Geoff Pilmoor Sims Moelich Associates Risk Assessment: Use and Applications
advertisement

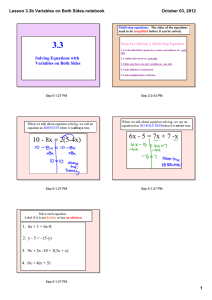
Risk Assessment: Use and Applications in Pharma and Biotech Manufacturing Operations Geoff Pilmoor Sims Moelich Associates September 29, 2005 Agenda Risk Risk assessment methods Use and Trends Operations Regulatory Compliance 05 Sep 29 2 Risk Combination of the probability of occurrence of harm and severity of that harm (ISO/IEC Guide 51) 05 Sep 29 3 Initiate Overall Approach Categorize Risk Assess Risk Communicate Risk Accept or Manage Risk Monitoring, Evaluation 05 Sep 29 4 ICH Q9 – Quality Risk Management Process 05 Sep 29 5 Risk Assessment Systematic process of organizing information to support a risk decision to be made within a risk management process (ICH Q9) 05 Sep 29 6 Initiators Standard Procedure Discrete event Change in environment 05 Sep 29 7 Categories Patient Product Process Project Business Continuity Strategic Financial Reputation …etc. 05 Sep 29 8 Assess Risk Identification / Listing of Potential Events Establish Informal approach Formal process Severity of Impact of Event Probability of Event Occurrence Qualitative versus Quantitative Consider Detection of Event can lead to Reduced Severity Reliability of Detection 05 Sep 29 9 Operational Risk Tools Process mapping HAZOP (Hazard and Operability Study) FTA (Fault Tree Analysis) FMEA (Failure Mode and Effect Analysis) System impact & component criticality assessment HACCP (Hazard Analysis and Critical Control Points) What if checklist Scenario analysis 05 Sep 29 10 HAZOP Generates a list of critical operations for risk management Team based, systematic brainstorming technique Assumes risk events are caused by deviations from design or operating intentions Considers “Guide words” against relevant parameters to identify potential deviations 05 Sep 29 11 HAZOP Start Explain overall design Select Section of System Explain design intent of Section Select operating condition Select guide word Credible deviation? Y Identify and document causes, consequences, actions N N Last Section? N Y Last condition? N Y Last guide word? Y *Example Guide words: Tabulate and report End 05 Sep 29 *NO FLOW *REVERSE FLOW *MORE FLOW *LESS FLOW *MORE LEVEL *LESS LEVEL *MORE PRESSURE *LESS PRESSURE *MORE TEMPERATURE *LESS TEMPERATURE *COMPOSITION CHANGE *CONTAMINATION *CORROSION/EROSION *SERVICE FAILURE *ABNORMAL OPERATION 12 FTA Generates a quantitative estimate of likelihood of each failure mode of a system Graphical depiction of all causal chains of failure of a system or sub-system Assumes failures follow from logical combinations of causes that occur with known probability 05 Sep 29 13 Bulb Fails No electricity Power Plant Fails Wind Breaks Line 05 Sep 29 Power Line Fails Glass Broken Connector Corroded FTA Filament Broken Impurities Vacuum Leak Vibrations Tree Breaks Line 14 FTA Complexity 05 Sep 29 15 Ranking & Filtering Qualitative rating Quantitative rating Look-up table Generally accepted rules / principles 05 Sep 29 16 Qualitative Probability Occurrence Probability A) High • Very likely will occur within a __ month/year period B) Medium • Likely will occur within a __ month/year period C) Low • May occur within a __ month/year period 05 Sep 29 17 Qualitative Severity Classification Interpretation I) High • Very significant negative impact (significant long-term / catastrophic short term) • Moderate negative impact over short to medium term II) Medium III) Low 05 Sep 29 • Minor negative impact over short term 18 Qualitative Classification Risk Classification Probability of Event Occurrence Severity of Event High High Medium Low 1 1 2 Medium 1 2 3 Low 2 3 3 05 Sep 29 19 Qualitative Prioritization Event Priority Likelihood of Detection Risk Classification High Low Medium High 1 1 2 Medium 1 2 3 Low 2 3 3 05 Sep 29 20 Bio-process Example ess p Error Area Error Item What happens to product? Likelihood L, M, H Severity L, M, H Risk Classification 1,2,3 Probability of Detection L, M, H Risk Priority L, M, H Mitigation strategy ing SD ment Comp AG 2101 Incorrectly agitated H M 1 M H Alarm on agitator T 2101 Incorrectly held L H 2 H L No action Severity: Probability: Detectability: H H H – Batch loss M – Batch Rework L - Minor 05 Sep 29 – < 1 event per 250 runs M – 1 event per 2,500 runs L - < 1 event per 2,500 runs – Each event M – 1 in 2 events L - ≤ 1 in 3 21 Operations Reliability Example Fluid leak from Roof Pipeline service in ceiling Upper Mfg Lower Mfg Weigh Outer RM Storage Inner RM Storage RO Mechanical Room 1 Mechanical Room 2 Microbiology Lab QC Lab 2nd Floor Washrooms Mfg B C Fluid leak from Roof Pipeline service in ceiling Upper Mfg Lower Mfg Weigh Outer RM Storage Inner RM Storage RO Mechanical Room 1 Mechanical Room 2 Microbiology Lab QC Lab 2nd Floor Washrooms Mfg 2 4 D C D C 5 4 5 4 Probability of Fluid leak into Mfg 2 Fill B D C A C Pack D < < < < < High Probable Low Improbable Not possible C C B C D D Risk Index of Fluid leak into Mfg 2 Fill 2 5 2 1 2 B C D D D > > > > > Mfg III III IV III IV III Impact of Fluid leak into Mfg 2 Fill III III I II II Pack IV II II II III III III IV IV IV IV IV Pack 5 2 2 1 4 5 5 Catastrophic Critical Nuisance Negligble Not possible Fluid leak from Roof Pipeline service in ceiling Upper Mfg Lower Mfg Weigh Outer RM Storage Inner RM Storage RO Mechanical Room 1 Mechanical Room 2 Microbiology Lab QC Lab 2nd Floor Washrooms Catastrophic Occurrence High Probability Probable Low Probability Improbable 1 1 2 3 Risk Classification Critical Nuisance 1 2 1 2 2 4 3 5 Negligible 4 4 5 5 4 5 5 5 5 Severity: Cat – Major portion of the business will be interrupted for one week or more Crit– Crit– Small portion of business will be interrupted for one week or more, more, or major portion of business will be interrupted for up to one week. Nuis -Small portion of business interrupted for up to one week NegNeg-Small portion of business interrupted for up to one shift 05 Sep 29 Probability: H –1 event per year highly likely M – 1 event per 3 years highly likely L - 1 event per 3 years may occur I – Likely will not occur in 3 years 22 Impact / Criticality Assessment Non-critical Critical Components in Components in Direct Impact Direct Impact Systems Systems All Components in All Systems Subject these items to Qualification Non-critical Components in Indirect Impact Systems Non-critical Components in No Impact Systems 05 Sep 29 23 Impact & Criticality A system is generally determined to be direct impact if it: Produces an ingredient or excipient. Has surfaces that direct contact with the product Produces or delivers a gas or liquid that has direct contact with the product Is used to clean or sanitize direct product contact surfaces of process equipment Preserves product status (e.g. environmental control such as a freezer) Produces data which is used to make Quality Operations decisions regarding product status Is a process control system that may affect product SISPQ, which has no independent verification of control system performance A component is generally determined to be critical if it: Contacts the product or product components Is used to demonstrate compliance with a registered process Presents information that becomes part of a GMP record, is recorded on a GMP document or is entered into as GMP data into a computer system Has a direct affect on product quality during normal operation or control Controls critical process elements that will affect product quality, where there is no independent verification of control system performance Provides an alarm for a condition that has a direct affect on product quality Creates or preserves a critical status of a system Has a direct affect on product quality if it fails 05 Sep 29 24 API Facility Example System No. System Description Direct Impact In-direct Impact No Impact HVAC and CONTAINMENT SYSTEMS AHU1 Manufacturing and storage area 9 FH01 Fume Hood 1 9 FH02 Fume Ho od 2 9 ISO1 Isolator 9 AHU2 Laboratory and offices 9 MECHANICAL and ELECTRICAL UTILITIES 9 N2 Gaseous nitrogen BST Building steam 9 CA Compressed air 9 CH4 Natural gas 9 CWS Chilled water 9 DCW Domestic cold water 9 DHW Domes tic recirculating hot water 9 05 Sep 29 25 Response to Risk Significant Risks Communicate to stakeholders Do what? Avoid 05 Sep 29 Transfer Mitigate Accept 26 Mitigation of Risk Identify actions to: Reduce probability of event Reduce severity of event Provide early/more reliable detection and initiation of response to event Re-assess risk assuming actions, until acceptable risk level is reached Implement actions Monitor and periodically re-assess 05 Sep 29 27 HACCP Systematic, proactive (preventative) risk management process Facilitates monitoring of critical manufacturing operations Most useful when expert product, process knowledge available 05 Sep 29 28 HACCP 1. Conduct hazard analysis; Identify preventive measures for each step of process 2. Determine critical control points (CCP) 3. Establish (measurable) critical limits 4. Establish system to monitor CCPs 5. Establish corrective action to be taken when monitoring indicates CCPs are not in control 6. Establish system to verify HACCP system is working effectively 7. Establish record-keeping system 05 Sep 29 29 Operational Trends Increasing use and internal acceptance of risk assessment tools in managing efforts Reliability Efficiency Widespread collection and charting of operational metrics Quantitative information available for risk assessment, management 05 Sep 29 30 Quality Compliance Risk Tools Standards / Definitions Site Risk / Inspection Prioritization Probabilistic Risk Assessment Compliance guides 05 Sep 29 31 Standards / Definitions HPFBI Guide 23 Definitions Classification Listings by Observation 05 Sep 29 32 Regulatory Trends Internal use of risk assessment tools in managing efforts International standardization, Quality Risk Management Increasing acceptance and use of Risk Assessment in regulation of industry 05 Sep 29 33 Site FDA Inspection Priority Quantitative approach Rating above threshold, set based on limited capacity FY 2005 pilot project Site Risk Potential Product Risk x Facility Risk x Process Risk 05 Sep 29 34 FDA Site Selection Model Site Risk Potential Top-Level Components Product Process Categories of Risk Factors CD1 CD2 Risk Facility CP2 CP1 CF1 CF2 Factors (quantitative or qualitative variables) 05 Sep 29 35 FDA Risk Factors Product: Process: Intrinsic Process Sterility Rx or OTC Dose Form Facility: Control Product type Unit Operation Contamination Recall History Frequency Severity 05 Sep 29 History GMP Violation Inspection Product volume Vulnerability Product type Unit Operation Operation type 36 Future Trends ? Risk-Informed regulation of industry will evolve (versus Risk Assessment) Risk determinations quantitatively based (versus qualitative basis) Regulatory environment will facilitate increased increased use of risk assessment and management tools in operations 05 Sep 29 37 Recap Risk and Approach Applications and Examples Where to get more information Questions? 05 Sep 29 38 References: Process Hazards CCPS, “Guidelines for Hazard Evaluation Procedures. Second Edition with Worked Examples”, 1992 WHO, “Application of Hazard Analysis and Critical Control Point (HACCP) methodology to pharmaceuticals” WHO Technical Report Series, No. 908, 2003 ISO, “Safety of machinery -- Principles of risk assessment” ISBN 0-8169-0491-X ISO 14121:1999 CSA, “Risk Analysis Requirements and Guidelines” CAN/CSA-Q634-91 05 Sep 29 39 References: GMP, Regulatory HPFBI, “Risk Classification of GMP Observations, 2003 Edition” ICH, “Quality Risk Management (draft consensus guideline)” Guide-0023 Q9, 2005 FDA, “Risk-based Method for Prioritizing cGMP Inspections of Pharmaceutical Manufacturing Sites – A Pilot Risk Ranking Model”, 2004 FDA, “Quality Systems Approach to Pharmaceutical Current Good Manufacturing Practice Regulations”, 2004 ASME, “Probabilistic Risk Assessment for Nuclear Power Plant Applications” RA-S-2002 05 Sep 29 40 References: ISPE ISPE Baseline® Pharmaceutical and Engineering Guide, “Volume 5 – Commissioning and Qualification”, First Edition Tran, Hasselbalch, Morgan, Claycamp, “Elicitation of Expert Knowledge about Risks Associated with Pharmaceutical Manufacturing Processes” Pharmaceutical Engineering, Jul/Aug 2005 Phoenix, Andrews, “Adopting a Risk-Based Approach to 21 CFR Part 11 Assessments” Pharmaceutical Engineering, Jul/Aug 2005 Coburn, Levinson, Weddel, “A Precedent for Risk-Based Regulation” ISPE, Mar. 2001 Pharmaceutical Engineering, Jul/Aug 2003 GAMP Forum, “Risk Assessment for Use of Automated Systems Supporting Manufacturing Processes” Pharmaceutical Engineering, May/Jun 2003 05 Sep 29 41 Contact the Speaker Geoff Pilmoor 905.849.1833 x413 Sims Moelich Associates 277 Lakeshore Road E., Oakville, ON L6J 1H9 Geoff.Pilmoor@SimsMoelich.com 05 Sep 29 42