Document 12071410
advertisement
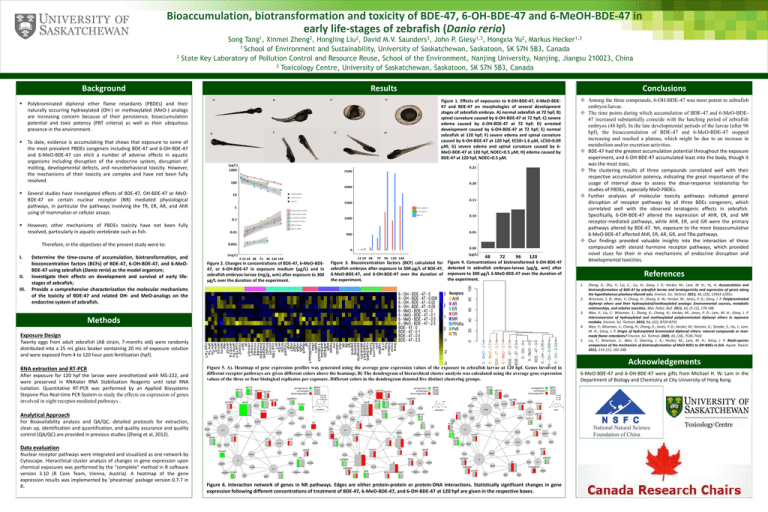
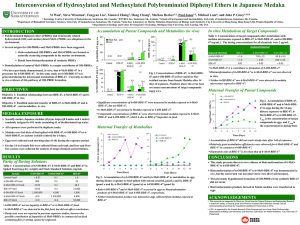
Bioaccumulation, biotransformation and toxicity of BDE-47, 6-OH-BDE-47 and 6-MeOH-BDE-47 in early life-stages of zebrafish (Danio rerio) 2 Song Tang1, Xinmei Zheng2, Hongling Liu2, David M.V. Saunders3, John P. Giesy1,3, Hongxia Yu2, Markus Hecker1,3 1 School of Environment and Sustainability, University of Saskatchewan, Saskatoon, SK S7N 5B3, Canada State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, Jiangsu 210023, China 3 Toxicology Centre, University of Saskatchewan, Saskatoon, SK S7N 5B3, Canada Background Polybrominated diphenyl ether flame retardants (PBDEs) and their naturally occurring hydroxylated (OH-) or methoxylated (MeO-) analogs are increasing concern because of their persistence, bioaccumulation potential and toxic potency (PBT criteria) as well as their ubiquitous presence in the environment. To date, evidence is accumulating that shows that exposure to some of the most prevalent PBDEs congeners including BDE-47 and 6-OH-BDE-47 and 6-MeO-BDE-47 can elicit a number of adverse effects in aquatic organisms including disruption of the endocrine system, disruption of molting, developmental defects, and neurobehavioral toxicity. However, the mechanisms of their toxicity are complex and have not been fully resolved. Several studies have investigated effects of BDE-47, OH-BDE-47 or MeOBDE-47 on certain nuclear receptor (NR) mediated physiological pathways, in particular the pathways involving the TR, ER, AR, and AhR using of mammalian or cellular assays. However, other mechanisms of PBDEs toxicity have not been fully resolved, particularly in aquatic vertebrate such as fish. Conclusions Results Figure 1. Effects of exposures to 6-OH-BDE-47, 6-MeO-BDE47 and BDE-47 on morphologies of several development stages of zebrafish embryo. A) normal zebrafish at 72 hpf; B) spinal curvature caused by 6-OH-BDE-47 at 72 hpf; C) severe edema caused by 6-OH-BDE-47 at 72 hpf; D) arrested development caused by 6-OH-BDE-47 at 72 hpf; E) normal zebrafish at 120 hpf; F) severe edema and spinal curvature caused by 6-OH-BDE-47 at 120 hpf, EC50=1.6 μM, LC50=0.09 μM; G) severe edema and spinal curvature caused by 6MeO-BDE-47 at 120 hpf, NOEC=0.5 μM; H) edema caused by BDE-47 at 120 hpf, NOEC=0.5 μM. Therefore, in the objectives of the present study were to: I. II. III. Determine the time-course of accumulation, biotransformation, and bioconcentration factors (BCFs) of BDE-47, 6-OH-BDE-47, and 6-MeOBDE-47 using zebrafish (Danio rerio) as the model organism; Investigate their effects on development and survival of early lifestages of zebrafish; Provide a comprehensive characterization the molecular mechanisms of the toxicity of BDE-47 and related OH- and MeO-analogs on the endocrine system of zebrafish. Figure 2. Changes in concentrations of BDE-47, 6-MeO-BDE47, or 6-OH-BDE-47 in exposure medium (µg/L) and in zebrafish embryos-larvae (mg/g, wm) after exposure to 300 μg/L over the duration of the experiment. Figure 3. Bioconcentration factors (BCF) calculated for zebrafish embryos after exposure to 300 μg/L of BDE-47, 6-MeO-BDE-47, and 6-OH-BDE-47 over the duration of the experiment. Figure 4. Concentrations of biotransformed 6-OH-BDE-47 detected in zebrafish embryos-larvae (μg/g, wm) after exposure to 300 μg/L 6-MeO-BDE-47 over the duration of the experiment. 4. 5. Twenty eggs from adult zebrafish (AB strain, 7-months old) were randomly distributed into a 25 mL glass beaker containing 20 mL of exposure solution and were exposed from 4 to 120 hour post-fertilization (hpf). Figure 5. A). Heatmap of gene expression profiles was generated using the average gene expression values of the exposure in zebrafish larvae at 120 hpf. Genes involved in different receptor pathways are given different colors above the heatmap; B) The dendrogram of hierarchical cluster analysis was calculated using the average gene expression values of the three or four biological replicates per exposure. Different colors in the dendrogram denoted five distinct clustering groups. Analytical Approach For Bioavailability analysis and QA/QC, detailed protocols for extraction, clean up, identification and quantification, and quality assurance and quality control (QA/QC) are provided in previous studies (Zheng et al, 2012). Data evaluation Nuclear receptor pathways were integrated and visualized as one network by Cytoscape. Hierarchical cluster analysis of changes in gene expression upon chemical exposures was performed by the "complete" method in R software version 3.10 (R Core Team, Vienna, Austria). A heatmap of the gene expression results was implemented by ‘pheatmap’ package version 0.7.7 in R. Created by Peter Downing – Educational Media Access and Production © 2011 1. 3. Exposure Design After exposure for 120 hpf the larvae were anesthetized with MS-222, and were preserved in RNAlater RNA Stabilization Reagents until total RNA isolation. Quantitative RT-PCR was performed by an Applied Biosystems Stepone Plus Real-time PCR System to study the effects on expression of genes involved in eight receptor-mediated pathways . References 2. Methods RNA extraction and RT-PCR Among the three compounds, 6-OH-BDE-47 was most potent to zebrafish embryos/larvae. The time points during which accumulation of BDE-47 and 6-MeO-BDE47 increased substantially coincide with the hatching period of zebrafish embryos (48 hpf). In the late developmental periods of the larvae (after 96 hpf), the bioaccumulation of BDE-47 and 6-MeO-BDE-47 stopped increasing and reached a plateau, which might be due to an increase in metabolism and/or excretion activities. BDE-47 had the greatest accumulation potential throughout the exposure experiment, and 6-OH-BDE-47 accumulated least into the body, though it was the most toxic. The clustering results of three compounds correlated well with their respective accumulation potency, indicating the great importance of the usage of internal dose to assess the dose-response relationship for studies of PBDEs, especially MeO-PBDEs. Further analyses of molecular toxicity pathways indicated general disruption of receptor pathways by all three BDEs congeners, which correlated well with the observed teratogenic effects in zebrafish. Specifically, 6-OH-BDE-47 altered the expression of AhR, ER, and MR receptor-mediated pathways, while AhR, ER, and GR were the primary pathways altered by BDE-47. Yet, exposure to the more bioaccumulative 6-MeO-BDE-47 affected AhR, ER, AR, GR, and TRα pathways. Our findings provided valuable insights into the interaction of these compounds with steroid hormone receptor pathways, which provided novel clues for their in vivo mechanisms of endocrine disruption and developmental toxicities. Figure 6. Interaction network of genes in NR pathways. Edges are either protein-protein or protein-DNA interactions. Statistically significant changes in gene expression following different concentrations of treatment of BDE-47, 6-MeO-BDE-47, and 6-OH-BDE-47 at 120 hpf are given in the respective boxes. Zheng, X.; Zhu, Y.; Liu, C.; Liu, H.; Giesy, J. P.; Hecker, M.; Lam, M. H.; Yu, H. Accumulation and biotransformation of BDE-47 by zebrafish larvae and teratogenicity and expression of genes along the hypothalamus-pituitary-thyroid axis. Environ. Sci. Technol. 2012, 46, (23), 12943-12951. Wiseman, S. B.; Wan, Y.; Chang, H.; Zhang, X. W.; Hecker, M.; Jones, P. D.; Giesy, J. P. Polybrominated diphenyl ethers and their hydroxylated/methoxylated analogs: Environmental sources, metabolic relationships, and relative toxicities. Mar. Pollut. Bull. 2011, 63, (5-12), 179-188. Wan, Y.; Liu, F.; Wiseman, S.; Zhang, X.; Chang, H.; Hecker, M.; Jones, P. D.; Lam, M. H.; Giesy, J. P. Interconversion of hydroxylated and methoxylated polybrominated diphenyl ethers in Japanese medaka. Environ. Sci. Technol. 2010, 44, (22), 8729-8735. Wan, Y.; Wiseman, S.; Chang, H.; Zhang, X.; Jones, P. D.; Hecker, M.; Kannan, K.; Tanabe, S.; Hu, J.; Lam, M. H.; Giesy, J. P. Origin of hydroxylated brominated diphenyl ethers: natural compounds or manmade flame retardants? Environ. Sci. Technol. 2009, 43, (19), 7536-7542. Liu, F.; Wiseman, S.; Wan, Y.; Doering, J. A.; Hecker, M.; Lam, M. H.; Giesy, J. P. Multi-species comparison of the mechanism of biotransformation of MeO-BDEs to OH-BDEs in fish. Aquat. Toxicol. 2012, 114-115, 182-188. Acknowledgements 6-MeO-BDE-47 and 6-OH-BDE-47 were gifts from Michael H. W. Lam in the Department of Biology and Chemistry at City University of Hong Kong.