. In-Line 35 EC + Metam Sodium 6. In-Line 35 EC + Metam Sodium 7. Methyl Bromide Root-gall index 14 November b c 8. Non-Treated Control N/A -----------4.8 a 7.0 b 16 Data are means of five replications. b Root Gall Index 1-5 scale: 1 = no galls, 2 = 1% to 25%, 3 = 26% to 50%, 4 = 51% to 75%, and 5 = 76% to 100% of roots galled. c Vigor was done a 1-10 scale with 10= live and healthy plants and 1=dead plants. d Maximum number of plants per plot = 17. Means in the same column followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant difference a -86- Table 3. Effect of soil chemical treatments on marketable yield of cucumbers -2001a Treatment Weight of marketable cucumbers per plot (35 lin. ft. row) November October 15 24 31 7 Total Mean Rate Method of Application 1. Telone II EC 18 gal/A Inject through drip tape 12.3 62.5 a 14.2 a 9.1 ab 98.1 a 2. In-Line 35 EC 20.5 gal/A Inject through drip tape 13.9 56.2 a 15.2 a 6.6 ab 91.8 a 3. In-Line 35 EC 35 gal/A Inject through drip tape 10.7 67.1 a 12.5 a 4. Telone C-35 35 gal/A Chisel Injection 14.9 64.7 a 13.8 a 10.3 a 103.8 a 5. In-Line 35 EC 20.5 gal/A Inject through drip tape 11.4 56.9 a 15.8 a 11.2 a 95.3 a 37.5 gal/A Inject through drip tape (3 days later) 20.5 gal/A 12.8 55.6 a 10.6 a 9.4 a 88.5 a 37.5 gal/A Inject through drip tape simultaneously (mix together in tank) 200 lbs ai/A Chisel Injection 15.3 61.6 a 12.4 a 9.3 ab 98.6 a + Metam Sodium 6. In-Line 35 EC + Metam Sodium 7. Methyl Bromide 7.5 ab 97.9 a 8. Non-Treated Control N/A -----------2.6 41.9 b 4.4 b 4.8 b 53.8 b Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant difference. a -87- Table 4. Effect of soil chemical treatments on marketable yield of cucumbers - 2001a Treatment Number of marketable cucumbers per plot (35 lin. ft. row) October November 15 24 31 7 Total Mean Rate Method of Application 1. Telone II EC 18 gal/A Inject through drip tape 29.2 a 88.0 a 34.4 a 18.4 ab 170.0 a 2. In-Line 35 EC 20.5 gal/A Inject through drip tape 31.6 a 81.8 a 35.2 a 13.6 ab 162.2 a 3. In-Line 35 EC 35 gal/A Inject through drip tape 24.2 a 94.2 a 26.0 a 13.4 ab 157.8 a 4. Telone C-35 35 gal/A Chisel Injection 31.2 a 90.8 a 29.0 a 20.0 a 171.0 a 5. In-Line 35 EC 20.5 gal/A Inject through drip tape 24.6 a 81.6 a 35.0 a 21.0 a 162.2 a 37.5 gal/A Inject through drip tape (3 days later) 20.5 gal/A 30.8 a 83.0 a 25.0 a 17.2 ab 156.0 a 37.5 gal/A Inject through drip tape simultaneously (mix together in tank) 200 lbs ai/A Chisel Injection 33.4 a 83.0 a 25.4 a 18.6 ab 160.4 a 11.4 b 82.6 b + Metam Sodium 6. In-Line 35 EC + Metam Sodium 7. Methyl Bromide 8. Non-Treated Control N/A -----------6.8 b 55.2 b 9.2 b Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. a -88- Table 5. Effect of soil chemical treatment on cull yield of cucumbers - 2001a Rate Method of Application Weight of cull cucumbers per plot (35 lin. ft. row) October November 15 24 31 7 1. Telone II EC 18 gal/A Inject through drip tape 0.1 1.2 b 0.2 1.3 2.7 ab 2. In-Line 35 EC 20.5 gal/A Inject through drip tape 0.0 1.1 b 0.9 0.8 2.8 ab 3. In-Line 35 EC 35 gal/A Inject through drip tape 0.1 1.3 b 0.8 1.5 3.7 a 4. Telone C-35 35 gal/A Chisel Injection 0.1 2.5 a 0.3 1.1 4.1 a 5. In-Line 35 EC 20.5 gal/A Inject through drip tape 0.0 1.2 b 0.9 1.0 3.0 ab 37.5 gal/A Inject through drip tape (3 days later) 20.5 gal/A 0.0 1.5 ab 0.9 1.0 3.0 ab 37.5 gal/A Inject through drip tape simultaneously (mix together in tank) 200 lbs ai/A Chisel Injection 0.1 0.9 b 1.2 0.9 2.9 ab Treatment + Metam Sodium 6. In-Line 35 EC + Metam Sodium 7. Methyl Bromide Total Mean 8. Non-Treated Control N/A -----------0.0 0.4 b 0.4 0.8 1.6 b Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non- significant difference. a -89- Table 6. Effect of soil chemical treatment on cull yield of cucumbers - 2001a Treatment Number of cull cucumbers per plot (35 lin. ft. row) October November 15 24 31 7 Total Mean Rate Method of Application 1. Telone II EC 18 gal/A Inject through drip tape 0.2 2.0 bcd 0.6 b 4.6 7.4 ab 2. In-Line 35 EC 20.5 gal/A Inject through drip tape 0.0 2.0 bcd 3.8 ab 2.6 8.4 ab 3. In-Line 35 EC 35 gal/A Inject through drip tape 0.2 2.4 abc 2.6 ab 4.0 9.2 a 4. Telone C-35 35 gal/A Chisel Injection 0.4 4.0 a 1.6 ab 3.4 9.4 a 5. In-Line 35 EC 20.5 gal/A Inject through drip tape 0.0 0.8 cd 3.2 ab 2.8 6.8 ab 37.5 gal/A Inject through drip tape (3 days later) 20.5 gal/A 0.0 2.8 ab 3.4 ab 3.0 8.6 ab 37.5 gal/A Inject through drip tape simultaneously (mix together in tank) 200 lbs ai/A Chisel Injection 0.2 1.6 bcd 4.8 a 3.2 9.8 a 2.6 4.8 b + Metam Sodium 6. In-Line 35 EC + Metam Sodium 7. Methyl Bromide 8. Non-Treated Control N/A -----------0.0 0.6 b 1.6 ab Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant difference. a -90- Table 7. Population densities of Pythium spp., Rhizoctonia spp., and Fusarium spp., and viability of Phytophthora capsici, Fusarium solani, Rhizoctonia solani, and yellow nutsedge in soil after treatment with alternatives to methyl bromide in August, 2001. Treatment Fungal populations a Fusarium Pythium Rhizoctonia PC Pathogen and weed viability b FS RS YN Rate Method of Application 1. Telone II EC 18 gal/A Inject through drip tape 1920 b 0.8 b 0 0 5.8 b 0.0 b 1.8 bc 2. In-Line 35 EC 20.5 gal/A Inject through drip tape 2032 b 0.8 b 0 0 4.4 b 0.0 b 2.6 b 3. In-Line 35 EC 35 gal/A Inject through drip tape 112 c 0.0 b 0 0 3.2 b 0.4 ab 0.0 c 4. Telone C-35 35 gal/A Chisel Injection 144 c 0.0 b 0 0 9.6 a 0.2 b 6.8 a 5. In-Line 35 EC 20.5 gal/A Inject through drip tape 64 c 0.0 b 0 1.8 5.2 b 0.0 b 0.0 c 37.5 gal/A Inject through drip tape (3 days later) 20.5 gal/A 336 c 3.2 b 0 0 5.6 b 0.0 b 0.0 c 37.5 gal/A Inject through drip tape simultaneously (mix together in tank) 200 lbs ai/A Chisel Injection 672 c 1.6 b 0 0 10.0 a 0.8 ab 8.6 a + Metam Sodium 6. In-Line 35 EC + Metam Sodium 7. Methyl Bromide 8. Non-Treated Control N/A -----------5504 a 34.4 a 0 0 10.0 a 1.2 a 9.2 a Total populations of Fusarium spp., Pythium spp., and Rhizoctonia spp. expressed as the number of colony forming units per gram of soil. b Number of pathogen-infested grains or yellow nutsedge nutlets (out of 10 per mesh bag) that were viable after removal from treated soil prior to transplanting of cucumbers on 5 September . PC=Phytophthora capsici, FS=Fusarium solani, RS=Rhizoctonia solani (AG-4), and YN=yellow nutsedge. Means followed by the same letter do not differ significantly as determined by Fisher’s protected least significant difference test (P#0.05).No letters indicate non-significant difference. a -91- Table 8. Population densities of Pythium spp., Rhizoctonia spp., and Fusarium spp. and viability of Phytophthora capsici, Fusarium solani, Rhizoctonia solani, and yellow nutsedge in soil on November 20th after treatment with alternatives to methyl bromide in August, 2001 Treatment Rate Method of Application 1. Telone II EC 18 gal/A Inject through drip tape 2064 b 19.2 b 0.0 2. In-Line 35 EC 20.5 gal/A Inject through drip tape 1152 c 6.4 cd 0.0 3. In-Line 35 EC 35 gal/A Inject through drip tape 6128 a 9.6 bcd 0.0 4. Telone C-35 35 gal/A Chisel Injection 1840 c 0.0 d 0.0 5. In-Line 35 EC 20.5 gal/A Inject through drip tape 5392 a 7.2 bcd 0.0 37.5 gal/A Inject through drip tape (3 days later) 20.5 gal/A 1552 c 13.6 bc 0.0 37.5 gal/A Inject through drip tape simultaneously (mix together in tank) 200 lbs ai/A Chisel Injection 1328 c 0.0 d 0.0 + Metam Sodium 6. In-Line 35 EC + Metam Sodium 7. Methyl Bromide Fusarium Fungal populations a Pythium 8. Non-Treated Control N/A -----------4784 ab 56.0 a Total populations of Fusarium spp., Pythium spp., and Rhizoctonia spp. expressed as the number of colony forming units per gram of soil. Means followed by the same letter do not differ significantly as determined by Fisher’s protected least significant difference test (P#0.05). No letters indicate non-significant difference. a -92- Rhizoctonia 0.03 EFFECTS OF VYDATE AND TELONE TREATMENTS FOR MANAGEMENT OF NEMATODES, DISEASES, AND YIELD OF CUCUMBER A. S. Csinos, Phytopathologist, Department of Plant Pathology, University of Georgia, Tifton Campus K. W. Seebold, Plant Pathologist, Department of Plant Pathology, University of Georgia, Tifton Campus P. Timper, Crop protection and Management Research Unit, USDA-ARS Tifton, GA R. F. Davis, Crop protection and Management Research Unit, USDA-ARS Tifton, GA J. E. Laska, Agricultural Research Coordinator, Plant Pathology University of Georgia, Tifton Campus Introduction Methyl bromide is a broad-spectrum biocide and is an important management tool for controlling nematodes, soilborne diseases and other pests in a wide range of crops including vegetable crops. The developing vegetable industry in Georgia is valued at over $635 million. By the year 2005 all uses of methyl bromide in the United states are scheduled to be terminated. Methyl bromide production and importation will be reduced from 1991 levels as follows: 25% in 1999, 50% in 2001, 70% in 2003, and 100% in 2005. Therefore, alternative pest management strategies must be developed before 2005. The objective of this study was to determine the effects of soil chemical treatments on a susceptible cultivar of cucumber on yield, nematodes, and residual effects on cucumber. Materials and Methods Cucumber Crop Field plots were established 8 August 2001 at the Blackshank Research Farm in Tifton Georgia, and maintained through November 2001 on a Tifton loamy sand (fine-loamy, siliceous thermic Plinthic Kandiudults: 85% sand, 10% silt, 5% clay; 0.5% organic matter; pH 5.4). The plots were naturally infested with Meloidogyne incognita, Patatrichodorus, Helicotylenchus, and Pythium spp. The experiment was a randomized block design with drip tape irrigation and chemical treatments injected with chemical injection rigs and replicated five times. Initially, the soil was disc-harrowed, plowed 10 to 12 inches deep with a moldboard plow and shaped into beds 30 inch wide and 8 inches high. Five hundred pounds per acre of 5-10-15 fertilizer was applied by broadcast to all plots and incorporated approximately four inches deep with a tractor-powered rototiller. Whole-plots were 30 inches wide and 25 feet long. All chisel injected treatments, were injected on 9 August. The Vydate treatments were applied during the growing season starting at planting and as follows. On 6 September the first Vydate (2qt/A) injection treatment, numbers 4 and 5, was applied through the drip tape. Total injection time 2 hours for 1/3 bed coverage (2.7ml per bed for 10 beds = 27.0 ml in 2 gal H2O). On 20 September the second Vydate (2qt/A) injection treatment, numbers 4, 5, 6, and 7, was applied through the drip tape. -93- Total injection time 3 hours for 1/2 bed coverage (2.7 ml per bed for 20 beds = 54.0 ml in 3 gal H2O). On 4 October the third Vydate (2qt/A) injection treatment, numbers 4, 5, 6, 7, and 8, was applied through drip tape. Total injection time 6 hours for full bed coverage (2.7 ml per bed for 25 beds = 67.5 ml in 6 gal H2O). On 18 October the forth Vydate (2qt/A) injection treatment, numbers 6, 7, and 8, was applied through drip tape. Total injection time 6 hours for full bed coverage (2.7 ml per bed for 15 beds = 40.5 ml in 6 gal H2O). On 1 November the fifth and final Vydate (2qt/A) injection treatment, number 8, was applied through drip tape. Total injection time 6 hours for full bed coverage (2.7 ml per bed for 5 beds= 13.5 ml in 6 gal H2O). Non-treated plots served as controls. The irrigation drip tubing, Toro “Aquatraxx” brand, with emitters spaced 12 inches apart, each emitter delivering 0.30 gallons per hour at 10 psi of pressure, was placed in the center of all beds as they were covered with 3 mil white polyethylene. Thirteen days after the last chisel injected treatment, 5 September, holes (2 inches diam.) were cut 18 inches apart in single rows using a mechanical-hand transplanter and a single greenhouse-grown cucumber seedling, cultivar “General Lee”, was planted in each hole per bed. The total number of living plants per plot was recorded on 17 September. A vigor rating was done on 27 September, with 1 to10 scale, 10 representing live and healthy plants and 1 representing dead plants. All cucumbers were hand-harvested, each harvest was separated into marketable and cull, counted, and weighed. There were a total of four harvests, they were as follows; 15, 24, and 31 October, and a final on 7 November. All plots were sprayed with Manex + Zinc (1.8 qts./A) and Kocide LF (5 pts./A) on a 7to 10- day schedule for foliage disease control and Ambush (10 oz./A), alternating with Pounce 3.2 (6 oz./A) for insect control on a 7 to 10 day interval. As per the recommendation of the University Of Georgia Extension service, all plots received liquid fertilizer (Miracle-Gro 15-3015) injected through the irrigation tubing, at a rate of 12 pounds of nitrogen per acre, per week through the growing season. Twenty cores of soil, 2.5-cm-diam. × 25-cm-deep, were collected from each row of each subplot on 5 September and 15 November. Soil cores were mixed, and nematodes were extracted from a 150-cm3 sub-sample with centrifugal flotation (Jenkins, 1964). Following final harvest on 14 November, ten plants were dug from each row and rated for root galling by M. incognita on a 1 to 5 scale: 1 = 0%, 2 = 1% to 25%, 3 = 26% to 50%, 4 = 51% to 75%, 5 = 76% to 100% roots galled (Barker et al., 1986). All data collected was analyzed with an analysis of variance (P = 0.05) and means were separated using Duncan’s Multiple range test. Two different injection rigs were used in this study. The first injector, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable products. The second injection rig, referred to as the Yetter rig, it is an injection unit designed for deep chemical injection 10-14 inches deep. It also is equipped with large 36 in. round disks which slice through field debris and trash such as old plastic and drip tape left behind after previous film mulch plantings. -94- Results and Discussion Methyl bromide and all treatments including Telone II suppressed root-knot nematode populations (Table 1) and root galling (Table 2) better than the control (treatment 1) or treatments receiving only Vydate (treatments 5 and 7). Vigor ratings (Table 2), cumulative weight of marketable cucumbers (Table 3), and cumulative number of marketable cucumbers (Table 4) generally were lower with treatments that had higher root-knot nematode populations and gall ratings. The combination of Telone II chiseled in, plus Vydate applied through drip tape provided yields equivalent to the methyl bromide standard (tables 3-6). Methyl bromide, chisel-injected at 200 lb a.i./A, was the only treatment to reduce populations of Fusarium spp. in soil samples taken prior to transplanting. By late November, Fusarium populations remained lowest in methyl bromide-fumigated plots and in those injected with Vydate at 2 qt./A at 2, 4, and 6 weeks after planting. The greatest reductions of Pythium spp. prior to transplanting were seen in plots treated with methyl bromide, Telone II (18 GPA), Telone II (18 GPA) followed by Vydate (2 qt/A) at 0, 2, and 4 weeks after transplanting, Vydate (2 qt/A) applied at 2, 4, and 6 weeks after transplanting, and Telone II (18 GPA) followed by Vydate (2 qt/A) applied at 4, 6, and 8 weeks after transplanting. A similar pattern was seen in samples taken in late November; however, Vydate applied alone at 2, 4, and 6 weeks after transplanting did not reduce Pythium populations as compared to the untreated check. Populations of Rhizoctonia spp. were lowest in methyl bromide-fumigated plots and in those injected with Vydate at 2 qt./A at 2, 4, and 6 weeks after planting. No differences were seen between any treatment and the untreated check in samples taken at the end of the season. Acknowledgments The authors wish to thank Unessee Hargett, Don Hickey, Lewis Mullis, Tonya Jo Cravens, Brandi Hogan, Bryan Horten, Eduvina McDonald, Donnie Starling, Matt McKinnon, Thomas H. Hilton, David L. Clements, Billy Wilson, and A. Kyle Monfort for technical support, Cindy H. LaHue for typing. Also, DuPont Chemical for financial support. -95- Table 1. Effects of soil chemical treatments on nematode population densities in cucumbers 2001a Treatment 1. Control a b Rate N/A Method of Applicationc Nematodes per 150-cm3 soil 5th September P.m. C.o. 15th M.i. November P.m. M.i.b C.o. 68 4 32 132 a 8 0 0 4 0 0b 2 0 12 0 64 10 b 6 0 36 0 16 6b 2 0 ------------------ 2. Methyl Bromide 98% 200lbs ai/A Chisel Inject (shallow application at 8") 3. Telone II 18 gal/A W/ Yetter Rig application at 12") 4. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") + Vydate 2qts/A 0, 2 & 4 weeks post plant 5. Vydate 2qts/A 0, 2 & 4 weeks post plant 12 8 16 140 a 2 0 6. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 12 0 48 0b 2 0 + Vydate 2qts/A 2, 4 & 6 weeks post plant 7. Vydate 2qts/A 2, 4 & 6 weeks post plant 236 16 20 160 a 4 0 8. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 252 20 20 24 b 6 0 + Vydate 2qts/A (deep 4, 6 & 8 weeks post plant Data are means of five replications. M.i. = Meloidogyne incognita, P.m. = Paratrichodorus, C.o. = Criconemoides. Chisel injected treatments were injected on 9 August, the Vydate treatments were applied through the drip tape as follows; 6 September (delivery time 2 hrs), and 20 September (delivery time 3 hrs), 4 October (delivery time 6 hrs), and 18 October (delivery time 6 hrs), and the final on 1 November (delivery time 6 hrs). Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant. c -96- Table 2. Effects of soil chemical treatments on root-gall indices, Vigor and stand count of cucumbers - 2001a Treatment 1. Control Rate N/A Method of Application Root-gall index b 14 November Vigor Rating c September 27 Stand count d September 17 ------------------ 4.4 a 8.4 ab 17 2. Methyl Bromide 98% 200lbs ai/A Chisel Inject (shallow application at 8") 1.3 b 9.4 a 17 3. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 1.7 b 7.8 ab 17 4. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 1.4 b 8.4 ab 17 + Vydate 2qts/A 0, 2 & 4 weeks post plant 5. Vydate 2qts/A 0, 2 & 4 weeks post plant 3.7 a 8.4 ab 17 6. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 1.3 b 9.0 a 17 + Vydate 2qts/A 2, 4 & 6 weeks post plant 7. Vydate 2qts/A 2, 4 & 6 weeks post plant 4.7 a 7.0 b 17 8. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 1.6 b 9.4 a 17 + Vydate 2qts/A 4, 6 & 8 weeks post plant Data are means of five replications. Root Gall Index 1-5 scale: 1 = no galls, 2 = 1% to 25%, 3 = 26% to 50%, 4 = 51% to 75%, and 5 = 76% to 100% of roots galled. c Vigor was done a 1-10 scale with 10= live and healthy plants and 1=dead plants. d Maximum number of plants per plot = 36. Means in the same column followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate nonsignificant difference. a b -97- Table 3. Effect of soil chemical treatments on marketable yield of cucumbers -2001a Treatment 1. Control a Rate N/A Method of Application ------------------ Weight (lbs.) of marketable cucumbers per plot (35 lin. ft. row) November Total October 15 24 31 7 Mean 9.1 c 50.5 ab 11.0 ab 6.5 cd 77.2 bc 2. Methyl Bromide 98% 200lbs ai/A Chisel Inject (shallow application at 8") 20.4 a 58.7 a 15.8 ab 10.2 abc 105.2 a 3. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 16.2 ab 45.1 ab 17.9 ab 8.3 bcd 87.4 abc 4. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 15.2 abc 46.1 ab 19.3 a 12.8 a 93.4 ab + Vydate 2qts/A 0, 2 & 4 weeks post plant 5. Vydate 2qts/A 0, 2 & 4 weeks post plant 13.2 bc 39.2 b 13.7 ab 6.7 cd 72.8 cd 6. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 16.6 ab 55.9 a 17.8 ab 12.3 ab 102.5 a + Vydate 2qts/A 2, 4 & 6 weeks post plant 7. Vydate 2qts/A 2, 4 & 6 weeks post plant 3.1 d 40.5 b 10.0 b 4.1 d 57.7 d 8. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 14.5 abc 50.5 ab 16.0 ab 9.8 abc + Vydate 2qts/A 4, 6 & 8 weeks post plant 90.8 abc Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. Table 4. Effect of soil chemical treatments on marketable yield of cucumber - 2001a -98- Treatment 1. Control a Rate N/A Method of Application Number of marketable cucumber per plot (35 lin. ft. row) October November Total 15 24 31 7 Mean ------------------ 21.6 c 64.4 abc 21.0 ab 13.8 bcd 120.8 b 2. Methyl Bromide 98% 200lbs ai/A Chisel Inject (shallow application at 8") 39.6 a 79.2 a 33.4 a 20.8 ab 173.0 a 3. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 36.3 ab 62.3 abc 34.3 a 19.0 abc 151.8 a 4. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 31.8 abc 60.4 bc 34.8 a 23.8 a 150.8 a + Vydate 2qts/A 0, 2 & 4 weeks post plant 5. Vydate 2qts/A 0, 2 & 4 weeks post plant 24.8 bc 52.2 c 27.6 ab 13.0 cd 117.6 b 6. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 36.4 ab 74.6 ab 35.2 a 22.2 a 168.4 a + Vydate 2qts/A 2, 4 & 6 weeks post plant 7. Vydate 2qts/A 2, 4 & 6 weeks post plant 7.8 d 53.6 c 16.8 b 8.2 d 86.4 c 8. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 31.0 abc 66.8 abc 29.8 ab 21.4 a 149.0 a + Vydate 2qts/A 4, 6 & 8 weeks post plant Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. -99- Table 5. Effect of soil chemical treatment on cull yield of cucumbers - 2001a Treatment 1. Control Rate N/A Method of Application ------------------ Weight (lbs.) of cull cucumbers per plot (35 lin. ft. row) October November 15 24 31 7 Total Mean 0.0 1.6 0.7 1.0 b 3.3 abc 2. Methyl Bromide 98% 200lbs ai/A Chisel Inject (shallow application at 8") 0.0 0.4 0.6 1.2 b 2.2 c 3. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 0.0 0.9 1.6 1.9 ab 4.4 ab 4. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 0.1 0.9 0.7 2.9 a 4.6 a + Vydate 2qts/A 0, 2 & 4 weeks post plant 5. Vydate 2qts/A 0, 2 & 4 weeks post plant 0.1 0.5 1.2 1.2 b 2.9 bc 6. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 0.1 0.6 0.9 1.5 b 3.1 abc + Vydate 2qts/A 2, 4 & 6 weeks post plant 7. Vydate 2qts/A 2, 4 & 6 weeks post plant 0.0 0.3 1.4 1.8 ab 3.5 abc 8. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 0.1 1.0 0.9 2.2 ab 4.1 ab + Vydate 2qts/A 4, 6 & 8 weeks post plant a Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non- significant difference. -100- Table 6. Effect of soil chemical treatment on cull yield of cucumbers - 2001a Treatment 1. Control Rate N/A Method of Application ------------------ Number of cull marketable cucumbers per plot (35 lin. ft. row) October November Total 15 24 31 7 Mean 0.0 1.2 3.4 4.2 b 8.8 abc 2. Methyl Bromide 98% 200lbs ai/A Chisel Inject (shallow application at 8") 0.0 0.8 2.2 3.4 b 6.4 c 3. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 0.0 1.5 5.5 5.3 ab 12.3 ab 4. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 0.4 1.2 2.6 9.0 a 13.2 a + Vydate 2qts/A 0, 2 & 4 weeks post plant 5. Vydate 2qts/A 0, 2 & 4 weeks post plant 0.4 0.8 3.8 3.2 b 8.2 bc 6. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 0.2 1.2 3.8 3.6 b 8.8 abc + Vydate 2qts/A 2, 4 & 6 weeks post plant 7. Vydate 2qts/A 2, 4 & 6 weeks post plant 0.0 0.4 5.6 4.4 b 10.4 abc 8. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 0.2 1.4 2.8 6.4 ab 10.8 abc + Vydate 2qts/A 4, 6 & 8 weeks post plant a Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. Means followed by the same letter do not differ significantly as determined by Fisher’s protected least significant difference test (P#0.05). No letters indicate non- significant difference. -101- Table 7. Population densities of Pythium spp., Rhizoctonia spp., and Fusarium spp. in soil after treatment with alternatives to methyl bromide in August, 2001 (cucumber test). Treatment 1. Control Rate N/A Method of Application ------------------ Fungal populations (9-11-01)a Fusarium Pythium Rhizoctonia Rhizoctonia Fungal populations(11-20-01) Fusarium Pythium 6128 c 36.8 a 0.16 ab 13584 b 25.6 c 0.03 2. Methyl Bromide 98% 200lbs ai/A Chisel Inject (shallow application at 8") 2128 d 4.0 d 0.0 c 8448 c 2.4 e 0.0 3. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 7520 bc 12.0 cd 0.05 bc 13392 b 11.2 de 0.03 4. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 6880 bc 8.0 d 0.05 bc 17408 a 4.0 e 0.03 + Vydate 2qts/A 0, 2 & 4 weeks post plant 5. Vydate 2qts/A 0, 2 & 4 weeks post plant 10784 a 25.6 ab 0.29 a 14944 ab 37.6 b 0.08 6. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 8688 b 24.8 abc 0.0 c 15936 ab 19.2 cd 0.0 + Vydate 2qts/A 2, 4 & 6 weeks post plant 7. Vydate 2qts/A 2, 4 & 6 weeks post plant 7248 bc 21.6 bc 0.05 bc 8544 c 51.2 a 0.03 8. Telone II 18 gal/A W/ Yetter Rig (deep application at 12") 11248 a 12.8 bcd 0.08 bc 14576 ab 5.6 e 0.03 + Vydate 2qts/A 4, 6 & 8 weeks post plant a Total populations of Fusarium spp., Pythium spp., and Rhizoctonia spp. expressed as the number of colony forming units per gram of soil. Means followed by the same letter do not differ significantly as determined by Fisher’s protected least significant difference test (P#0.05). No letters indicate non- significant difference. -102- EVALUATION OF CHISEL PLACEMENT OF TELONE PRODUCTS AND DRIP INJECTION FOR EFFICACY AND YIELD IN BELL PEPPER A. S. Csinos, Phytopathologist, Department of Plant Pathology, University of Georgia, Tifton Campus K. W. Seebold, Plant Pathologist, Department of Plant Pathology, University of Georgia, Tifton Campus P. Timper, Crop Protection and Management Research Unit, USDA-ARS Tifton, GA J. E. Laska, Agricultural Research Coordinator, Plant Pathology University of Georgia, Tifton Campus Introduction Methyl bromide is a broad-spectrum biocide and is an important management tool for controlling nematodes, soilborne diseases and other pests in a wide range of crops including vegetable crops. The developing vegetable industry in Georgia is valued at over $635 million. By the year 2005 all uses of methyl bromide in the United states are scheduled to be terminated. Methyl bromide production and importation will be reduced from 1991 levels as follows: 25% in 1999, 50% in 2001, 70% in 2003, and 100% in 2005. Therefore, alternative pest management strategies must be developed before year 2005. The objective of this study was to determine the effects of soil chemical treatments on a susceptible cultivar of bell pepper on yield, nematodes, and residual effects on bell pepper. Materials and Methods Bell Pepper Crop The study was located at the Blackshank Farm, CPES, Tifton, GA. The area had a history of soybeans, tobacco, and assorted vegetables. The area was prepared using all current University of Georgia Extension Service recommendations. The plot design was a randomized complete block consisting of single bed plots replicated five times. Each plot was 35 feet long. On 27 March, chisel injection treatments, 2, 3, 4a, and 5a were applied to the test plot area as indicated in footnotes of table 1. On 9 April chisel injection treatments 1, 4b, 5b, 6, and 8 were then applied. All plots were then shaped to a 30 in. wide X 8 in. high bed then covered with 6 mil black polyethylene with drip tape in the center of the bed approximately 1in. deep. The final treatment number 7, 1,3-D-C35 “Inline”, an emulsifiable concentrate, was applied through drip tape on 11 April using a Milton Roy injection pump over a 6 hour period at 12 psi pressure with Aquatraxx brand dripline with emitters spaced 12 inches apart, each emitter delivering 0.30 gallons per hour at 10 psi of pressure. Two different injection rigs were used in this study. The first injector, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable products. The second injection rig, referred to as the Yetter rig, it is an injection unit designed for deep chemical injection 10-14 -103- inches deep. It also is equipped with large 36 in. round disks which slice through field debris and trash such as old plastic and drip tape left behind after previous film mulch plantings. Bell pepper, cultivar “Capistrano”, a variety susceptible to M. incognita, seedlings were produced in nutrient tray system to the 4-leaf stage. A single pepper plant was transplanted into holes cut into the center of the plastic bed adjacent to the drip tape on 19 April. Stand counts were made to record live plants on 26 May. A vigor rating was done on 1 June and rated with 1 to 10 scale, 10 representing live and healthy plants and 1 representing dead plants. As per the recommendation of the University Of Georgia Extension service, all plots received 145 lbs. of N, and 145 lbs. of K, in the form of liquid fertilizer (Miracle-Gro15-0-14) injected through the irrigation tubing during the growing season. All pepper plots were sprayed with Manex with Zinc (2.4 qt/A) plus Kocide LF (0.5 gal/A) for control of foliar diseases and Ambush (10 oz./A), alternating with Pounce 3.2 (6 oz./A) for insect control on a 7 to 10 day interval. Twelve cores of soil, 2.5-cm-diam× 25-cm-deep, were collected from each row of each plot on 26 March, 20 April, and 3 August, 2001.Replications for treatments on each data were mixed, and nematodes were extracted from a 150-cm3 sub-sample using a centrifugal flotation technique. All pepper pods were hand-harvested, each harvest was separated into marketable and cull, counted, and weighed. There were a total of six harvests, they were as follows; 26 June, and 5, 11, 18, and 24 July, and a final on 1 August. Following final harvest on 1 August, ten plants were dug from each row and rated for root galling by M. incognita on a 1 to 5 scale: 1 = 0%, 2 = 1% to 25%, 3 = 26% to 50%, 4 = 51% to 75%, 5 = 76% to 100% roots galled. All data collected was analyzed with an analysis of variance (P = 0.05) and means were separated using Duncan’s Multiple range test. Results and Discussion Not all chemical treatments were effective in reducing root gall indices or nematode populations (tables 1-3). Only Telone C-17 plus metam sodium had superior vigour ratings and yield aspects equaled to methyl bromide. Soil populations of Fusarium spp., Pythium spp. and Rhizoctonia spp. at transplanting were significantly reduced by applications of Telone C-17 at 10 GPA in combination with Vapam at 37.5GPA 3 weeks prior to transplanting. Application depth of Telone C-35 did not affect the ability of the fumigant to suppress populations of Fusarium and Rhizoctonia spp.; however, populations of Pythium were higher when Telone C35 was injected at 12 inches compared to 8 inches. No differences in fungal populations were seen between plots treated with Telone II (no chloropicrin) followed by chloropicrin and Telone C-17 followed by chloropicrin. Telone C-35 Inline reduced fungal populations as effectively as methyl bromide. At three months after fumigation, no differences were observed between any treatment and the untreated check with regard to populations of Rhizoctonia spp. All fumigation treatments had significantly lower populations of Pythium spp. than the untreated check. Only those plots treated with Telone C-17 in combination with Vapam and Telone C-35 Inline had Fusarium populations greater than or equal to the untreated check. Telone C-35 Inline was the only treatment to reduce the survival of Fusarium solani, Rhizoctonia solani AG-4, and Cyperus esculentus (yellow nutsedge). No differences in viability of propagules were seen between any fumigation treatment and the untreated check. -104- Acknowledgments The authors wish to thank Unessee Hargett, Don Hickey, Lewis Mullis, Tonya Jo Cravens, Bryan Horten, Eduvina McDonald, Donnie Starling, Matt McKinnon, Thomas H. Hilton, David L. Clements, Billy Wilson, and A. Kyle Monfort for technical support, Cindy H. LaHue for typing. Also, Dow Agrosciences for financial support. -105- Table 1. Effects of soil chemical treatments on nematode population densities in bell pepper 2001a Treatment c Rate Nematodes per 150-cm3 soil Method of Application 26 March P.m. C.o. M.i. 50 14 ab 0 2b 2 0 146 b 0 0 b M.i. 1. Telone C-17 20 April P.m. C.o. 3 August M.i. P.m. C.o. 10 gal/A Chisel Injected 8 in. 37.5 gal/A Rototilled 4 in. 2. Telone C-35 35 gal/A Chisel Injected 8 in. 46 4 ab 0 0b 2 0 28 b 2 0 3. Telone C-35 35 gal/A Yetter d Injected 12 in. 38 4 ab 0 0b 0 0 26 b 2 2 4. Telone II 18 gal/A Yetter Injected 12 in. 38 6 ab 0 0b 4 4 24 b 2 0 9 gal/A Chisel Injected 8 in. 14 days later 21.6 gal/A Yetter Injected 12 in. 20 2b 0 0b 2 2 308 a 0 0 9 gal/A Chisel Injected 8 in. 14 days later 6. Methyl Bromide 89% 200 lb ai/A Chisel Injected 8 in. 10 26 a 0 2b 4 0 50 b 2 0 7. In-Line (C-35 EC) 35 gal/A Through Drip Tape 34 8 ab 0 2b 4 2 8b 0 0 8.Non-Treated Control N/A ------------------------ 36 20 ab 0 8a 4 0 424 a 0 0 + Metam Sodium + Chloropicrin 5. Telone C-17 + Chloropicrin a Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant. b M.i. = Meloidogyne incognita, P.m. = Paratrichodorus, C.o. = Criconemoides c Treatments 2, 3, 4a, and 5a were applied on 27 March, treatments 1, 4b, 5b, 6 and 8 were all applied 14 days later on 9 April. The final treatment 7, “Inline”, an emulsifiable concentrate, was applied through the drip system on 11 April using a Milton Roy injection pump over a 6 hour period at 10 psi pressure with Aquatraxx brand dripline with emitters spaced 12 inches apart, each emitter delivering 0.30 gallons per hour at 10 psi of pressure. d Yetter rig is an injection unit designed for deep chemical injection 10-14 inches deep. It also is equipped with large 36 in. round disks which slice through field debris and trash such as old plastic and drip tape left behind after previous plantings. -106- Table 2. Effects of soil chemical treatments on root-gall indices, Vigor and stand count of bell pepper - 2001a Method of Application Treatment 1. Telone C-17 Rate Root-gall index 3 August 10 gal/A Chisel Injected 8 in. 37.5 gal/A Rototilled 4 in. 2. Telone C-35 35 gal/A 3. Telone C-35 4. Telone II b Vigor Rating 1 June d Stand count c 26 May 3.1 a 9.0 a 36 Chisel Injected 8 in. 1.4 b 6.8 c 35 35 gal/A Yetter Injected 12 in. 1.6 b 7.0 bc 36 18 gal/A Yetter Injected 12 in. 1.6 b 8.8 ab 36 9 gal/A Chisel Injected 8 in. 14 days later 21.6 gal/A Yetter Injected 12 in. 2.8 ab 7.6 abc 36 9 gal/A Chisel Injected 8 in. 14 days later 6. Methyl Bromide 89% 200 lb ai/A Chisel Injected 8 in. 2.0 ab 8.0 abc 36 7. In-Line (C-35 EC) 35 gal/A Through Drip Tape 1.3 b 6.4 c 36 + Metam Sodium + Chloropicrin 5. Telone C-17 + Chloropicrin 8.Non-Treated Control N/A -----------------------3.2 a 6.2 c 36 Data are means of five replications. Means in the same column followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant difference. b Root Gall Index 1-5 scale: 1 = no galls, 2 = 1% to 25%, 3 = 26% to 50%, 4 = 51% to 75%, and 5 = 76% to 100% of roots galled. c Maximum number of plants per plot = 36. d Vigor was done a 1-10 scale with 10= live and healthy plants and 1=dead plants. a -107- Table 3. Effect of soil chemical treatments on marketable yield of bell pepper -2001a Treatment 1. Telone C-17 Rate Method of Application 10 gal/A Chisel Injected 8 in. 37.5 gal/A Rototilled 4 in. 2. Telone C-35 35 gal/A 3. Telone C-35 4. Telone II Weight (lbs.) of June 26 5 marketable bell pepper per plot July 11 18 24 (35 lin. ft. row) August 1 Total Mean 23.9 a 4.4 a 5.2 b 7.0 ab 7.3 b 6.7 54.6 a Chisel Injected 8 in. 10.4 c 1.1 b 4.2 b 3.8 b 5.4 b 5.4 30.3 d 35 gal/A Yetter Injected 12 in. 10.2 c 2.0 ab 4.3 b 6.5 ab 5.3 b 5.6 33.9 cd 18 gal/A Yetter Injected 12 in. 13.9 bc 2.7 ab 6.5 ab 6.1 ab 6.4 b 7.7 43.4 bc 9 gal/A Chisel Injected 8 in. 14 days later 21.6 gal/A Yetter Injected 12 in. 11.4 c 2.2 ab 6.4 ab 5.8 ab 14.0 a 8.4 48.2 ab 9 gal/A Chisel Injected 8 in. 14 days later 6. Methyl Bromide 89% 200 lb ai/A Chisel Injected 8 in. 22.2 ab 4.2 a 9.26 a 5.0 b 6.2 55.9 a 7. In-Line (C-35 EC) 35 gal/A Through Drip Tape 6.1 c 2.5 ab 6.0 ab 4.4 b 5.6 b 8.6 33.1 cd 8.Non-Treated Control N/A ------------------------ 11.2 c 2.5 ab 6.1 ab 6.0 ab 5.1 b 8.5 39.5 bcd + Metam Sodium + Chloropicrin 5. Telone C-17 + Chloropicrin a 8.96 a Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant difference. Table 4. Effect of soil chemical treatments on marketable yield of bell pepper - 2001a -108- Treatment Rate Method of Application Number of marketable bell pepper per plot July June 26 5 11 18 (35 lin. ft. row) August Total 24 1 Mean 80.0 a 15 a 22.6 ab 35.8 ab 41.0 ab 54.2 248.6 a Chisel Injected 8 in. 44.6 b 4.2 b 21.4 ab 20.2 b 30.6 b 34.2 155.2 d 35 gal/A Yetter Injected 12 in. 41.8 b 8.6 ab 18.8 b 30.6 b 29.0 b 41.0 169.8 cd 18 gal/A Yetter Injected 12 in. 49.4 ab 10.6 ab 26.2 ab 32.6 b 34.0 b 54.4 207.2 abc 9 gal/A Chisel Injected 8 in. 14 days later 21.6 gal/A Yetter Injected 12 in. 8.4 ab 27.0 ab 66.6 a 53.8 224.0 ab 9 gal/A Chisel Injected 8 in. 14 days later 28.4 b 6. Methyl Bromide 89% 200 lb ai/A Chisel Injected 8 in. 77.8 a 38.0 a 52.2 a 28.8 b 42.0 253.6 a 7. In-Line (C-35 EC) 35 gal/A Through Drip Tape 26.2 b 9.6 ab 30.2 ab 21.8 b 35.4 b 40.4 163.6 cd 8.Non-Treated Control N/A ------------------------ 43.0 b 10.0 ab 26.6 ab 29.0 b 26.0 b 48.6 183.2 bcd 1. Telone C-17 10 gal/A Chisel Injected 8 in. 37.5 gal/A Rototilled 4 in. 2. Telone C-35 35 gal/A 3. Telone C-35 4. Telone II + Metam Sodium + Chloropicrin 5. Telone C-17 + Chloropicrin 39.8 c a 14.8 a Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant difference. -109- Table 5. Effect of soil chemical treatment on cull yield of bell pepper - 2001a Treatment 1. Telone C-17 Rate Method of Application 10 gal/A Chisel Injected 8 in. 37.5 gal/A Rototilled 4 in. 2. Telone C-35 35 gal/A 3. Telone C-35 4. Telone II Weight (lbs.) of cull bell pepper per plot July June 26 5 11 18 (35 lin. ft. row) August 24 1 Total Mean 0.5 b 0.3 ab 0.8 bc 0.5 ab 1.9 a 1.0 5.0 ab Chisel Injected 8 in. 0.3 b 0.1 ab 0.5 c 0.7 ab 0.7 b 0.4 2.7 b 35 gal/A Yetter Injected 12 in. 0.1 b 0.1 ab 0.1 c 0.4 ab 0.8 b 0.3 1.8 b 18 gal/A Yetter Injected 12 in. 3.0 a 0.6 a 2.0 ab 1.1 ab 0.7 b 1.0 8.2 a 9 gal/A Chisel Injected 8 in. 14 days later 21.6 gal/A Yetter Injected 12 in. 1.6 ab 0.4 ab 2.3 a 1.3 a 1.9 a 0.6 8.1 a 9 gal/A Chisel Injected 8 in. 14 days later 6. Methyl Bromide 89% 200 lb ai/A Chisel Injected 8 in. 1.1 b 0.2 ab 0.8 bc 0.8 ab 0.7 b 0.8 4.4 ab 7. In-Line (C-35 EC) 35 gal/A Through Drip Tape 0.2 b 0.2 ab 0.4 c 0.7 ab 1.1 ab 0.6 3.1 b 8.Non-Treated Control N/A ------------------------ 0.1 b 0.1 b 0.4 c 0.2 b 0.2 b 0.5 1.4 b + Metam Sodium + Chloropicrin 5. Telone C-17 + Chloropicrin a Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non- significant difference. -110- Table 6. Effect of soil chemical treatment on cull yield of bell pepper - 2001a Treatment Rate Method of Application Number of Cull bell pepper per plot (35 lin. ft. row) July June 26 5 11 18 24 August Total 1 Mean 3.4 b 1.4 ab 5.8 abc 4.0 ab 15.4 a 8.0 38.0 ab Chisel Injected 8 in. 1.6 b .4 b 3.8 bc 5.2 ab 4.8 bc 3.2 19.0 bc 35 gal/A Yetter Injected 12 in. .4 b .6 ab 0.8 c 2.2 ab 5.4 bc 1.8 11.2 bc 18 gal/A Yetter Injected 12 in. 16.0 a 3.2 a 12.2 ab 7.2 ab 5.4 bc 7.2 51.2 a 9 gal/A Chisel Injected 8 in. 14 days later 21.6 gal/A Yetter Injected 12 in. 7.8 ab 1.8 ab 13.4 a 9.8 a 12.2 ab 4.0 49.0 a 9 gal/A Chisel Injected 8 in. 14 days later 6. Methyl Bromide 89% 200 lb ai/A Chisel Injected 8 in. 6.0 b 1.0 ab 4.8 abc 6.6 ab 7.0 bc 5.2 30.6 abc 7. In-Line (C-35 EC) 35 gal/A Through Drip Tape 1.0 b 1.0 ab 3.0 bc 5.0 ab 8.8 abc 5.0 23.8 abc 8.Non-Treated Control N/A ------------------------ 1.2 b 0.2 b 2.2 c 1.0 b 1.8 c 3.2 9.6 c 1. Telone C-17 10 gal/A Chisel Injected 8 in. 37.5 gal/A Rototilled 4 in. 2. Telone C-35 35 gal/A 3. Telone C-35 4. Telone II + Metam Sodium + Chloropicrin 5. Telone C-17 + Chloropicrin a Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant difference. -111- Table 7. Population densities of Pythium spp., Rhizoctonia spp., and Fusarium spp., and viability of Pythium irregulare, Fusarium solani, Rhizoctonia solani, and yellow nutsedge in soil 3 weeks after treatment with alternatives to methyl bromide in April, 2001 (pepper test). Treatment 1. Telone C-17 Rate Method of Application 10 gal/A Chisel Injected 8 in. 37.5 gal/A Rototilled 4 in. 2. Telone C-35 35 gal/A Chisel Injected 8 in. 3. Telone C-35 35 gal/A Yetter Injected 12 in. 4. Telone II 18 gal/A Yetter Injected 12 in. 9 gal/A Chisel Injected 8 in. 14 days later 21.6 gal/A Yetter Injected 12 in. 9 gal/A Chisel Injected 8 in. 14 days later 6. Methyl Bromide 89% 200 lb ai/A 7. In-Line (C-35 EC) 8.Non-Treated Control + Metam Sodium + Chloropicrin 5. Telone C-17 + Chloropicrin Fungal populations a Fusarium Pythium Rhizoctonia Pathogen and weed viability b PI FS RS YN 272 d 0d 0b 36 a 100 a 100 a 82 a 992 bc 22 c 0.05 b 38 a 100 a 80 a 74 a 40 b 0.11 b 40 a 100 a 82 a 78 a 960 bc 0d 0b 46 a 100 a 100 a 78 a 464 cd 0d 0.03 b 42 a 100 a 100 a 54 a Chisel Injected 8 in. 608 cd 0.8 d 0.03 b 30 a 100 a 100 a 72 a 35 gal/A Through Drip Tape 720 cd 3.2 d 0b 34 a 72 b 18 b 14 b N/A ------------------------ 64 a 0.45 a 42 a 100 a 86 a 62 a 1536 b 5232 a Means followed by the same letter do not differ significantly as determined by Fisher’s protected least significant difference test (P#0.05). a Total populations of Fusarium spp., Pythium spp., and Rhizoctonia spp. expressed as the number of colony forming units per gram of soil. b Percentage of pathogen-infested grains or yellow nutsedge nutlets that were viable after removal from treated soil prior to transplanting of peppers. PI=Pythium irregulare, FS=Fusarium solani, RS=Rhizoctonia solani (AG-4), and YN=yellow nutsedge. -112- Table 8. Population densities of Pythium spp., Rhizoctonia spp., and Fusarium spp. in soil 3 months after treatment with alternatives to methyl bromide in April, 2001 (pepper test). Treatment 1. Telone C-17 Rate Method of Application 10 gal/A Chisel Injected 8 in. 37.5 gal/A Rototilled 4 in. 2. Telone C-35 35 gal/A Chisel Injected 8 in. 3. Telone C-35 35 gal/A 4. Telone II Fusarium Rhizoctonia 4b 0.03 a 368 cd 0b 0.03 a Yetter Injected 12 in. 176 d 4b 0.05 a 18 gal/A Yetter Injected 12 in. 144 d 2.4 b 0.05 a 9 gal/A Chisel Injected 8 in. 14 days later 21.6 gal/A Yetter Injected 12 in. 208 d 0b 0.03 a 9 gal/A Chisel Injected 8 in. 14 days later 6. Methyl Bromide 89% 200 lb ai/A Chisel Injected 8 in. 608 cd 3.2 b 0.03 a 7. In-Line (C-35 EC) 35 gal/A Through Drip Tape 1824 ab 3.2 b 0.05 a 8.Non-Treated Control N/A ------------------------ 1136 bc 11.2 a 0.11 a + Metam Sodium + Chloropicrin 5. Telone C-17 + Chloropicrin 2160 a Fungal populations a Pythium Means followed by the same letter do not differ significantly as determined by Fisher’s protected least significant difference test (P#0.05). a Total populations of Fusarium spp., Pythium spp., and Rhizoctonia spp. expressed as the number of colony forming units per gram of soil. -113- -114- TELONE C-17, METAM SODIUM COMBINATIONS FOR EFFICACY AND EVALUATION OF PHYTOTOXICITY IN SQUASH A. S. Csinos, Phytopathologist, Department of Plant Pathology, University of Georgia, Tifton Campus K. W. Seebold, Plant Pathologist, Department of Plant Pathology, University of Georgia, Tifton Campus P. Timper, Crop Protection and Management Research Unit, USDA-ARS Tifton, GA J. E. Laska, Agricultural Research Coordinator, Plant Pathology University of Georgia, Tifton Campus Introduction Methyl bromide is a broad-spectrum biocide and is an important management tool for controlling nematodes, soilborne diseases and other pests in a wide range of crops including vegetable crops. The developing vegetable industry in Georgia is valued at over $635 million. By the year 2005 all uses of methyl bromide in the United states are scheduled to be terminated. Methyl bromide production and importation will be reduced from 1991 levels as follows: 25% in 1999, 50% in 2001, 70% in 2003, and 100% in 2005. Therefore, alternative pest management strategies must be developed before year 2005. The objective of this study was to determine how soon greenhouse produced transplants could be safely transplanted into a chemically injected plastic covered bed, also the effects of soil chemical treatments on yield of squash, nematodes, and plant vigour on squash. Materials and Methods Squash Crop The study was located at the Blackshank Farm, CPES, Tifton, GA. The area had a history of soybeans, tobacco, and assorted vegetables. The area was prepared using all current University of Georgia Extension Service recommendations. The plot design was a randomized complete block consisting of single bed plots replicated five times. Each plot was 35 feet long. On 8 March, the first of the chisel injection treatments, 1, 5, 6, and 7 were applied to the test plot area as indicated in tables. On 19 March chisel injection treatment 2 was then applied followed by treatment 3 on 26 March. The final treatment 4 was applied on 9 April. At the time of each injection, the specific plots were then shaped to a 30 in. wide X 8 in. high bed then covered with 6 mil black polyethylene with drip tape in the center of the bed approximately 1in. deep. The injector used in this study, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable soil fumigant products. Squash, cultivar “Prelude”, a variety susceptible to M. incognita, seedlings were produced in nutrient tray system to the 4-leaf stage. A single squash plant was transplanted into holes cut into the center of the plastic bed adjacent to the drip tape on 19 April. Stand counts were made to record live plants on 26 April. Two vigor ratings were done on 25 April and 14 May and rated with 1 to 10 scale, 10 representing live and healthy plants and 1 representing dead plants. -115- As per the recommendation of the University Of Georgia Extension service, all plots received 145 lbs. of N, and 145 lbs. of K, in the form of liquid fertilizer (Miracle-Gro15-0-14) injected through the irrigation tubing during the growing season. All squash plots were sprayed with Manex with Zinc (2.4 qt/A) plus Kocide LF (0.5 gal/A) for control of foliar diseases and Ambush (10 oz./A), alternating with Pounce 3.2 (6 oz./A) for insect control on a 7 to 10 day interval. Twelve cores of soil, 2.5-cm-diam. × 25-cm-deep, were collected from each row of each plot on 8 March, 13 April, and 15 July, 2001. Replications for treatments on each data were mixed, and nematodes were extracted from a 150-cm3 sub-sample using a centrifugal flotation technique. All squash were hand-harvested, each harvest was separated into marketable and cull, counted, and weighed. There were a total of eight harvests, they were as follows;15, 18, 22, 25, 29, May, 1, 5, and a final on 8 June. Following final harvest on 8 June, ten plants were dug from each row and rated for root galling by M. incognita on a 1 to 5 scale: 1 = 0%, 2 = 1% to 25%, 3 = 26% to 50%, 4 = 51% to 75%, 5 = 76% to 100% roots galled. All data collected was analyzed with an analysis of variance (P = 0.05) and means were separated using Duncan’s Multiple range test. Results and Discussion Root gall indices and nematode numbers were high in all treatments including the methyl bromide standard. Vigour ratings were highest in treatments which were allowed to fumigate the longest, suggesting either phytotoxicity or lack of pest control. All treatments were effective in increasing yield over the control and all treatments performed as well as or better than the methyl bromide standard (tables 3-6). In general, soil populations of Pythium spp. and Rhizoctonia spp. at transplanting were significantly reduced by applications of Telone C-17 at 10 GPA in combination with Vapam at 37.5GPA, regardless of the interval between application and transplanting. Total populations of Fusarium spp. were reduced in comparison to the untreated control at all fumigation-transplant intervals except 35 days. Combinations of Telone C-17 plus Vapam, and chloropicrin plus Vapam, performed as well as methyl bromide in reducing populations of Pythium spp. and Rhizoctonia spp. at transplanting. Populations of Pythium spp. and Rhizoctonia spp. remained low for all fumigants three months after transplanting, and were significantly lower than in the untreated controls. However, populations of Fusarium spp. were, for the most part, highest where fumigants had been applied. It is likely that the “biological vacuum” created by soil fumigation created a non-competitive environment for the species of Fusarium present and allowed for rapid population growth. Time of exposure to the combination of Telone C-17 and Vapam affected the survival of Fusarium solani, Rhizoctonia solani AG-4, and Cyperus esculentus (yellow nutsedge). Propagules of these organisms were least viable when exposed to the Telone/Vapam combinations for 22 and 35 days. Viability of propagules was higher as the exposure interval decreased, indicating that longer (> 3 weeks) periods of exposure are needed for maximum efficacy. -116- Acknowledgments The authors wish to thank Unessee Hargett, Don Hickey, Lewis Mullis, Tonya Jo Cravens, Bryan Horten, Eduvina McDonald, Donnie Starling, Matt McKinnon, Thomas H. Hilton, David L. Clements, Billy Wilson, and A. Kyle Monfort for technical support. Also Cindy H. LaHue for typing. -117- Table 1. Effects of soil chemical treatments on nematode population densities in Prelude Yellow Crook Neck Squash 2001c Treatment / Rate Method b / Time before Planting a M.i. 1. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 2. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 3. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 4. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 5. Vapam, 37.5gal/A + Chloropicrin, 9 gal/A 6. Methyl Bromide 98%, 200 lbs. ai/A 8 March P.m. C.o. Nematodes per 150-cm3 soil 13 April 15 July M.i. P.m. C.o. M.i. P.m. C.o. Chisel Injected 8" / 35 days rototilled 4" to 6" 106 a 2 0 8 0 0 110 0 0 44 ab 8 0 2 0 0 28 0 0 20 b 2 0 2 0 0 74 0 0 24 b 0 0 2 0 0 24 6 0 22 b 12 0 2 2 0 48 0 0 16 b 6 0 6 0 0 94 4 0 Chisel Injected 8" / 22 days rototilled 4" to 6" Chisel Injected 8" / 17 days rototilled 4" to 6" Chisel Injected 8" / 5 days rototilled 4" to 6" rototilled 4" to 6" Chisel Injected 8" / 35 days Chisel Injected 8" / 35 days 7. Non-treated Control NA / 35 days 20 b 0 0 2 0 0 144 0 0 M.i. = Meloidogyne incognita, P.m. = Paratrichodorus, C.o. = Criconemoides b The injector used in this study, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable soil fumigant products. a c Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. indicate non-significant. -118- No letters Table 2. Effects of soil chemical treatments on root-gall indices, Vigor and stand count of Prelude Yellow Crook Neck Squash- 2001e Treatment / Rate 1. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 2. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 3. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 4. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 5. Vapam, 37.5gal/A + Chloropicrin, 9 gal/A Method d / Time before Planting Stand count b 26 April Root-gall index a 18 June Vigor Ratingc 25 April Vigor Ratingc 14 May 4.8 a 9.0 a 9.4 a 36 4.3 ab 6.4 b 6.6 b 36 3.5 b 6.2 b 5.2 bc 36 3.6 b 3.8 b 4.4 c 36 4.9 a 9.2 a 10.0 a 36 Chisel Injected 8" / 35 days rototilled 4" to 6" Chisel Injected 8" / 22 days rototilled 4" to 6" Chisel Injected 8" / 17 days rototilled 4" to 6" Chisel Injected 8" / 5 days rototilled 4" to 6" rototilled 4" to 6" Chisel Injected 8" / 35 days 6. Methyl Bromide 98%, 200 lbs. ai/A Chisel Injected 8" / 35 days 4.6 a 8.8 a 5.0 bc 36 7. Non-treated Control NA / 35 days 4.9 a 8.6 a 4.0 c 36 a Root Gall Index 1-5 scale: 1 = no galls, 2 = 1% to 25%, 3 = 26% to 50%, 4 = 51% to 75%, and 5 = 76% to 100% of roots galled. Maximum number of plants per plot = 36. c Vigor was done a 1-10 scale with 10= live and healthy plants and 1=dead plants. d The injector used in this study, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable soil fumigant products. e Data are means of five replications. Means in the same column followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant difference. b -119- Table 3. Effect of soil chemical treatments on marketable yield of Prelude Yellow Crook Neck Squash -2001b Treatment / Rate 1. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 2. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 3. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 4. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 5. Vapam, 37.5gal/A + Chloropicrin, 9 gal/A a Method / Time before Planting Chisel Injected 8" / 35 days rototilled 4" to 6" Chisel Injected 8" / 22 days rototilled 4" to 6" Chisel Injected 8" / 17 days rototilled 4" to 6" Chisel Injected 8" / 5 days rototilled 4" to 6" rototilled 4" to 6" Chisel Injected 8" / 35 days 15 Number of marketable Yellow Crook Neck Squash plot (35 lin. ft. row) May _______ June______ 18 22 25 29 1 5 8 Total Mean 38.8 ab 27.2 a 21.4 ab 21.4 ab 25.4 a 20.6 ab 21.6 a 21.6 a 198.0 ab 26.0 bc 24.8 a 19.0 ab 19.2 ab 20.8 abc 20.6 ab 23.8 a 20.6 a 174.8 bc 18.0 c 20.4 ab 14.0 bc 17.4 b 19.4 abc 14.4 bc 23.0 a 19.6 ab 146.2 cd 2.8 d 14.4 b 19.0 ab 15.0 bc 15.8 c 17.4 abc 26.4 a 13.4 bc 124.2 d 50.0 a 28.4 a 27.2 a 26.6 a 23.0 ab 26.4 a 26.0 a 14.6 abc 222.2 a 6. Methyl Bromide 98%, 200 lbs. ai/A Chisel Injected 8" / 35 days 27.4 bc 21.4 ab 14.0 bc 13.6 bc 17.2 bc 22.0 ab 21.2 a 10.4 c 147.2 cd 7. Non-treated Control NA / 35 days 15.8 cd 13.2 b 5.4 c 8.2 c 18.6 bc 10.4 c 13.2 b 11.0 c 95.8 e a The injector used in this study, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable soil fumigant products. b Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. Table 4. Effect of soil chemical treatments on marketable yield of Prelude Yellow Crook Neck Squash - 2001b -120- Treatment / Rate 1. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 2. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 3. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 4. Telone C-17, 10 gal/A + Vapam, 37.5gal/A a Method / Time before Planting Chisel Injected 8" / 35 days 15 Weight (lbs.) of marketable Yellow Crook Neck Squash plot (35 lin. ft. row) May June 18 22 25 29 1 5 8 ___ Total Mean 15.2 a 9.6 a 8.3 ab 6.0 ab 10.5 a 8.9 ab 11.7 ab 8.9 ab 77.7 a 6.1 b 5.9 b 4.8 bc 4.9 abc 7.6 b 7.2 abc 11.4 ab 9.3 a 57.0 b 3.6 bc 4.4 bc 3.6 c 4.2 bcd 6.3 bc 4.1 cd 9.1 abc 7.5 ab 43.0 c 0.4 c 2.7 c 3.9 c 2.8 cd 4.5 c 5.5 bcd 14.1 a 5.5 bc 39.4 c 18.6 a 10.4 a 10.4 a 6.6 a 8.1 ab 10.7 a 11.4 ab 6.4 abc 82.6 a rototilled 4" to 6" Chisel Injected 8" / 22 days rototilled 4" to 6" Chisel Injected 8" / 17 days rototilled 4" to 6" Chisel Injected 8" / 5 days rototilled 4" to 6" 5. Vapam, 37.5gal/A rototilled 4" to 6" + Chloropicrin, 9 gal/A Chisel Injected 8" / 35 days 6. Methyl Bromide 98%, 200 lbs. ai/A Chisel Injected 8" / 35 days 6.6 b 5.4 bc 3.6 c 2.4 cd 4.7 c 7.4 abc 8.9 bc 4.1 c 43.1 c 7. Non-treated Control NA / 35 days 3.3 bc 2.6 c 1.1 c 2.0 d 5.9 bc 3.1 d 4.5 c 3.7 c 26.2 d a The injector used in this study, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable soil fumigant products. b Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. -121- Table 5. Effect of soil chemical treatment on cull yield of Prelude Yellow Crook Neck Squash - 2001b Treatment / Rate 1. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 2. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 3. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 4. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 5. Vapam, 37.5gal/A + Chloropicrin, 9 gal/A Method a / Time before Planting 15 May 18 Number of Cull Yellow Crook Neck Squash plot (35 lin. ft. row) June Total 22 25 29 1 5 8 Mean Chisel Injected 8" / 35 days rototilled 4" to 6" 1.0 0.0 b 0.0 0.8 0.2 1.2 0.2 1.8 a 5.2 abc 1.0 0.2 ab 0.8 1.6 0.2 0.0 0.6 1.6 ab 6.0 ab 0.0 0.2 ab 0.0 1.0 1.6 0.0 0.2 0.4 ab 3.4 bc 0.6 0.6 ab 1.0 1.0 1.0 0.2 0.6 0.0 b 5.0 abc 0.6 0.8 ab 1.8 1.0 2.2 1.0 0.2 0.8 ab 8.4 a Chisel Injected 8" / 22 days rototilled 4" to 6" Chisel Injected 8" / 17 days rototilled 4" to 6" Chisel Injected 8" / 5 days rototilled 4" to 6" rototilled 4" to 6" Chisel Injected 8" / 35 days 6. Methyl Bromide 98%, 200 lbs. ai/A Chisel Injected 8" / 35 days 0.0 1.0 a 1.0 1.8 0.2 0.2 1.0 1.2 ab 6.4 ab 7. Non-treated Control NA / 35 days 0.0 0.0 b 0.2 0.6 0.6 0.0 0.6 0.0 b 2.0 c a The injector used in this study, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable soil fumigant products. b Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non- significant difference. -122- Table 6. Effect of soil chemical treatment on cull yield of Prelude Yellow Crook Neck Squash - 2001b Treatment / Rate 1. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 2. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 3. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 4. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 5. Vapam, 37.5gal/A + Chloropicrin, 9 gal/A 6. Methyl Bromide 98%, 200 lbs. ai/A Method a / Time before Planting 15 Weight (lbs) of Cull Yellow Crook Neck Squash plot (35 lin. ft. row) May June Total 18 22 25 29 1 5 8 Mean Chisel Injected 8" / 35 days rototilled 4" to 6" 0.14 0.0 b 0.0 0.06 0.02 0.2 a 0.04 0.24 5.2 abc 0.08 0.02 b 0.1 0.1 0.02 0.0 b 0.04 0.2 6.0 ab 0.0 0.06 ab 0.0 0.06 0.12 0.0 b 0.08 0.06 3.4 bc 0.06 0.12 ab 0.14 0.14 0.14 0.04 b 0.14 0.0 5.0 abc 0.12 0.22 a 0.32 0.2 0.12 0.06 b 0.02 0.26 8.4 a 0.0 0.14 ab 0.16 0.1 0.02 0.02 b 0.08 0.22 6.4 ab Chisel Injected 8" / 22 days rototilled 4" to 6" Chisel Injected 8" / 17 days rototilled 4" to 6" Chisel Injected 8" / 5 days rototilled 4" to 6" rototilled 4" to 6" Chisel Injected 8" / 35 days Chisel Injected 8" / 35 days 7. Non-treated Control NA / 35 days 0.0 0.0 b 0.02 0.12 0.1 0.0 b 0.04 0.0 2.0 c a The injector used in this study, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable soil fumigant products. b Data are means of five replications. Means followed by the same letter are not different (P = 0.05) according to Duncan’s multiple range test. No letters indicate non-significant difference. -123- Table 7. Population densities of Pythium spp., Rhizoctonia spp., and Fusarium spp., and viability of Pythium irregulare, Fusarium solani, Rhizoctonia solani, and yellow nutsedge in soil 3 weeks after treatment with alternatives to methyl bromide in April, 2001 (Prelude Yellow Crook Neck Squash). d Treatment / Rate 1. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 2. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 3. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 4. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 5. Vapam, 37.5gal/A + Chloropicrin, 9 gal/A 6. Methyl Bromide 98%, 200 lbs. ai/A Method b / Time before Planting Chisel Injected 8" / 35 days Fusarium Fungal populations a Pythium Rhizoctonia PI Pathogen and weed viability c FS RS YN 528 ab 4.0 b 0.03 b 0b 48 b 10 c 0c 112 cd 0.8 b 0.05 b 0b 20 b 0c 0c 320 bc 0b 0b 0b 64 ab 52 ab 10 c 32 d 0b 0b 0b 64 ab 86 a 68 b 16 d 6.4 b 0.05 b 0b 18 b 0c 0c 16 d 0b 0.08 b 0b 100 a 20 bc 2c rototilled 4" to 6" Chisel Injected 8" / 22 days rototilled 4" to 6" Chisel Injected 8" / 17 days rototilled 4" to 6" Chisel Injected 8" / 5 days rototilled 4" to 6" rototilled 4" to 6" Chisel Injected 8" / 35 days Chisel Injected 8" / 35 days 7. Non-treated Control NA / 35 days 608 a 140 a 0.5 a 80 a 100 a 74 a 88 a Total populations of Fusarium spp., Pythium spp., and Rhizoctonia spp. expressed as the number of colony forming units per gram of soil. b The injector used in this study, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable soil fumigant products. c Percentage of pathogen-infested grains or yellow nutsedge nutlets that were viable after removal from treated soil prior to transplanting of peppers. PI=Pythium irregulare, FS=Fusarium solani, RS=Rhizoctonia solani (AG-4), and YN=yellow nutsedge. d Data are means of five replications. Means followed by the same letter do not differ significantly as determined by Fisher’s protected least significant difference test (P#0.05). a -124- Table 8. Population densities of Pythium spp., Rhizoctonia spp., and Fusarium spp. in soil 3 months after treatment with alternatives to methyl bromide in April, 2001 (Prelude Yellow Crook Neck Squash.)c Treatment / Rate Method b / Time before Planting Fungal populations a Pythium Rhizoctonia 8672 bc 4.8 b 0b 9408 ab 2.4 b 0b 11488 a 0.8 b 0b 9840 ab 0.8 b 0b 11056 a 2.4 b 0b 10960 ab 2.4 b 0b Fusarium 1. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 2. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 3. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 4. Telone C-17, 10 gal/A + Vapam, 37.5gal/A 5. Vapam, 37.5gal/A + Chloropicrin, 9 gal/A 6. Methyl Bromide 98%, 200 lbs. ai/A Chisel Injected 8" / 35 days rototilled 4" to 6" Chisel Injected 8" / 22 days rototilled 4" to 6" Chisel Injected 8" / 17 days rototilled 4" to 6" Chisel Injected 8" / 5 days rototilled 4" to 6" rototilled 4" to 6" Chisel Injected 8" / 35 days Chisel Injected 8" / 35 days 7. Non-treated Control NA / 35 days 6496 c 45.6 a 0.08 a Total populations of Fusarium spp., Pythium spp., and Rhizoctonia spp. expressed as the number of colony forming units per gram of soil. b The injector used in this study, we refer to as the chisel injector, was specially built for these applications. It has chisel shanks for injecting chemicals 8-10 inches deep and is equipped with a combination rototiller for applying chemicals such as metam sodium in combination with injectable soil fumigant products. c Data are means of five replications. Means followed by the same letter do not differ significantly as determined by Fisher’s protected least significant difference test (P#0.05). a -125- Effects of Tomato Spotted Wilt Virus on Plant Growth, Yield and Fruit Quality of Drip-Irrigated Tomato Plants Juan C. Diaz-Perez, Dean Batal, Denne Bertrand and David Giddings Dept of Horticulture, Coastal Plain Experiment Station, Tifton, GA 31794 Introduction Tomato spotted wilt virus (TSWV) can cause serious damage to tomato, pepper, lettuce and other crops. The virus is transmitted by several species of thrips. Because the virus has wide host range, it is difficult to eliminate the disease. Chemical control of the vector is often used as a means to reduce the incidence of TSWV. However, chemical control may not fully control TSWV. Besides excessive use of pesticides increases the cost of production and may have an impact on the environment. Effective strategies of disease management require an understanding of the relationship between the host (tomato plant) and the vector (thrips). The objective of this study was to determine the effect of the time after transplanting when TSWV symptoms first appeared on tomato plant size, fruit yield and fruit quality. Materials and Methods This study was conducted at the Horticulture Farm, Coastal Plain Experiment Station, Tifton, Georgia during the spring of 1999. Tomato (cv. ‘Florida-47',Asgrow) plants were dripirrigated and planted over black plastic mulch. Fumigation, fertilization and irrigation were performed according to standard practices (University of Georgia, Extension Service). Six-week old transplants were planted in 0.9-m beds, formed on 1.8-m centers. Transplants were planted in a single row per bed and a 60-cm spacing between transplants. Determination of TSWV incidence Tomato plants acquired TSWV under conditions of natural infection. During the season, there was a high incidence of thrips and TSWV in the region. Individual plants were visually monitored every 4-7 d for TSWV symptoms for the whole season, and the date when each plant first showed TSWV symptoms was determined. The presence of TSWV in symptomatic plants was confirmed through ELISA. The percent of asymptomatic plats was not determined. Determination of TSWV severity in fruit Severity of TSWV symptoms in fruit was determined visually based on a 5-point scale (see below). Fruit were graded according to USDA standards. Those fruit with a TSWV score of < 3 were considered marketable. Effect of TSWV on quality of ripe fruit -126- Fruit from individual plants were harvested and graded. Marketable fruit with TSWV severity of # 3 were allowed to ripen at 20 °C (68 °F). After ripening, fruit were graded again for TSWV severity. TSWV Severity Scale in Fruit 1 = NO SYMPTOMS. 2 = MILD. Small specks or irregular discoloration in <10% fruit surface; fruit surface free from scars or other defects. 3 = MODERATE. More than 10% fruit surface with specks or patches of different skin color; <10% fruit surface with minor scars on fruit surface). 4 = MODERATELY SEVERE. More than 10% fruit surface with specks or patches of different skin color and scars; concentric rings in the skin (bull’s eye); moderate fruit deformation. 5 = SEVERE. Fruit are small, highly deformed with abundant necrotic area. Results and Discussion Dynamics of TSWV symptoms appearance The first plants with TSWV symptoms where found 28 DAT. The incidence of TSWV was close to 100% by 70 DAT. High populations of thrips were detected since early stages of plant development, which were probably associated to the high incidence of TSWV. Effect of TSWV on plant growth Plant fresh weight was higher the later in plant development TSWV symptoms first appeared. Plants infected 30 DAT reached a final weight of 0.5 kg, while those infected 70 DAT weighed about 5.0 kg (Fig. 1). Plant leaf area was also positively correlated with the time of first symptom expression (data not shown). Effect of TSWV on fruit yield The time of first symptom expression was also positively correlated with total fruit yield (Fig. 1), marketable yield, and fruit number and size (Table 1). This decrease in fruit number and size associated to early TSWV infection was probably a result of a reduced capacity of the infected plants to produce enough photosynthates to sustain fruit growth. Effect of TSWV on fruit quality after ripening Fruit from TSWV-infected plants ripened abnormally, showing irregular red color development of the skin. TSWV scores after ripening were 3.15, 3.34, and 3.57 for fruit previously graded as 1, 2, and 3 respectively. This indicates that fruit from TSWV-infected plants with no apparent TSWV symptoms before ripening, may not ripen properly during storage or at the retail level and thus be of poor quality. Conclusions -127- TSWV infection was deleterious to plant growth and fruit production. Plants were susceptible to damage from TSWV infection from transplanting to harvest. The impact of TSWV on plant growth and fruit yield was more severe the sooner after transplanting tomato plants first showed TSWV symptoms. Presence of TSWV in the plant was associated with a reduction in fruit quality (fruit deformation and irregular fruit ripening). Irregular ripening was observed in several mature-green fruit with no TSWV symptoms at harvest time. Table 1. Correlation coefficients (r) for the relationships of time (DAT) for TSWV symptom expression with various tomato plant growth and fruit yield attributes (n = 214 plants). Plant attribute r p>F 0.805 < 0.0001 Total 0.798 < 0.0001 Marketable Large Medium Small Culls 0.713 0.640 0.512 0.457 0.756 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 0.824 0.704 0.223 0.640 0.523 0.477 0.741 < 0.0001 < 0.0001 0.021 < 0.0001 < 0.0001 < 0.0001 < 0.0001 Plant growth Plant fresh weight Number of fruit per plant Fruit yield per plant Total Marketable Extra-large Large Medium Small Culls -128- Plant fresh weight (Kg) 6 y = 0.0885x - 1.7484 2 R = 0.648 5 4 3 2 1 0 0 20 40 60 80 40 60 80 Fruit yield per plant (kg) 12 y = 0.2679x - 8.8358 2 R = 0.679 10 8 6 4 2 0 0 20 Time of TSWV symptom expression (DAT) Figure 1. Effect of time of TSWV symptom expression on tomato plant weight and fruit yield. -129- Evaluation of Fungicides and Biological Control Materials for Control of Cercospora Leaf Spot of Turnip D. B. Langston, Jr.1, R. T. Boland, Jr.2, J. G Price3 Cercospora Leaf Spot; Cercospora brassicicolaUniv. of Georgia Coop. Ext. Ser., Depts. 1Plant Pathology, Tifton, GA 31793, 2Brantley Co. CEC, Nahunta, GA 31553 and Camden Co. CEA, Woodbine, Ga, 31569 Introduction Leaf spot diseases directly affect leafy green crops because the plant part that is marketed is the part that is affected. According to the U.S. Department of Agriculture standards for mustard and turnip greens, leaves with more than 10% of the surface area discolored are unsalable. Also, most leafy greens are harvested mechanically by once-over cutting which means that all foliage must be removed. Hand separation of unacceptable leaves and decayed tissue is time-consuming and expensive. Fungicides that can be used to control foliar diseases of leafy greens are few. Benlate had been an excellent option for control of Cercospora leaf spot of turnip greens and has been used under a Section 24C in several southeastern states. DuPont has recently voluntarily removed Benlate from the market within the past 2 years. Maneb has a Section 24C state label for Georgia and Tennessee for leafy greens. However, Maneb is only partially effective in controlling these leaf spot diseases under high disease pressure. Quadris has recently received a full federal label for use on leafy greens and has been shown to be an excellent fungicide for control of many foliar pathogens. However, fungicide resistance to Quadris is becoming more of a problem and there exists no effective rotational fungicide partners in leafy greens to aid in resistance management. New, effective fungicide options must be labeled soon to provide adequate protection for leafy green production in the southeast. Materials and Methods Turnip seed were planted in 36 in rows at 4 lb/A on 24 Oct in Hortense, GA. Standard practices for management of fertility, weeds, nematodes and insects were followed throughout the season. The experiment utilized a randomized complete block design with 4 replications. Each fungicide plot consisted of two 15-ft long rows that utilized a 3-ft buffer zone between plot ends. Foliar fungicide treatments were initiated at the first symptoms of disease (26 Nov). Fungicides were applied using a CO2-pressurized backpack sprayer calibrated to deliver 40 gal/A at 75 psi through TX-18 hollow cone nozzles. Results -130- Weather during the experiment was warm and very dry with rainfall accumulations during the test period of only 0.92 in. Treatments that significantly reduced leaf spot compared to the non-treated check on the 04 Dec rating date were both BAS500 treatments, BAS516, Quadris at 5.0 fl oz/A, and Benlate (Table 1). All fungicide treatments significantly reduced leaf spot by 17 Dec. No phytotoxicity was observed with any of the treatments. -131- Table 1. Leaf Spot Ratings2 Treatment, rate/A and spray timing1 04 Dec 17 Dec BAS500 20WG, 0.75 lb/A (2, 4) . . . . . . . . . . . . . . . . . . 5.5 d3 2.0 e BAS500 20WG, 1.0 lb/A (2, 4) . . . . . . . . . . . . . . . . . . . 4.5 d 2.3 e BAS516 38WG, 0.66 lb/A (2, 4) . . . . . . . . . . . . . . . . . 7.8 cd 4.8 de Quadris 2.08F, 5.0 fl oz/A (2, 4) . . . . . . . . . . . . . . . . . 14.0 b-d 13.8 cd Quadris 2.08F, 8.0 fl oz/A (2, 4) . . . . . . . . . . . . . . . . . 19.0 a-c 15.0 cd Benlate 50WP, 0.5 lb/A (2, 4) . . . . . . . . . . . . . . . . . . . . 9.3 cd 9.4 de Bravo Weatherstik 720F, 1.5 pt/A (2, 4) . . . . . . . . . . . 26.5 a 23.8 c Maneb 75DF, 2.0 lb/A (2, 4) . . . . . . . . . . . . . . . . . . . . 24.0 ab 35.0 b Serenade 10WP, 4.0 lb/A (2, 4) . . . . . . . . . . . . . . . . . . 21.3 ab 45.0 b Non-treated check . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30.0 a 60.0 a 1 Spray dates are as follows: 1=26 Nov; 2=4 Dec. 2 Percent leaf area affected by leaf spot. 3 Means in columns with letter(s) in common are not significantly different according to Fisher’s Protected LSD test at P -132- Evaluation of Fungicides and Spray Programs for Control of Gummy Stem Blight of Watermelon. D. B. Langston, Jr. and K.W. Seebold, University of Georgia Dept. of Plant Pathology, CPES, Tifton, GA 31793 Introduction Gummy stem blight, caused by the fungus Didymella bryoniae is the most widespread and destructive disease of watermelon in Georgia. Management options for this disease are rotation, deep turning diseased tissue, avoiding irrigating that prolongs leaf wetness, and preventive fungicide applications. Of these management options, preventive fungicide applications is the most effective. Fungicides labeled for control of gummy stem blight are primarily ethylenebisdithiocarbamates (Dithane, Maneb, Manzate, Penncozeb, ect), chlorothalonil (Bravo, Echo, Equus), benomyl (Benlate), thiophanate methyl (Topsin M), and just recently, the strobilurin azoxystrobin (Quadris). Benomyl or thiophanate methyl tank-mixed with EBDC’s and alternated with chlorothalonil products had proven to offer good control of gummy stem blight until resistance to the benzimidazoles (benomyl and thiophanate methyl) was observed in the early 1990's. Chlorothalonil products have shown good efficacy on gummy stem blight but are not used because they have been implicated in causing phytotoxicity to mature watermelon rinds. Azoxystrobin was shown to have excellent efficacy on gummy stem blight by several researchers in the early 1990's and was granted Section 18 emergency exemption status in Georgia in 1997 and 1998 specifically for gummy stem blight control. A full Section 3 national label was granted for azoxystrobin use on the cucurbit crop grouping in March of 1999 which led to the widespread and routine use of the fungicide to control a broad spectrum of foliar cucurbit diseases. However, resistance to azoxystrobin in gummy stem blight has been documented across Georgia. Studies that evaluate the efficacy of new and emerging products along with the strobilurins are needed to develop effective spray programs for watermelons. Materials and Methods Watermelon seed (Citrullus lanatus ‘Stargazer’) were planted on 6 Jun in a bare-ground field every 42 in. rows spaced 6-ft apart. Experimental plots were 30-ft long, utilized 10-ft borders between plot ends, and were arranged in a randomized complete block with 4 replications. Each treated plot was alternated with a non-treated row. Standard practices for management of fertility, weeds, nematodes and insects were followed throughout the season. Fungicides were applied using a CO2-pressurized backpack sprayer calibrated to deliver 40 gal/A at 40 psi through TX-26 hollow cone nozzles. Plots were harvested on 13 Sep. Results Rainfall accumulation from Jun through Sep was 4.1 in. above normal, which was conducive to disease development. Gummy stem blight was first observed on 8 Aug and completely defoliated some plots by 4 Sep. Bravo Weatherstik alone, Folicur applied alone at 8.0 oz/A, Folicur applied -133- at 8.0 oz/A alternated with Bravo Weatherstik, and TD 2435-01 applied at the 1.75 lb/A rate significantly reduced gummy stem blight severity on 4 Sep and the AUDPC compared to the nontreated check (Table 1). Folicur at 8.0 oz/A was the only treatment to significantly improve yield over the non-treated check. -134- Table 1. Gummy Stem Blight2 AUDPC3 Treatment, rate/A, spray timing1 Yield lbs/plot Bravo Weatherstik 720 6F, 2.0 pt/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 78.8 d4 39.7 h 67.9 a-c Folicur 3.6F, 6.0 fl oz/A + Induce, 6.4 fl oz/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . 97.8 ab 49.7 e-h 45.2 c-h Folicur 3.6F, 8.0 oz/A + Induce, 6.4 fl oz/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . 86.0 b-d 40.3 h 74.5 a Folicur 3.6F, 6.0 fl oz/A + Induce, 6.4 fl oz/A (1, 4, 6) Bravo Weatherstik 720 F, 2.0 pt/A (2, 5) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 90.0 a-d 42.7 gh 51.6 a-e Folicur 3.6F, 8.0 fl oz/A + Induce, 6.4 fl oz/A (1, 4, 6) Bravo Weatherstik 720 F, 2.0 pt/A (2, 5) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 83.3 cd 41.4 h 69.0 ab Dithane Rainshield 75DF, 3.0 lb/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99.5 a 60.5 b-f 45.4 b-h Topsin M 70WP, 0.5 lb/A, (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 97.8 a 64.2 a-d 31.3 d-i Topsin M 70WP, 0.5 lb/A + Dithane Rainshield 75DF, 2.0 lb/A (1, 4, 6) Bravo Weatherstik 720 6F, 2.0 pt/A (2, 5) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 97.0 ab 61.3 b-e 43.9 d-h TD 2435-01 90DF, 0.75 lb/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99.5 a 62.7 a-e 49.6 b-f TD 2435-01 90DF, 1.25 lb/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 96.0 ab 60.6 b-f 47.0 b-g TD 2435-01 90DF, 1.75 lb/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 82.3 cd 40.3 h 51.1 a-e Switch 62.5WG, 13.5 oz/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 90.0 a-d 48.6 e-h 54.0 a-d Nova 40WP, 5.0 oz/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 98.7 a 52.6 d-h 52.2 a-e Flint 50WG, 2.0 oz/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 96.0 ab 62.6 a-e 22.7 hi Flint 50WG, 4.0 oz/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99.3 a 70.4 a-c 26.2 f-i BAS500 20WG, 12.0 oz/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99.0 a 65.5 a-d 25.4 g-i -135- Table 1. continued Gummy Stem Blight AUDPC Yield lbs/plot BAS500 20WG, 12.0 oz/A (1, 4, 6) Bravo Weatherstik 720 6F, 2.0 pt/A (2, 5) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 93.0 a-c 46.8 f-h 41.9 d-h Quadris 2.08SC, 12.3 fl oz/A (1, 2, 4, 5, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 100.0 a 70.7 a-c 25.8 g-i Quadris 2.08SC, 12.3 fl oz/A (1, 4, 6) Bravo Weatherstik 720 6F, 2.0 pt/A (2, 5) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99.5 a 71.5 ab 46.7 b-g Quadris 2.08SC, 6.2 fl oz/A + Actigard 50WG, 0.5 oz/A (1, 6) Bravo Weatherstik 720 6F, 2.0 pt/A (4) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99.5 a 69.0 a-c 25.8 g-i Quadris 2.08SC, 6.2 fl oz/A + Actigard 50WG, 0.5 oz/A (1, 8) Bravo Weatherstik 720 6F, 2.0 pt/A (5) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99.0 a 60.1 b-f 41.4 d-h Quadris 2.08SC, 9.2 fl oz/A + Actigard 50WG, 0.5 oz/A (1, 4) Bravo Weatherstik 720 6F, 2.0 pt/A (3, 6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 100.0 a 60.0 b-f 29.1 e-i Quadris 2.08SC, 9.2 fl oz/A + Actigard 50WG, 0.5 oz/A (1, 6) Bravo Weatherstik 720 6F, 2.0 pt/A (4) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99.3 a 75.9 a 35.9 d-h Quadris 2.08SC, 6.2 fl oz/A + Actigard 50WG, 0.5 oz/A (1, 4, 6) Bravo Weatherstik 720 6F, 2.0 pt/A + Actigard 50WG, 0.5 oz/A (2, 5) . . . . . . . . . . . . . . 100.0 a 61.7 b-e 13.9 i Quadris 2.08SC, 6.2 fl oz/A + Actigard 50WG, 0.5 oz/A (1, 4) Switch 62.5WG, 13.5 oz/A (2, 5) Bravo Weatherstik 720 6F, 2.0 pt/A (6) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 100.0 a 62.2 a-e 41.4 d-h Non-treated check . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99.8 a 56.6 c-g 47.4 b-g Treatment, rate/A, and spray timing 1 Fungicide treatments were applied on the following dates: 1=23 Jul; 2=31 Jul; 3=3 Aug; 4=8 Aug; 5=14 Aug; 6=21 Aug; 7=23 Aug; and 8=28 Aug. 2 Percentage of leaf area affected by gummy stem blight on 4 Sep. 3 Area under the disease progress curve for gummy stem blight calculated from four weekly evaluations beginning on 8 Aug to 4 Sep. -136- 4 Means in columns with letter(s) in common are not significantly different according to Fisher’s protected LSD test at P Evaluation of Actigard, Messenger, and Vacciplant for Suppression of Bacterial Spot of Tomato 1 D. B. Langston, Jr.1, and J. T. Flanders2, University of Georgia Coop. Ext. Ser., Dept. Plant Pathology, Tifton, GA 31793 and 2Grady Co. CEC., Cairo, GA 31728 Introduction Bacterial spot, caused by the bacterium Xanthomonas campestris pv. vesicatoria, is one of the most troublesome diseases of tomato in Georgia. Warm weather coupled with wet conditions are favorable for development of this disease. Standard control practices are using disease-free seed and transplants, avoiding working in wet foliage, and weekly copper sprays. However, severe losses to bacterial spot are still observed despite these measures. Part of the problem is resistance of the bacterium to copper. Copper tank-mixed with maneb or mancozeb has helped overcome some of the resistance problem but some isolates have become less sensitive to this strategy as well. New biological control materials and plant activators have shown promise against bacterial pathogens in other systems. This study was conducted to determine the effectiveness of those compounds when added to a normal copper + maneb spray program. Materials and Methods A commercial tomato field in Grady Co. GA was chosen for this study. Tomato plants (Lycopersicon esculentum “Florida 47”) were treated in the field with Actiguard, Messenger and Vacciplant using a CO2 pressurized backpack sprayer calibrated to deliver 40 GPA at 75 psi. Plots were arranged in a randomized complete block design, replicated 4 times, were 50-ft long × 6-ft apart, contained approximately 25 plants/plot. Results The weather during the experiment was warm and dry but was favorable enough to support a natural epidemic of bacterial spot in the field. Actigard at 0.75 oz/acre was the only treatment to significantly reduce the severity of bacterial spot by the 9 September rating date. No differences in yield or disease severity were noted in later assessments. -137- Treatment, rate/A and spray timing1 Bacterial Spot Ratings2 9/09 9/27 Bacterial Spot No.3 9/27 Total4 Yield Actigard, 0.75 oz/A (1, 3, 5, 7) . . . . . . . . . . . . 5.0 b3 40.0 a 35.5 a 109.3 a Messenger, 30 ppm, (1-10) . . . . . . . . . . . . . . 11.3 a 57.5 a 48.0 a 104.1 a Vacciplant, 12.8 fl oz/A (1, 3, 5, 7) . . . . . . . 15.0 a 47.5 a 46.8 a 106.0 a Vacciplant, 25.6 fl oz/A (1, 3, 5, 7) . . . . . . . . 15.0 a 61.2 a 37.3 a 100.3 a Industry Standard (copper + maneb weekly) . 11.3 a 57.5 a 75.3 a 96.1 a 1 Spray dates are as follows: 1=27 Jul; 2=3 Aug; 3=11 Aug; 4=17 Aug; 5=25 Aug; 6=1 Sep; 7=10 Sep; 8=18 Sep; 9=25 Sep; 10=2 Oct. 2 Percent leaf area affected by leaf spot. 3 Number of spots per 5 leaves per plot. 4 Total marketable yield from 10 Oct, 24 Oct, 21 Nov. 5 Means in columns with letter(s) in common are not significantly different according to Fisher’s Protected LSD test at P=0.05. -138- Evaluation of Fungicides and Biological Control Materials for Control of Powdery Mildew in Transgenic Yellow Crookneck Squash D. B. Langston, Jr.1 and W. T. Kelley2, Univ. of Georgia Coop. Ext. Ser., Depts. 1Plant Pathology and 2Horticulture, Tifton, GA 31793. Introduction Powdery mildew, caused by the fungus Sphaerotheca fuliginea, is on of the most costly diseases of cucurbits both in yield loss and cost of control. Several fungicides have been developed through the years which have been used to suppress losses to powdery mildew but have become ineffective due to a shift towards more aggressive, less fungicide sensitive pathogen populations and because of development of resistance to certain fungicidal modes of action. Cucurbit growers have few fungicide options open to them that provide adequate powdery mildew suppression that can also serve as alternative modes of action for fungicide resistance management. Several fungicides with high levels of efficacy against powdery mildew and with different modes of action are needed to provide adequate disease control and to maintain the effectiveness of powdery mildew fungicides. Materials and Methods Squash seed (‘Destiny III’) were planted on 30 Aug black plastic-mulched beds that had been previously been planted to bell pepper in Tifton, GA. Seed were planted every 18 in. in rows spaced 6 ft apart. Standard practices for management of fertility, weeds, nematodes and insects were followed throughout the season. The experiment utilized a randomized complete block design with 4 replications. Each fungicide plot consisted of a single 15-ft long row that utilized a 3-ft buffer zone between plot ends. Foliar fungicide treatments were initiated at anthesis (27 Sep). Fungicides were applied using a CO2-pressurized backpack sprayer calibrated to deliver 40 gal/A at 75 psi through TX-18 hollow cone nozzles. Results Weather during the experiment was cool with rainfall accumulations from Sep through Oct near the 77 year average of 5.6 in. Powdery mildew was first observed on 10 Oct and increased to high levels by the 26 Oct rating date. Disease assessments on 16 Oct indicated that none of the treatments fungicides significantly reduced powdery mildew severity on the upper leaf surface (ULS) (Table 1). Lower leaf surface (LLS) assessments on 16 Oct indicated that all treatments significantly reduced powdery mildew severity compared to the non-treated check with the exception of the biological material, Serenade, applied at the 2.0 lb/A rate. All fungicides and fungicide/Serenade combinations significantly outperformed Serenade applied alone at 4.0 lb/A. Serenade alternated with Nova significantly reduced disease severity compared to Serenade alternated with Flint. Disease assessments recorded on 26 Oct -139- demonstrated that all fungicide treatments significantly reduced disease on the ULS and LLS except the 2.0 rate of Serenade. Treatments containing Procure, Nova or Flint either alone or in combination demonstrated the greatest levels of control. However, when applied alone, both Procure and Nova significantly outperformed Flint. Nova also significantly outperformed Flint when alternated with Serenade. -140- Table 1. Powdery Mildew Ratings2 16 Oct 26 Oct Treatment, rate/A and spray timing1 ULS LLS ULS LLS Procure 50WS, 6.0 oz/A (1, 3) Flint 50WG, 2.0 oz/A (2, 4) . . . . . . . . . . . . . . . . . . . . . . . . 0.0 a3 3.8 cd 0.0 b 3.1 de Procure 50WS, 6.0 oz/A + Silwet L-77, 0.05% v/v (1, 3) Flint 50WG, 2.0 oz/A (2,4) . . . . . . . . . . . . . . . . . . . . . . . . 0.0 a 3.1 cd 0.0 b 1.8 de Procure 50WS, 8.0 oz/A (1, 3) Flint 50WG, 2.0 oz/A (2, 4) . . . . . . . . . . . . . . . . . . . . . . . 0.0 a 4.0 cd 0.0 b 3.6 de Nova 40W, 5.0 oz/A (1, 3) Flint 50WG, 2.0 oz/A (2, 4) . . . . . . . . . . . . . . . . . . . . . . . . 0.0 a 3.4 cd 0.0 b 2.1 de Procure 50WS, 6.0 oz/A (1, 2, 3, 4) . . . . . . . . . . . . . . . . . 0.0 a 1.0 d 0.0 b 1.0 e Nova 40W, 5.0 oz/A (1, 2, 3, 4) . . . . . . . . . . . . . . . . . . . . 0.0 a 1.0 d 0.0 b 1.0 e Flint 50WG, 2.0 oz/A (1, 2, 3, 4) . . . . . . . . . . . . . . . . . . . . 0.0 a 5.0 cd 0.0 b 15.3 d Serenade 10WP, 2.0 lb/A (1, 2, 3, 4) . . . . . . . . . . . . . . . . . 5.8 a 35.0 a 19.4 a 77.5 a Serenade 10WP, 4.0 lb/A (1, 2, 3, 4) . . . . . . . . . . . . . . . . . 2.5 a 26.3 b 6.5 b 61.3 b Serenade 10WP, 2.0 lb/A (1, 3) Flint 50WG, 2.0 oz/A (2, 4) . . . . . . . . . . . . . . . . . . . . . . . . 0.3 a 9.4 c 6.3 b 42.5 c Serenade 10WP, 2.0 lb/A (1, 3) Nova 40W, 5.0 oz/A (2, 4) . . . . . . . . . . . . . . . . . . . . . . . . . 0.0 a 2.1 d 0.9 b 13.1 de Non-treated check . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.6 a 38.8 a 25.0 a 91.3 a 1 Spray dates are as follows: 1=27 Sep; 2=5 Oct; 3=15 Oct; 4=22 Oct. Percent leaf area affected by powdery mildew on both upper (ULS) and lower (LLS) leaf surfaces. 3 Means in columns with letter(s) in common are not significantly different according to Fisher’s Protected LSD test at P=0.05. 2 -141- Evaluation of Fungicides and Biological Materials for Control of Downy Mildew and Plectosporium Blight of Pumpkin D. B. Langston, Jr. and K.W. Seebold, University of Georgia Dept. of Plant Pathology, CPES, Tifton, GA 31793 Introduction Downy Mildew (Pseudoperonospora cubensis) and Plectosporium blight (Plectosporium tabacinum) are two of the most destructive diseases plauging pumpkin growers in North Georgia. Fungicide trials were conducted at the Mountain Branch Research Station in Blairsville Georgia to evaluate certain fungicides for efficacy against both of these pumpkin diseases. Materials and Methods Pumpkin seed (‘Oz’) were planted on 13 Jun in a bare-ground field every 2 ft in rows spaced 8 ft apart. Experimental plots were 16-ft long and utilized a randomized complete block design with 4 replications. Standard practices for management of fertility, weeds, nematodes and insects were followed throughout the season. Nova 40WP was applied at 5.0 oz/A on 4 and 11 Aug to suppress powdery mildew. Fungicides were applied using a CO2-pressurized backpack sprayer calibrated to deliver 40 gal/A at 40 psi through TX-26 hollow cone nozzles. Plots were harvested on 1 Oct. Results Rainfall accumulation from Jul through Sep was only 0.36 in. below normal. Downy mildew was first observed on 16 Aug and increased rapidly to cause complete defoliation in some plots by harvest. Microdochium blight was observed first on 30 Aug and progressed rapidly until harvest. Microdochium blight severity was significantly reduced compared to the non-treated check by most treatments except the Ranman/Silwet tank-mix, KP 481at 8.0 oz/A tank-mixed with Dithane Rainshield, QRD 282 applied alone, and Aliette. Applications of BAS500, Flint, Quadris and Bravo Weatherstik produced the lowest Microdochium blight ratings. Significant suppression of downy mildew was achieved by all fungicides and fungicide programs compared to the non-treated check except Aliette and two rates of QRD 282 applied alone. The greatest reductions in downy mildew severity and AUDPC were observed in plots treated with BAS500, Ranman, Omega 500, Tattoo C, and KP 481 + Dithane Rainshield. -142- Table 1. Plectosporium Downy blight2 mildew3 Treatment, rate/A, spray timing1 BAS500 20WG, 8.0 oz/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.5 hi5 34.0 g-j BAS 500 20WG, 12.0 oz/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.8 i 19.0 j Flint 50WG, 4.0 oz/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.1 g-i AUDPC4 Yield (tons/A) 17.0 h-l 11.2 b-f 9.1 kl 13.5 a-c 60.0 cd 37.7 de 6.1 h-k Quadris 2.08SC, 15.0 oz/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.1 g-i 51.8 d-f 27.1 e-i 10.7 b-g Acrobat MZ 69WP, 2.25 lb/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.5 d-f 60.0 cd 34.3 d-f 7.4 f-j Gavel 75DF, 2.25 lb/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.9 c-f 51.3 d-g 23.1 f-j 12.3 a-d Ridomil Gold Bravo 76WP, 2.0 lb/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.8 e-g 59.3 cd 28.0 e-i 8.3 e-i Bravo Weatherstik 720F, 2.0 pt/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.1 g-i 62.5 cd 33.6 d-f 10.0 c-h Dithane Rainshield 75DF, 3.0 lb/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.1 f-h 58.3 cd 31.9 d-g 7.1 g-k Omega 500F, 1.0 pt/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.5 d-f 18.8 j 8.9 kl 10.5 b-g Tattoo C, 2.5 pt/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.6 c-f 22.5 ij 10.1 kl 11.2 b-f Ranman 400SC, 2.1 fl oz/A + Silwett L-77, 4:3 Ranman:Silwet (1-7) . . . . . . 8.1 ab 37.8 e-i 17.7 h-l 9.1 d-h Ranman 400SC, 2.75 fl oz/A + Silwett L-77, 4:3 Ranman:Silwett (1-7) . . . . 8.1 ab 34.3 f-j 17.5 h-l 8.5 d-h BAS 500 20WG, 8.0 oz/A (1, 3, 5, 7) Ranman 400SC, 2.1 fl oz/A + Silwett L-77, 4:3 Ranman:Silwet (2, 4, 6) . . . 5.6 c-f 25.3 ij 12.6 j-l 11.1 b-f BAS 500 20WG, 16.0 oz/A (1, 3, 5, 7) Ranman 400SC, 2.75 fl oz/A + Silwett L-77, 4:3 Ranman:Silwet (2, 4, 6) . . 4.1 f-h 16.8 j 7.9 l 14.2 ab BAS 500 20WG, 8.0 oz/A (1, 3, 5, 7) Ranman 400SC, 2.1 fl oz/A + Silwett L-77, 4:3 Ranman:Silwet + Bravo Weatherstik 720F, 1.0 pt/A (2, 4, 6) . . . . . . . . . . . . . . . . . . . . . . . . . 4.4 e-h 27.0 ij 13.4 j-l 15.1 a BAS 500 20WG, 16.0 oz/A (1, 3, 5, 7) Ranman 400SC, 2.75 fl oz/A + Silwett L-77, 4:3 Ranman:Silwet + Bravo Weatherstik 720F, 1.0 pt/A (2, 4, 6) . . . . . . . . . . . . . . . . . . . . . . . . . 2.1 i 21.3 ij 10.3 kl 12.1 a-e KQ 667 68.75WG, 24 oz/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.1 c-e 51.3 d-g 29.0 e-h 8.0 f-i -143- Plectosporium Blight Mildew Table 1. continued Downy Yield AUDPC (tons/A) KQ 667 68.75WG, 32.1 oz/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.6 c-f 45.3 d-h 21.2 g-k 15.4 a KP 481 50WG, 8.0 oz/A + Dithane Rainshield 75DF, 2.0 lb/A (1-7) . . . . . . . 6.9 a-d 52.0 de 28.1 e-i 6.6 h-k KP 481 50WG, 12.2 oz/A + Dithane Rainshield 75DF, 2.0 lb/A (1-7) . . . . . . 5.3 d-f 31.8 h-j 15.9 i-l 9.3 d-h Aliette 80WDG, 3.0 lb/A (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.5 a-c 91.7 a 52.9 bc 4.4 i-k QRD 282 AS, 1% v/v (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.6 ab 88.8 a 60.3 ab 3.4 jk QRD 282 AS, 2% v/v (1-7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.8 a 94.5 a 65.8 a 4.0 jk QRD 282 AS, 1% v/v (1, 3, 5, 7) Ridomil Gold Bravo 76WP, 2.0 lb/A (2, 4, 6) . . . . . . . . . . . . . . . . . . . . . . . . . 6.8 b-d 70.8 bc 43.9 cd 8.2 f-i Non-treated check . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.7 a 87.7 ab 60.7 ab 3.3 k 1 Fungicide treatments were applied on the following dates: 1=24 Jul; 2=31 Jul; 3=8 Aug; 4=16 Aug; 5=22 Aug; 6=30 Aug; and 7=6 Sep. 2 Microdochium blight was evaluated on 12 Sep using a 0-10 scale where 0=no symptoms and 10=total infestation of crowns and stems. 3 Percentage of leaf area with symptoms of downy mildew. 4 Area under the disease progress curve for downy mildew calculated from four weekly evaluations beginning on 22 Aug to 12 Sep. 5 Means in columns with letter(s) in common are not significantly different according to Fisher’s protected LSD test at P=0.05. -144- Evaluation of Fungicides and Protection Intervals for Control of Alternaria Leaf Spot of Carrot Using Two Nitrogen Fertility Regimes D. B. Langston, Jr.1 and J. E. Hudgins 2, Univ. of Georgia Coop. Ext. Ser., Depts. 1Plant Pathology, Tifton, GA 31793. and 2CEA, Decatur Co., Bainbridge , GA 31718 Introduction Carrot production has become very significant in Georgia in recent years. In 2001, approximately 5,328 acres of carrot were grown in Georgia that were valued at 25,796,788. The soil types and climatic conditions in Georgia are conducive for production of high yielding, good quality carrots. Like most vegetable crops, carrots are susceptible to attack by several diseases. The primary disease of carrots in Georgia is Alternaria leaf blight (caused by Alternaria dauci). This disease causes a foliar blight of carrot tops which reduces root size and harvesting efficiency. The main controls for this disease have been rotation, using disease-free seed, and applying fungicides in a preventive manner. Fungicides that are labeled on carrot for suppressing Alternaria leaf blight are chlorothalonil (Bravo, Echo, Equus), azoxystrobin (Quadris), pyraclostrobin (Cabrio), and iprodione (Rovral). It has been hypothesized that overfertilization with nitrogen can cause increased susceptibility of carrot tops to Alternaria leaf blight. Materials & Methods Carrot seed (‘Choctaw’) were planted on 6 Nov 00 on 6-ft beds in Bainbridge, GA. Seed were planted every 1.5 in. in rows spaced 8 in. apart. Standard practices for management of irrigation, weeds, nematodes and insects for carrots grown in Georgia were followed throughout the season. The experiment utilized a split plot design with 4 replications, with fertility treatments as the main plots and fungicide treatments as sub-plots. The standard nitrogen (N) fertility regime received 173 lbs of N/A which consisted of a pre-plant application of 15 lbs/A of 5-20-20 (5 Nov 00), 50 lbs of 14-4-14 (12 Dec 00), 30 lbs/A of 28-0-0-5 (5 Jan 01 and 15 Feb 01), and 48 lbs/A of 28-0-0-5 (8 Mar 01). The high N regime received 223 lb of N/A and differed from the standard only by applying an extra 50 lb/A of 14-4-14 on 23 Jan, 01. Fungicide plots were 20-ft long with a 10-ft buffer between tiers and a 15-ft buffer between replications. Fungicides were applied using a CO2- pressurized backpack sprayer calibrated to deliver 40 gal/A at 75 psi through TX-18 hollow cone nozzles. Results Weather conditions during the experiment were dry with rainfall accumulations of 8.3 in. below the 99 year average for Nov through Apr. The onset of Alternaria leaf blight occurred late and symptom development was not severe until after the last application of fungicide. No significant differences in disease severity were noted between fertility regimes and no significant interactions were detected between fungicide treatment and fertility regime. Therefore fungicide treatments were averaged across fertility regimes. All fungicide treatments significantly reduced disease severity compared to the non-treated check (Table 1). Generally, fungicide programs utilizing the shortest protection interval received more fungicide sprays and had the lower disease ratings for Alternaria leaf spot. -145- Table 1. Alternaria Treatment, rate/A and spray timing1 leaf spot2 Bravo Weatherstik 720 6L, 1.5 pt/A (1-8) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.5 b-f3 Bravo Weatherstik 720 6L, 1.5 pt/A. (1, 3, 5, 7) . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.7 b-e Bravo Weatherstik 720 6L, 1.5 pt/A (1, 4, 7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.8 b-e Bravo Weatherstik 720 6L, 1.5 pt/A (1, 3, 5, 7) Quadris 2.08 SC, 15 oz/A (2, 4, 6, 8) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.1 ef Bravo Weatherstik 720 6L, 1.5 pt/A (1, 5) Quadris 2.08 SC, 15 oz/A (2, 7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.7 b-e Bravo Weatherstik 720 6L, 1.5 pt/A (1, 7) Quadris 2.08 SC 15 oz/A, (4) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.9 b-d Quadris 2.08 SC, 15 oz/A (1-8) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.8 f Quadris 2.08 SC, 15 oz/A (1, 3, 5, 7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.1 bc Quadris 2.08 SC, 15 oz/A (1, 4, 7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.6 b-e Rovral 4F, 1.5 pt/A (1-8) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.4 c-f Rovral 4F, 1.5 pt/A (1, 3, 5, 7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.7 b-e Rovral 4F, 1.5 pt/A (1, 4, 7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.6 b-e Bravo Weatherstik 720 6L, 1.5 pt/A (1, 3, 5, 7) Rovral 4F, 1.5 pt/A (2, 4, 6, 8) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.3 d-f Bravo Weatherstik 720 6L, 1.5 pt/A (1, 5) Rovral 4F, 1.5 pt/A (2, 7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.7 b-e Bravo Weatherstik 720 6L, 1.5 pt/A (1, 7) Rovral 4F, 1.5 pt/A (4) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.3 b Non-treated check . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.2 a 1 Spray dates are as follows: 1=8 Feb; 2=15 Feb; 3=23 Feb; 4=8 Mar; 5=17 Mar; 6=23 Mar; 7=2 Apr; 8=10 Apr. 2 Alternaria leaf blight ratings recorded on 8 May according to a 0-10 scale where 0=no symptoms and 10=total defoliation. 3 Means in columns with letter(s) in common are not significantly different according to Fisher’s Protected LSD test at P=0.05. -146- Screening Fungicides for Control of Phytophthora capsici in Summer Squash Kenneth W. Seebold, Jr., David B. Langston, Jr., and T. Bryan Horten University of Georgia, Dept. of Plant Pathology, Tifton GA Introduction An experiment was conducted at the Blackshank Research Farm in Tifton, GA in a field that had previously been inoculated with Phytophthora capsici to establish a disease nursery. The soil was a Fuquay loamy sand, and was prepared with a rototiller prior to planting. Guidelines established by the University of Georgia Cooperative Extension Service were followed for land preparation, fertility, weed management, and insect control. Summer squash (cv. ‘Dixie’) were planted on 23 April 01 into non-mulched, raised beds with a Stanhay vegetable seeder set to deliver seed every 12 in. Each plot consisted of two 20 ft rows, spaced 36 in. apart, with 5 ft borders between blocks. The experiment was laid out in a randomized complete-block design with 4 replications. The number of plants emerged at 21 days after planting (DAP) was counted immediately prior to initiation of fungicide applications. Fungicides were applied over the rows with a CO2-powered backpack sprayer through a 4-nozzle boom (18 in. spacing) fitted with TSX-26 hollow cone nozzles and set to deliver 40 gallons at 40 psi. Fungicide applications were initiated at 21 DAP and continued weekly until maximum disease incidence was reached in the untreated check (total of 4 sprays). Supplemental overhead irrigation was applied to the test site after planting (0.5 in.), and daily thereafter to achieve optimal moisture conditions for development of disease. Plant mortality was recorded in plots weekly beginning on 8 June 01 and ending on 22 June 01, when maximum mortality was observed in untreated plots. Results and Discussion The combination of warm temperatures, frequent irrigation, and late-season rain resulted in severe damage to fruit by Phytophthora capsici, Pythium spp. (cottony leak), Sclerotium rolfsii, and Choanephora cucurbitarum. Yields were therefore not taken for the experiment. Incidence of crown rot was heaviest at the final evaluation in the untreated checks and in plots treated with Quadris 2.08F (1 pt/A) (Table 1). The combination of Ranman 400SC (2.75 fl oz/A), BAS500 21.9WG (9 oz/A), and Silwet (2.1 fl oz/A) gave significantly better control of Phytophthora crown rot than Ridomil Gold Bravo at 2 lb/A, Ranman 400SC (2.75 fl oz/A) tank-mixed with Penetrator Plus (1 pt/A), and Ranman 400SC (2.75 fl oz/A) plus BAS550 50WP (6.4 oz/A) tank mixed with Silwet (2.1 fl oz/A). No phytotoxicity was observed in the experiment. -147- Table 1. Effect of fungicides on the severity of Phytophthora crown rot in yellow squash, 2001. Final disease Treatment and rate/A (6/22/01)a AUDPCa Ranman 400SC 2.75 fl oz + Silwet 2.1 oz ……………………………… 21.14 cd 0.99 b Ranman 400SC 2.75 fl oz + Penetrator Plus 1 pt ………………………. 49.32 bc 3.38 ab Ranman 400SC 2.75 fl oz + BAS550 50WP 6.4 oz + Silwet 2.1 oz …… 39.95 bc 2.23 ab Ranman 400SC 2.75 fl oz + BAS500 21.9WG 1 lb + Silwet 2.1 oz …… 19.82 cd 0.90 b Ranman 400SC 2.75 fl oz + BAS500 21.9WG 9 oz + Silwet 2.1 oz …... 3.56 d 0.12 b Omega 500F 1 pt ……………………………………………………….. 18.13 cd 0.80 b Ridomil Gold Bravo 76WP 2.0 lb ……………………………………… 41.06 bc 1.99 b BAS 500 21.9WG 1 lb ………………………………………………….. 18.66 cd 1.12 b Quadris 2.08F 1 pt ……………………………………………………… 64.74 ab 5.50 a Ridomil Gold 4EC 1 pt …………………………………………………. 22.87 cd 0.84 b Untreated check ………………………………………………………… 84.95 a 5.44 a Means followed by the same letter do not differ significantly as determined by Fisher’s protected least significant difference test (P#0.05). a Final disease rating (incidence of plants with symptoms of Phytophthora crown rot) taken on 6/22/01; AUDPC=area under the disease progress curve, constructed from evaluations taken on 6/08/01, 6/15/01, and 6/22/01. -148- Evaluation of Experimental Fungicides for the Control of Phytophthora Crown rot of Summer Squash Kenneth W. Seebold, Jr., David B. Langston, Jr., and T. Bryan Horten. University of Georgia, Dept. of Plant Pathology, Tifton GA Introduction An experiment was conducted at the Blackshank Research Farm in Tifton, GA in a field that had previously been inoculated with Phytophthora capsici to establish a disease nursery. The soil was a Fuquay loamy sand, and was prepared with a rototiller prior to planting. Guidelines established by the University of Georgia Cooperative Extension Service were followed for land preparation, fertility, weed management, and insect control. Summer squash (cv. ‘Dixie’) were planted on 23 April 01 into non-mulched, raised beds with a Stanhay vegetable seeder set to deliver seed every 12 in. Each plot consisted of two 20 ft rows, spaced 36 in. apart, with 5 ft borders between blocks. The experiment was laid out in a randomized complete-block design with 4 replications. The number of plants emerged at 21 days after planting (DAP) was counted immediately prior to initiation of fungicide applications. Fungicides were applied over the rows with a CO2-powered backpack sprayer through a 4-nozzle boom (18 in. spacing) fitted with TSX-26 hollow cone nozzles and set to deliver 40 gallons at 40 psi. Fungicide applications were initiated at 21 DAP and continued weekly until maximum disease incidence was reached in the untreated check (total of 4 sprays). Supplemental overhead irrigation was applied to the test site after planting (0.5 in.), and daily thereafter to achieve optimal moisture conditions for development of disease. Plant mortality was recorded in plots weekly beginning on 8 June 01 and ending on 22 June 01, when maximum mortality was observed in untreated plots. Results and Discussion High rainfall, supplemental irrigation, and warm temperatures resulted in high incidence of Phytophthora crown rot in the experiment. Fruit rots caused by Pythium spp. and Choanephora cucurbitarum severe and widespread, resulting in a total loss of yield. All treatments reduced disease incidence early in the course of the epidemic, with KQ 667 68.75WG (2 lb/A), KP 481 (0.5 and 0.75 lb/A) + Dithane 75DF (2 lb/A), and Ridomil Gold 4EC (1 pt/A) giving highest levels of suppression (Table 1). At the final evaluation, no significant differences in disease control were found between any treatment. None of the experimental fungicides performed as well as Ridomil Gold 4EC (1 pt/A). Area under the disease progress curve was lowest for Ridomil Gold 4EC. No phytotoxicity was observed with any fungicide treatment. -149- Table 1. Effects of experimental fungicides on the severity of Phytophthora crown rot on yellow squash, 2001. Treatment and rate/A KQ 667 68.75WG 1.5 lb KQ 667 68.75WG 2.0 lb Percent incidence of diseased plants 6/08/2001 6/22/2001 AUDPCa 33.8 a 92.4 a 10.8 a 1.3 c 84.7 a 8.6 ab KP 481 50WG 0.5 lb + Dithane 75DF 2 lb 9.5 bc 81.8 a 8.6 ab KP 481 50WG 0.75 lb + Dithane 75DF 2 lb 7.6 bc 89.2 a 8.6 ab Ridomil Gold 4EC 1 pt 1.5 c 66.9 a 5.9 b Untreated check 23.8 ab 87.6 a 9.9 a Means followed by the same letter do not differ significantly as determined by Fisher’s protected least significant difference test (P=0.05). a AUDPC=area under disease progress curve, calculated from plant mortality counts made on 08 June, 15 June, and 22 June 01. -150- -151- Insect Control -152- -153- INSECT PEST CONTROL TRIALS IN VEGETABLES IN 2000-2001 David G. Riley Coastal Plain Experiment Station Dept. Entomology, P.O. Box 748 Tifton, GA 31793 Introduction In 2000-2001, several insecticide efficacy trials were conducted to evaluate various chemicals for the control of insect pests of vegetable crops in Tift County, Georgia. The following results summarizes efficacy data for insect pests Which occurred in significant numbers in two snap bean tests, two cabbage tests, a pepper test and a tomato test. Insects cause millions of dollars in damage to Georgia vegetable crops each year and the use of effective insecticides is essential to the short term viability of the vegetable industry. Materials and Methods Evaluation of Insecticide Treatments in Snap Bean Against Whitefly, 2000: Snap beans were planted into two 6-ft beds by 30 ft length on 1 Aug 2000 and maintained with standard cultural practices at the Lang Farm, Georgia Coastal Plain Experiment Station at Tifton. A total of 275 lbs of 10-10-10 and 150 lbs of 34-0-0 were applied to Tift sandy clay loam field plots and irrigation regularly with an overhead sprinkler system. Scouting was initiated on 9 Aug and continued weekly until 27 Sep. Four foliar applications of insecticide were made on 11, 22, 31 Aug and11 Sep and the drench applications of Admire and Platinum were made on 9 Aug. Three 1 mm dia. leaf disks from 3 leaf samples were taken for whitefly egg and nymph counts and 3 randomly sampled leaf turn counts were scouted on 8/21, 8/23, 9/15, 9/27. Eggs, small (1st + 2nd instar), and large (3rd +4th instar) larvae of SLWF were recorded per 0.8 mm2 of leaf area sample. Beans were harvested in a single picking from 5 plants at the center of the 30 ft plot on 28 Sep and were categorized as marketable or unmarketable and the weight of fresh plant tops, cut at ground level, was also recorded. Evaluation of Insecticide Treatments in Snap Bean Against Whiteflies, 2001: Snap beans were planted similar to the 2000 snap bean test. A total of 250 lbs of 5-10-15 were applied to Tift sandy clay loam field plots and irrigation regularly with an overhead sprinkler system. Scouting was initiated on 21 Aug and continued weekly until 25 Sep. Four foliar applications of insecticide were made on 23, 30 Aug and 6, 13 Sep and the drench applications of Admire and Platinum were made on 9 Aug. Three 1 mm dia. leaf disks from 3 leaf samples were taken for whitefly egg and nymph counts and 3 randomly sampled leaf turn counts were scouted on 8/21, 8/28, 9/4, 9/11, 9/18, 9/25. Eggs, small (1st + 2nd instar), and large (3rd +4th instar) larvae of SLWF were recorded per 0.8 mm2 of leaf area sample. Beans were harvested in a single picking -154- from 5 plants at the center of the 30 ft plot on 28 Sep and were categorized as marketable or unmarketable and the weight of fresh plant tops, cut at ground level, was also recorded. Evaluation of Insecticide Treatments in Cabbage, 2000: Cabbage was transplanted into 2 rows per 6-ft beds on 21 Feb 2000. A total of 500 lbs of 10-10-10 was applied to Tift sandy clay loam field plots and irrigation regularly with an overhead sprinkler system. Scouting was initiated on 8 Mar and continued weekly until harvest. Five weekly applications of insecticide were made from 21 Mar to17 May and 1 sample of 6 plants were scouted per plot approximately 24-48 h after weekly applications. Small (1st - 3rd instar) and large (4th - 6th instar) larvae of DBM, CL, and ICW were recorded per 3-plant sample. Cabbage was harvested from 10 ft of row (10 heads) on 25 May and heads and wrapper leaves were categorized as severely, slightly, or not damaged and percent marketable was estimated as the percent of cabbage heads with no damage (% marketable). Damage ratings for cabbage heads (0=none, 3=severe) and weight (lbs) of marketable heads were reported. Data was analyzed using GLM and LSD tests for separation of means (SAS Institute 1985). Evaluation of Insecticide Treatments in Cabbage, 2001: Cabbage was transplanted into 2 rows per 6-ft beds on 21 Feb 2001 and a total of 500 lbs of 10-10-10 was applied to Tift pebbly clay loam field plots and irrigated regularly. Scouting was initiated on 13 Mar and continued weekly until harvest. Seven applications of insecticide were made from 21 Mar to25 May and 1 sample of 6 plants were scouted per plot approximately 48 h after weekly applications. Small (1st - 3rd instar) and large (4th - 6th instar) larvae of DBM, CL, and ICW were recorded per 3-plant sample. Cabbage was harvested from 10 ft of row (10 heads) on 23 May to 4 Jun and heads and wrapper leaves were categorized as severely, slightly, or not damaged and the average weight of heads was measured. Damage ratings for cabbage heads (0=none, 3=severe) and weight (lbs) of marketable heads were reported. Evaluation of Insecticide Treatments in Pepper, 2000: Pepper was transplanted into 2 rows per 6-ft beds on 10 Aug 2000 and maintained with standard cultural practices at the Lang Farm, Georgia Coastal Plain Experiment Station at Tifton. A total of 500 lbs of 8-8-13 per acre was applied to Tift sandy clay loam field plots and irrigation regularly with an overhead sprinkler system. Scouting was initiated in Aug and continued weekly until harvest. Eight applications of insecticide were made from 16 Aug to31 Oct and 1 sample of 6 plants were scouted per plot approximately 24-48 h after weekly applications. Lepidoptera larvae and other insects were recorded per 6-plant sample. Pepper was harvested from 35 ft of row (60-70 plants) on 23 Oct and 16 Nov and fruit were categorized as marketable or unmarketable and all fruit were cut to inspect for pepper weevil larvae or pupae inside the pod. Evaluation of Insecticide Treatments in 2001: Tomato, hyb. Florida 47, was transplanted with 2-ft spacing into 1 row per 6-ft wide beds in 30-ft long plots on 15 Aug and maintained with standard cultural practices at the Georgia Coastal Plain Experiment Station at Tifton. A total of 350 lbs of 8-8-13 was applied to Tift pebbly clay loam field plots and irrigated -155- regularly with drip irrigation system. Scouting was initiated on 5 Sep and continued weekly until harvest. All plots were on metallic-silver mulch (ReflecTek Foils, Inc. Lake Zurich, IL) except treatment 10 which was white-painted plastic mulch. Nine applications of insecticide were made from 31 Aug to 24 Oct and 1 sample of 6 plants were scouted per plot after weekly applications. Tomatoes were harvested from 10 ft of row (5 plants) on 1 Nov and fruit were categorized as marketable or unmarketable (primarily Lepidoptera larval damage) and the average weight was measured. Composite (all species) measurements of Lepitoptera damage were reported. In all of the above tests, each treatment was replicated four times in a randomized complete block design and data were analyzed using ANOVA and LSD tests for separation of means (SAS Institute 1985). Results were summarized in Tables 1-6. Results and Discussion In the 2000 Snap Bean Trial, the lowest number of total whitefly nymphs and adults occurred in the Knack and Knack plus Admire treatments. At the end of the season Knack provided very good control, but there did not appear to be a rate response. The best treatments in terms of percent marketable weight were the Admire plus Warrior/Monitor, Admire plus Knack, high rates of Admire alone, the Warrior/Monitor and low rate of Knack. In the 2001 trial, the best treatments in terms of lowest number of total whitefly nymphs at the end of the season, were the high rate of Knack, Knack plus Admire and Thiodan treatments. Knack provided good control of whitefly nymphs and there appeared to be a slight rate response. In the 2000 Cabbage Trial the best treatments in terms of percent marketable heads were the Novaluron, Spintor-Dipel rotation, Avaunt, Avaunt-Spintor-Dipel rotation, Avaunt-DipelSpintor rotation, and Spintor. In terms of lowest number of total Lepidoptera larvae, the best treatments were the same. Avaunt, Spintor and Novaluron treatments all had the lowest damage to heads. In the 2001 trial, the best treatments in terms of undamaged heads were the Avaunt, Novaluron, Spintor, and Proclaim treatments. These treatments also had the lowest damage to wrapper leaves. In terms of lowest number of total Lepidoptera larvae, the best treatments were Avaunt, Karate, Spintor, Proclaim and Novaluron treatments. Avaunt, Spintor, and Karate treatments significantly reduced small (1st and 2nd instar) diamondback moth compared to the untreated check. The pest pressure from all Lepidoptera larvae was moderate (averaged approximately one per plant over the sampling period in the untreated check). The Pepper Trial treatments that had the lowest cucumber beetle numbers were the high rates of S-1812 plus Orthene and Admire plus Confirm. The treatments that had the lowest pepper weevil infestation were S-1812 at 0.2 lb ai, TD 2344 at 0.03 lb ai, but treatment effects were not significantly different from the untreated check. -156- All of the insecticide treatments in the Tomato Trial treatments provided good Lepidoptera control. The untreated checks were severely damaged by various species of Lepidoptera larvae, producing less than 50% of the yield of the treated plots. The spray treatments that also had the lowest damage from Lepidoptera were the S-1812 combination treatments. In terms of lowest number of total whitefly, all metallic silver mulch treatments had lower numbers compared to the one white mulch check. Also, the white mulch treatment had significantly greater THW larvae. The best treatment in terms of the highest yield was the S1812 plus Asana XL treatment, followed by the high rate of Baythroid, then the S-1812 plus Dipel DF treatment. -157- Table 1a. Snap Bean 2000 trial results. Treatments (application method) Rate lb ai/a SLWF eggs 8/21 SLWF eggs 9/15 Small nymphs 9/15 Large nymphs 9/15 SLWF eggs 9/27 Small nymphs 9/27 Large nymphs 9/27 1. Admire 2SC (drench) 0.25 23.17cd 106.83a 63.17ab 5.92a 43.75cde 84.67a 1.17abc 2. Admire 2SC (drench) 0.375 3.33d 63.17c 24.67cd 0.42b 32.17de 40.50bc 0.92bc 3. Admire 2SC (drench) 0.50 1.67d 74.33abc 8.50de 0.42b 65.58abcd 58.00abc 0.00c 4. Platinum 2SC (drench) 0.14 8.08d 78.67abc 43.17bc 1.50b 30.58de 57.42abc 4.83ab 5. Admire 2SC (drench), 0.25 21.92cd 109.25a 7.25de 0.58b 80.42abc 2.75d 0.00c Knack 0.86 EC (foliar spray) 0.067 6. Warrior 1EC (foliar spray), 0.015 102.25a 88.33abc 34.17c 0.08b 47.75bcd 51.58bc 0.33c Monitor 4 L (foliar spray) 0.75 7. Admire 2SC (drench), 0.25 11.00cd 65.83bc 0.83e 0.08b 9.25e 32.83cd 0.58c Warrior 1EC (foliar spray), Monitor 4 L (foliar spray) 0.015 8. Knack 0.86 EC (foliar spray) 0.054 66.50b 64.08c 10.83de 0.67b 84.58ab 2.00d 0.00c 9. Knack 0.86 EC (foliar spray) 0.067 37.42c 103.00ab 5.17de 1.67b 86.92a 4.92d 0.42c 10. Untreated check - 70.92b 86.00abc 72.25a 2.83ab 40.42de 65.92ab 5.25a 0.75 * Means within columns followed by the same letter are not significantly different (LSD, P<0.05). -158- Table 1b. Snap Bean 2000 trial results. Treatments (application method) Rate lb ai/a SLWF adults 8/21 SLWF adults 8/23 SLWF adults 9/15 SLWF adults 9/27 Bean marketable wt (lb) Cut bean plant top wt (lb) 1. Admire 2SC (drench) 0.25 20.58a 15.08abc 34.58a 0.75bc 0.226b 1.969abc 2. Admire 2SC (drench) 0.375 7.67d 9.92abc 32.58a 1.83abc 0.332ab 2.594a 3. Admire 2SC (drench) 0.50 8.00cd 14.58abc 34.33a 2.33ab 0.273ab 2.594a 4. Platinum 2SC (drench) 0.14 13.67abcd 16.08abc 37.75a 1.33bc 0.177b 2.063abc 5. Admire 2SC (drench), 0.25 16.58ab 18.47ab 44.08a 0.58bc 0.353ab 2.219abc Knack 0.86 EC (foliar spray) 0.067 6. Warrior 1EC (foliar spray), 0.015 15.83abc 9.17cd 38.58a 3.33a 0.275ab 1.875bc Monitor 4 L (foliar spray) 0.75 7. Admire 2SC (drench), 0.25 13.33abcd 4.67d 30.08a 1.17bc 0.496a 2.563ab Warrior 1EC (foliar spray), Monitor 4 L (foliar spray) 0.015 8. Knack 0.86 EC (foliar spray) 0.054 19.67a 21.17a 39.58a 0.00c 0.254ab 1.969abc 9. Knack 0.86 EC (foliar spray) 0.067 9.25bcd 17.08abc 36.17a 0.50bc 0.169b 1.875bc 10. Untreated check - 14.75abcd 17.67abc 30.42a 0.92bc 0.109b 1.594c 0.75 * Means within columns followed by the same letter are not significantly different (LSD, P<0.05). -159- Table 2. Snap Bean 2001 trial results. Treatments (application method) Rate lb ai/a SLWF eggs on 8/28/01 SLWF eggs on 9/11/01 SLWF nymphs on 8/21/01 SLWF nymphs on 9/18/01 SLWF nymphs overall SLWF adults overall 1. Knack 0.86 EC (foliar spray) 0.054 0.38bc 3.08bc 0.21b 0.13b 0.26abc 1.89a 2. Knack 0.86 EC (foliar spray) 0.067 0.96ab 3.38ab 0.21b 0.04b 0.15bc 1.51a 3. Admire 2SC (drench), 0.187 0.75bc 2.92bc 0.00b 0.00b 0.07c 1.57a Knack 0.86 EC (foliar spray) 0.067 4. Admire 2SC (drench) 0.187 1.54a 2.08bc 0.25b 0.67a 0.42ab 1.74a 5. Thiodan 3EC 1.0 0.17c 0.58c 0.21b 0.00b 0.12bc 1.47a 6. Untreated check - 0.54bc 6.00a 0.88a 0.63a 0.52a 1.98a Treatments (application method) Rate lb ai/a SLWF adults on 8/28/01 SLWF adults on 9/04/01 SLWF adults on 9/25/01 Bean marketable wt (lb) Number of light-colored beans 1. Knack 0.86 EC (foliar spray) 0.054 2.21a 2.29a 0.96b 7.3a 5.25a 2. Knack 0.86 EC (foliar spray) 0.067 1.08a 2.67a 0.92b 3.8a 4.25a 3. Admire 2SC (drench), 0.187 1.58a 1.38a 0.08b 1.5a 2.00a Knack 0.86 EC (foliar spray) 0.067 4. Admire 2SC (drench) 0.187 1.42a 2.92a 0.96b 3.8a 2.75a 5. Thiodan 3EC 1.0 1.46a 2.13a 1.21b 11.1a 8.00a 6. Untreated check - 1.63a 2.71a 2.50a 4.1a 7.25a -160- * Means within columns followed by the same letter are not significantly different (LSD, P<0.05). Table 3. Cabbage 2000 trial results. Total Lep. DBM total DBM large CL ICW total Damage rating for heads Damage rating for wrapper leaves Wt. Good Heads per 10 ft of row Percent marketable total 16.Untreated Check 6.50 a 3.19 a 1.81 a 0.31 2.88 a 1.78 a 2.75 a 5.40 f 0.10 f 9.Pounce 3.2EC 0.1 lb ai/a 1.31 b 0.69 b 0.56 b 0.19 0.13 b 0.60 b 1.40 b 25.0 cde 0.50 e 10. Confirm 2F 0.094ai + Ketch Bt 8oz prod 0.88 b 0.44 b 0.13 bc 0.25 0.00 b 0.30 cd 1.20 bc 33.0 cde 0.73 d 3. Proclaim 5SG 0.01 lb ai/a 0.81 b 0.38 b 0.13 bc 0.06 0.25 b 0.25 de 0.98 cd 43.9 ab 0.75 cd 13.RH-2485 80WP 0.10 ai +Ketch Bt 8oz prod 0.81 b 0.50 b 0.25 bc 0.06 0.00 b 0.23 de 0.98 cd 36.1 cd 0.78 bcd 12.RH-2485 80WP 0.05 ai +Ketch Bt 8oz prod 0.69 b 0.56 b 0.31 bc 0.00 0.00 b 0.53 bc 1.33 bc 18.6 ef 0.50 e 11. Confirm 2F 0.125ai +Ketch Bt 8oz prod 0.56 b 0.38 b 0.19 bc 0.00 0.19 b 0.68 b 1.28 bc 24.7 de 0.53 e 8. Spintor 0.067, Dipel 1 0.50 b 0.19 b 0.00 c 0.06 0.13 b 0.05 e 0.48 efg 39.7 bcd 0.95 ab 15. Novaluron 0.833EC 0.08 0.50 b 0.25 b 0.06 bc 0.00 0.25 b 0.15 de 0.63 defg 39.9 bc 0.90 abcd 14. Novaluron 0.833EC 0.04 0.44 b 0.13 b 0.06 bc 0.13 0.06 b 0.03 e 0.78 de 57.3 a 0.98 a Treatment -161- Total Lep. DBM total DBM large CL ICW total Damage rating for heads Damage rating for wrapper leaves Wt. Good Heads per 10 ft of row Percent marketable total 7. Avaunt 0.065, Spintor, Dipel 2 0.44 b 0.19 b 0.13 bc 0.06 0.00 b 0.10 de 0.48 efg 37.5 bcd 0.90 abcd 2.Avaunt 30WDG 0.065 0.44 b 0.13 b 0.13 bc 0.00 0.06 b 0.08 de 0.28 g 40.5 b 0.93 abc 4. Spintor 2SC 0.067 lb ai/a 0.31 b 0.06 b 0.00 c 0.06 0.00 b 0.15 de 0.38 fg 38.5 bcd 0.88 abcd 1. Avaunt 30WDG 0.045 0.25 b 0.06 b 0.06 bc 0.00 0.06 b 0.13 de 0.55 efg 39.5 bcd 0.88 abcd 5. Avaunt 0.065, Dipel, Spintor 3 0.25 b 0.06 b 0.00 c 0.00 0.06 b 0.13 de 0.73 def 40.0 bc 0.90 abcd 6. Avaunt 0.045, Spintor, Dipel 4 0.13 b 0.00 b 0.00 c 0.06 0.06 b 0.15 de 0.70 def 42.8 ab 0.85 abcd Treatment lbs ai/a lbs ai/a * Means within columns followed by the same letter are not significantly different (LSD, P<0.05). SPRAY ROTATIONS: 1 alternate a) and b): a)Spintor 2SC 0.067 lb ai/a, b)Dipel Df 1lb prod/a 2 1 of a),1 of b), 2 of c),1 of a),1 of b),1of a),1of b): a)Avaunt 30WDG 0.065lbs ai/a, b)Spintor 2SC 0.067lb ai/a, c)Dipel Df 1lb prod/a 3 2 of a), 2 of b), 2 of c), then 2 of a): a)Avaunt 30WDG 0.065lbs ai/a, b)Dipel Df 1lb prod/a, c)Spintor 2SC 0.047lb ai/a 4 2 of a), 2 of b), 2 of c), then 2 of a): a)Avaunt 30WDG 0.045lbs ai/a, b)Dipel Df 1lb prod/a, c)Spintor 2SC 0.047lb ai/a -162- Table 4. Cabbage 2001 trial results. small Damage rating for heads Damage rating for wrapper leaves Avg. wt. (lb) heads each 0.14a 0.11ab 1.85a 1.30a 3.87bc 0.25cd 0.08a 0.00b 0.75fgh 0.35efgh 4.55a 0.03c 0.31bcd 0.00a 0.00b 1.20cd 0.90bc 4.30ab 0.78bc 0.00c 0.33bcd 0.06a 0.19a 1.13cde 0.65cde 4.31a 5. Baythroid 20WP - 0.025 lb ai 1.67a 0.28a 0.36bcd 0.14a 0.19a 1.13cde 0.85cd 4.64a 6. Baythroid 20WP - 0.05 lb ai 0.83bc 0.08bc 0.44bcd 0.06a 0.08ab 1.30bc 0.93bc 4.33a 7. Leverage 2.7SC - 0.08 lb ai 1.22ab 0.08bc 0.92a 0.06a 0.00b 1.55ab 1.20ab 3.64c 8. Novaluron 0.833EC - 0.039 ai 0.75bc 0.03c 0.39bcd 0.03a 0.00b 0.22ij 0.13gh 3.47c 9. Novaluron 0.833EC - 0.059 lb ai 1.00ab 0.17ab 0.67abc 0.00a 0.06b 0.43hij 0.20fgh 3.78c 10. Novaluron 0.833EC - 0.078 lb ai 0.94bc 0.03c 0.58abcd 0.11a 0.00b 0.53ghi 0.35efgh 4.70a 11. Karate Z - 0.03 lb ai 0.56bc 0.06bc 0.22d 0.06a 0.00b 0.80efg 0.43efg 4.50a 12. Avaunt 30WDG 0.065 lb ai/a 0.50c 0.05bc 0.25cd 0.08a 0.06b 0.18j 0.10h 4.70a 13. Proclaim 5SG - 0.0075 lb ai 0.833bc 0.00c 0.33bcd 0.03a 0.00b 0.95def 0.48ef 4.38a 14. Proclaim 5SG - 0.01 lb ai 0.67bc 0.08bc 0.36bcd 0.06a 0.03b 0.60gh 0.55de 4.31a CL Treatment - rate per acre Total Lep. DBM ICW total DBM small large 1.Untreated Check 1.19ab 0.05bc 0.69ab 2. Intrepid (RH 2485) 80WP - 0.10 ai/a then Spintor 2SC - 0.067 ai 0.67bc 0.05bc 3. Confirm 2F - 0.125 ai then Spintor 2SC - 0.067 ai 0.801bc 4. Baythroid 2EC - 0.025 lb ai -163- 15. Spintor 2SC - 0.067 lb ai 0.64bc 0.11bc 0.22d 0.03a 0.00b 0.48ghij 0.40efgh 4.64a * Means within columns followed by the same letter are not significantly different (LSD, P<0.05) except for total Lep. (P<0.1). Table 5. Pepper 2001 trial results. Treatments BAW per 6 plants CB per 6 plants PW in harvested fruit 1. Avaunt 30WDG 0.045 lbs ai/a 0.000a 0.03bc 0.25a 2. Avaunt 30WDG 0.065 lbs ai/a 0.000a 0.03bc 0.25a 3. Proclaim 5SG 0.01 lb ai/a 0.031a 0.13ab 0.38a 4. Spintor 2SC 0.067 lb ai/a 0.000a 0.03bc 0.88a 0.000a 0.03bc 0.25a 0.000a 0.00c 0.13a 0.063a 0.09abc 0.13a 0.063a 0.06abc 0.38a 9. TD2344-03 0.83E 0.03 lb ai/a 0.094a 0.03bc 0.00a 10. S-1812 35WP 0.1 lb ai/a 0.000a 0.03bc 0.38a 11. S-1812 35WP 0.15 lb ai/a 0.000a 0.03bc 0.13a 5. Platinum 0.140 lb ai/a drench +Confirm 2F 0.125 ai 6. Admire 2F 0.25 lb ai/a drench +Confirm 2F 0.125 ai 7. Kryocide 96W 7.68 lb ai/a (first 3 sprays then spray TD2344-03 0.83E 0.03 lb ai/a) 8. Kryocide 96W 11.52 lb ai/a (first 2 sprays then spray TD2344-03 0.83E 0.03 lb ai/a) -164- 12. S-1812 35WP 0.2 lb ai/a 0.000a 0.16a 0.00a 0.000a 0.00c 2.38a 0.000a 0.16a 1.63a 15. Orthene 90SP 0.5 lb ai/a 0.000a 0.06abc 0.50a 16. UNTREATED CHECK 0.000a 0.03bc 0.75a 13. S-1812 35WP 0.1 lb ai/a +Orthene 90SP 0.5 lb ai/a 14. S-1812 35WP 0.1 lb ai/a +Orthene 90SP 0.75 lb ai/a * Means within columns followed by the same letter are not significantly using LSD Test (P>0.10) -165- Table 6. Tomato 2001 trial results. SAW larvae overall TFW larvae overall CL larvae overall Total Lep. overall Whitefly adults Predators Treatment - formulation (lb AI/a) THW larvae + eggs overall (mainly spiders) Yield (lbs/5 plants) Lepidoptera Damaged fruit 1. S1812 - 35WP (0.15) 0.13c 0.00b 0.04 0.08b 0.13b 1.21b 0.04ab 25.19abc 13.50bc 2. S1812 - 35WP (0.20) 0.21c 0.00b 0.00b 0.00b 0.08b 1.17b 0.00b 27.99abc 5.50bc 3. S1812 - 35WP (0.15) + Orthene - 97PE (0.63) 0.04c 0.00b 0.00b 0.04b 0.42b 2.58b 0.00b 23.09bc 4.42c 4. S1812 - 35WP (0.15) + Asana XL - 0.66EC (0.02) 0.13c 0.04b 0.00b 0.17b 2.00b 2.46b 0.08ab 36.33a 3.75c 5. S1812 - 35WP (0.15) + Dipel DF (1 lb product) 0.13c 0.00b 0.00b 0.63b 0.63b 1.96b 0.08ab 28.31ab 4.50c 6. V10101 - 2.25 EC (0.18) 0.33c 0.00b 0.00b 0.21b 0.75b 0.96b 0.04ab 26.75abc 7.75bc 7. Baythroid - 2 EC (0.02) 0.21c 0.00b 0.42ab 0.33b 0.42b 2.08b 0.04ab 28.20ab 7.50bc 8. Baythroid - 20WP (0.04) 0.38c 0.00b 0.00b 0.33b 0.88b 1.83b 0.00b 32.34ab 7.75bc 9. Untreated Check (silver mulch) 1.04b 12.25a 0.13a 1.75a 15.08a 2.25b 0.04ab 8.66d 34.75a 10.Untreated Check (white mulch) 1.54c 19.21a 0.13a 1.54a 21.83a 7.88a 0.13a 15.52cd 20.00ab -166- * Means within columns followed by the same letter are not significantly different (LSD, P<0.05). ABBREVIATIONS: Silverleaf whitefly (SLWF); Bemisia argentifolii Bellows&Perring Diamondback moth (DBM); Plutella xylostella (L.) Cabbage looper (CL); Trichoplusia ni (Hübner) Imported cabbageworm (ICW); Artegeia rapae (L.) Pepper weevil (PW); Anthonomus eugenii Beet armyworm (BAW); Spodoptera exigua Cucumber beetles (CB); Diabrotica spp. Beet armyworm (BAW); Spodoptera exigua (Hübner) Tomato fruitworm (TFW); Helicoverpa zea (Boddie) Cabbage looper (CL); Trichoplusia ni (Hübner) Southern armyworm (SAW); Spodoptera eridania (Stoll) Tobacco hornworm (THW); Manduca sexta (Linnaeus) -167- ECONOMICS AND MARKETING -168- -169- COMMERCIAL VEGETABLE PRICE OUTLOOK Greg E. Fonsah Assistant Professor and Extension Economist Fruits, Vegetables and Pecans Department of Agricultural and Applied Economics University of Georgia, P.O. Box 1209 – RDC Tifton, Ga 31793 Tel: 229 386 3512 Fax: 229 386 3440 Introduction Commercial vegetable production is on the rise in Georgia with about 33 different vegetables crops currently being produced. More-so, fruits and vegetables are amongst the fastest growing sector in the consumer-related high value products industry. As any other agricultural commodity, commercial vegetable production requires several inputs such as, seeds or plants, fertility, insecticides, fungicides, nematicides, herbicides, labor, irrigation, packaging material, machinery, brokers commissions, transportation and freight. All these inputs cost several dollars to the farmer and eventually increase cost of production. An increased production cost reduces profit margin and renders commercial vegetables less competitive in the market place. However, one of the major factors in determining profit margin is the price obtained by the growers of vegetables. If the prices are good, the gap caused by the high cost of production is narrowed and profitability is improved. The reverse is true when depressing prices are obtained. The primary objective of this research is to investigate price trend for commercial vegetables. The specific objective are: (a) to analyze Georgia vegetable 5 years average prices; (b) to analyze wholesales prices for selected fresh market vegetables at shipping-point; and (c) to analyze the 5 years monthly-average f.o.b. prices for selected US fresh vegetables. Material and Methods Descriptive statistics such as graphs and/or time-series will be used to illustrate price trend for the enumerated specific objectives. Secondary data will be collected from various sources of the Economic Research Service, USDA and Georgia Statistics respectively. -170- Results and Discussions Prices for Georgia commercial vegetables have been fluctuating for the past five. Snap beans, cucumber, eggplant, and carrot experience an increase in price from 1998 and 1999, a drop in year 2000, and another increase in 2001 as shown in fig. 1. Prices for corn dropped in 1998 and 2000. There was a slight increase in the price of corn in 1999 but year 2001 was much better. Incidentally, cantaloupe prices have remained constant for the past five years. Fig 1 Georgia Vegetables 5 Years Average Prices: 1997-2001 14 12 10 dollars 8 6 4 2 0 1997 1998 1999 2000 2001 5YR AVG Years Bean, snap Cabbage Cantaloupe Cucumber Eggplant Okra Source: Eastern Vegetable and Fruit Report (USDA), various issues -171- Carrots Corn Figure 2 shows that Vidalia onion experienced a price boom from 1998 to 1999, a drastic fall in price in 2000 and another increase in 2001. Prices for Vidalia onion is expected to escalate in year 2002 due to production shortages experienced by Southeast Georgia growers, due to fluctuating climate during the growing season that caused serious damage to the crop (Torrance and Dasher, 2002). Incidentally, the anticipated price hike will not be enough to upset the loss in productivity. Squash, zucchini and pepper continue to encounter depressing prices. Fig. 2 Georgia Vegetables 5 Years Average Prices Cont.:1997-2001 18 16 14 Dollars 12 10 8 6 4 2 0 1997 1998 1999 2000 2001 5YR AVG Pepper Potatoes Squash Sw eet Potatoes Tomatoes Vidalia Onion Watermelon Zuccini Source: Eastern Vegetable and Fruit Report (USDA), various issues (1997-2001) -172- Figure 3 below shows that wholesales price per bushel/cartons in Chicago shipping point for Florida, Georgia and Michigan snap bean (round green, handpicked) was erratic. Prices as high as $40 per bushel/carton were observed from February 2001 with worse prices in June of the same year. Prices for greens (collards, turnips), onion and tomatoes were not as erratic. The greens from California and Georgia were sold in cartons of 24s whereas green onions from California and Mexico were sold in cartons or bunched of 48s. Tomatoes (mature green and large) prices were sold in 25 lb/carton containers. Fig 3. Selected Wholesales prices for Fresh Market Vegetables in Chicago: 2001 2002 45.00 40.00 35.00 $/bu/ctn 30.00 25.00 20.00 15.00 10.00 5.00 0.00 2- 25611- 2-Jul 64153Jan Feb Mar Apr May Jun Aug Sep Oct Nov Dec Jan 4622 Feb Mar Apr Months Snap beans Greens, Collards Onion, green Tomatoes Source: Fruit & Vegetable Market News, Agricultural Marketing Service, USDA (2002) Vegetable and Melon Outlook/VGS-290/April 18. -173- Figure 4 shows the price trend for US selected fresh vegetables, 5 years monthly average, f.o.b. shipping point from 1997 to 2002. Cauliflower, sweet corn and broccoli are extremely erratic. The average price for year 2002 is only for three months (January, February and March), thus is expected to level off in subsequent months. Fig 4. Selected Fresh Vegetables 5 Years Monthly-Average F.O.B. Shipping-Point Prices: 1997-2002 60 50 (dollars/ cwt) 40 30 20 10 0 1997 1998 1999 2000 2001 2002 Years Fig. 4 Broccoli Cantaloups Carrots Cauliflower Corn Sweet Source: Economic Research Service, USDA (2002) Vegetables and Melons Outlook/VGS-290, April, 18. Figure 5 shows that the average price for cucumbers for year 2002 is the price recorded for March. Onions, snap beans and tomatoes are for three months averages (January, February and -174- March), and does not reflect or represent the whole year. The twelve months average is expected to follow the same trend as previous years. Fig. 5 Selected Fresh Vegetables Prices: U.S. 5 Years-Average F.O.B. Shipping-Point: 1997-2002 Cont. 60 50 (Dollars/Cwt) 40 30 20 10 0 1997 1998 1999 2000 2001 2002 Years Cucumbers Onions Snap beans Tomatoes Source: Economic Research Service, USDA (2002) Vegetables and Melons Outlook/VGS-290, April, 18. Conclusion -175- The Georgia 5 years average price for most commercial vegetables is downward slopping and this price trend is expected to maintain status-quo due to the erratic nature. The wholesales prices for fresh market vegetable in the Chicago shipping point shows no significant improvement either. The US 5 years average f.o.b. shipping-point prices show a similar pattern. Price trend for commercial vegetables will remain the same in the years to come except new market outlets (export) are developed. Reference 1 Atlanta Wholesale Fruit and Vegetable Report, USDA (1997-2002). 2 Economic Research Service, USDA (2002). Vegetables and Melon Outlook/VGS- 290/April 18. 3 Eastern Vegetable and Fruit Report, USDA (1997-2002). 4 Torrance, R. and S. Dasher (2002) “Growers Lose Most of Vidalia Onion Crop” CAES News Center, UGA College of Agricultural and Environmental Sciences. Author Index Page Number -176- Batal, D.......................................................................................................................................125 Bertrand, D................................................................................................................................. 125 Boland, R.T................................................................................................................................ 129 Boyhan, G.............................................................................................................................. 19, 21 Csinos, A. S.......................................................................................................71, 82, 93, 103, 114 Curry, D. .................................................................................................................................32, 45 Davis, R.F..........................................................................................................................71, 82, 93 Diaz-Perez, J................................................................................................................................125 Flanders, J.T.................................................................................................................................136 Fonsah, G.....................................................................................................................................169 Giddings, D..................................................................................................................................125 Granberry, D............................................................................................................................19, 21 Hardison, G..............................................................................................................................32, 45 Hill, R.......................................................................................................................................19, 21 Horten, T.B......................................................................................................................... 146, 148 Hudgins,J.E..................................................................................................................................144 Johnson,W.C..................................................................................................................................51 Kelley, W.T............................................................................11, 13, 15, 19, 21, 24, 28, 32, 45, 138 Langston, D.B................................................................. ....129, 132, 136, 138, 141, 144, 146, 148 Laska, J.E...........................................................................................................71, 82, 93, 103, 114 Price,J.G.......................................................................................................................................129 Riley,D.........................................................................................................................................153 Seebold, K.W.....................................................................71, 82, 93, 103, 114, 132, 141, 146, 148 Sumner,P..........................................................................................................................................3 Timper, P............................................................................................................71, 82, 93, 103, 114 Webster, T.M. .........................................................................................................................51, 65 -177-