fic water quality criteria for aquatic ecosystems: A case study of Site-speci pentachlorophenol for Tai Lake, China
advertisement

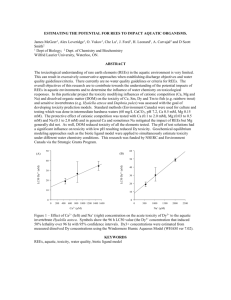
Science of the Total Environment 541 (2016) 65–73 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Site-specific water quality criteria for aquatic ecosystems: A case study of pentachlorophenol for Tai Lake, China Yi Chen a,b, Shuangying Yu c, Song Tang d, Yabing Li a, Hongling Liu a,⁎, Xiaohui Zhang a, Guanyong Su a, Bing Li b, Hongxia Yu a, John P. Giesy a,e,f,g a State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, Jiangsu 210023, China Jiangsu Provincial Academy of Environmental Sciences, Nanjing, Jiangsu 210036, China Savannah River Ecology Laboratory, University of Georgia, Aiken, SC 29808, USA d School of Environment and Sustainability, University of Saskatchewan, Saskatoon, SK S7N 5B3, Canada e Toxicology Centre, University of Saskatchewan, Saskatoon, SK S7N 5B3, Canada f Department of Veterinary Biomedical Sciences, University of Saskatchewan, Saskatoon, SK S7N 5B3, Canada g School of Biological Sciences, University of Hong Kong, Hong Kong, SAR, China b c H I G H L I G H T S G R A P H I C A L A B S T R A C T • Acute toxicity tests of nine native species were performed. • Species compositions in the 1980s were considered to restore the healthier ecological community in Tai Lake. • In situ toxicity tests were performed to consider the bioavailability of PCP in Tai Lake. • Like criteria in some developed countries, criteria of PCP were expressed as functions of pH. a r t i c l e i n f o Article history: Received 7 May 2015 Received in revised form 30 August 2015 Accepted 1 September 2015 Available online xxxx Editor: D. Barcelo a b s t r a c t Given the widely varying types of aquatic ecosystems and bioavailability of chemicals, it is important to develop site-specific water quality criteria (WQC) to ensure criteria are neither over- nor under-protective. In the study, using pentachlorophenol (PCP) as an example, several approaches to derive site-specific WQC were investigated, including the conventional species sensitivity distribution (SSD), weighted SSD based on the proportion of each trophic level, and water effect ratio (WER) method. When corrected to a pH of 7.8, the conventional SSD approach resulted in criteria maximum concentration (CMC) and criteria continuous concentration (CCC) of 18.11 and 1.74 μg/L, respectively. If SSD was weighted according to the current species composition in Tai Lake, the CMC and CCC were 32.81 and 4.48 μg/L, respectively. However, available data suggest that many Abbreviations: WQC, water quality criteria; PCP, pentachlorophenol; 2, 4-DCP, 2, 4-dichlorophenol; SSDs, species sensitivity distributions; CMC, criteria maximum concentration; CCC, criteria continuous concentration; WER, water effect ratio; ACR, acute-chronic ratio; NOEC, no observed effect concentration; LOEC, lowest observed effect concentration; MATC, maximum acceptable toxicant concentration; EC50, 50% effective concentration; LC50, 50% lethal concentration; EC10, 10% effective concentration; WQS, water quality standard. ⁎ Corresponding author at: School of the Environment, Nanjing University, Nanjing, Jiangsu 210023, China. E-mail address: hlliu@nju.edu.cn (H. Liu). http://dx.doi.org/10.1016/j.scitotenv.2015.09.006 0048-9697/© 2015 Elsevier B.V. All rights reserved. 66 Keywords: Site-specific criteria Historic species composition Criteria maximum concentration (CMC) Criteria continuous concentration (CCC) Water-effect ratio Y. Chen et al. / Science of the Total Environment 541 (2016) 65–73 sensitive species inhabiting Tai Lake during 1980s were disappeared. Considering the species composition of the healthier ecosystem in 1980s, the CMC and CCC were 10.99 and 0.38 μg/L, respectively, which provide more protective water quality standards. Water effect ratio (WER) was further used to correct for co-occurrence of other toxicants and factors affecting bioavailability of PCP. A final WER of 4.72 was applied to adjust the criteria derived by using the weighted SSD for the 1980s aquatic community, and the final CMC and CCC obtained were 51.87 and 1.79 μg/L, respectively, at a pH of 7.8. Water quality criteria derived using the 1980s species composition and adjusted with WER were deemed the most appropriate WQC for water management and aquatic life protection. Merits of the various approaches for developing WQC for protection of aquatic species were discussed. © 2015 Elsevier B.V. All rights reserved. 1. Introduction Aquatic ecosystems vary dramatically in their physicochemical properties, which in turn affect bioavailability of some metal and organic chemical contaminants. In addition, species compositions and their relative sensitivities to chemical contaminants vary widely among ecosystems, which might lead the national criteria to be over- or under-protective for specific basin. There are two basic uncertainties that could be made when applying national WQC. First, the presence of other contaminants might result in the WQC being underprotective. In addition, the bioavailable fraction or forms in which contaminants can occur might affect relative potencies of contaminants under various conditions. Therefore, it is of great significance for a range of water quality criteria (WQC) that are adjusted for conditions among ecoregions (USEPA, 2002, 2004, 2009). In general, due to adsorption to particulates or coordination interaction of the organic or in-organic ligands, chemical activities, the available fractions of contaminants are generally less under field conditions than in tests conducted under laboratory conditions where contaminants are normally nearly 100% available. Therefore, hardness-adjusted WQC criteria have been developed for metals and more recently, the Biotic Ligand Model has been applied to correct for the forms of metals present under varying geochemical conditions. Furthermore, for sediment acid volatile sulfides (AVS) and organic carbon normalization have been used to modify sediment quality criteria (SQC) based on site-specific conditions. In addition, the U.S. EPA published guidelines for deriving numerical sitespecific WQC by considering the effects of physical and chemical parameters (e.g., pH, hardness, temperature, etc.) and proposed use of the water effect ratio (WER) (Carlson, 1984). Tai Lake (Ch: Taihu), one of the largest freshwater lakes in China, has been contaminated with municipal, industrial and agricultural runoff due to rapid economic growth and intensive industrialization in the region. It is unique in its geological characteristics, assemblage of species and pattern of contaminants (Wu et al., 2008). The current WQC used for Tai Lake were developed based on national water quality standards and therefore might not be appropriate for protecting the local aquatic community. Chlorophenols (CPs) are an important group of contaminants in China and other parts of the world. As raw materials in production of textile additives, pharmaceutical products, chemicals and pesticides, large amounts of CPs have the potential to enter aquatic environments and are frequently detected (Gao et al., 2008). Chlorophenols are especially problematic because of their resistance to degradation, toxicity to aquatic life, and potential to bioaccumulate (Bello et al., 2013; Jin et al., 2012a; Martí et al., 2011). One of the more common CPs is pentachlorophenol (PCP), a priority pollutant in China (Zhou et al., 1990). It has been widely detected in environmental media including air, water, sediment, soil, and tissues of wildlife, and humans (Hodin et al., 1991; Mari et al., 2007). Use of PCP as a molluscicide in Dongting Lake resulted in concentrations as great as 104 μg/L in water and 4.8 × 104 μg/kg dry mass (dm) in sediment (Yin et al., 2008; Zheng et al., 2012). The maximum concentrations of PCP in water of two major river basins (Yellow and Yangtze) were 29 and 0.59 μg/L, respectively (Gao et al., 2008). The median concentrations of PCP in the Yangtze River and Tai Lake were 60 and 12 ng/L (Qu et al., 2004). Pentachlorophenol has also been found in aquatic organisms (e.g., fish, shrimp, carp) at concentrations as great as 6.3 × 102 μg/kg wet mass (wm) in grass carp (Ctenopharyngodon idellus) (Zheng et al., 2000), posing potential risks to humans via consumption of fish. Several studies demonstrated the toxic potency of PCP. Concentrations from 0.025 to 5 mg/L resulted in lesser hatchability and greater mortality and deformities in embryos of zebrafish (Danio rerio) (Zheng and Zhu, 2005). The 96-h median lethal concentration (LC50) for the catfish (Ictalurus punctatus) was 68 μg/L (Luo et al., 2009). Fishes were the most sensitive species, followed by invertebrates and aquatic plants (Luo et al., 2009). Several WQC have been developed for CPs in China, including 2, 4dichlorophenol (2,4-DCP) (Yin et al., 2002) and 2, 4, 6-trichlorophenol (2,4,6-TCP) (Yin et al., 2003). The criteria maximum concentration (CMC) and the criteria continuous concentration (CCC) for 2, 4-DCP were 1.25 × 103 μg/L, and 2.12 × 102 μg/L, respectively (Yin et al., 2002). For 2, 4, 6-TCP, the CMC and CCC were 1.01 × 103 μg/L and 2.3 × 102 μg/L, respectively (Yin et al., 2003). pH-dependent WQC for 2, 4-DCP, 2, 4, 6-TCP and PCP (CMC2,4-DCP = exp.(0.6274 × pH + 0.7630), CCC2,4-DCP = exp.(0.6274 × pH-2.1056); CMC2,4,6-TCP = exp.(0.8937 × pH-1.1374), CCC2,4,6-TCP = exp.(0.8937 × pH-2.9706); CMCPCP = exp.(0.7330 × pH-3.2847), CCCPCP = exp.(0.7330 × pH4.3564); μg/L) were derived by considering species compositions among three trophic levels (Xing et al., 2012a). Because of the unique regional characteristics and importance of the resources provided by Tai Lake, it was deemed necessary to develop site-specific WQC for this Lake that would be more certain to effectively protect native biota more effectively by adjusting WQC to local geochemical and ecological conditions as well as local water quality. The objective of the present study was to develop site-specific WQC for PCP in order to protect the aquatic community that inhabited Tai Lake prior to its current more contaminated state half a decade ago. Several WQC were generated based on (1) laboratory toxicity testing of native aquatic biota; (2) species composition and structure of the aquatic community specific to Tai Lake, and (3) geochemical characteristics of water in Tai Lake. 2. Materials and methods 2.1. Toxicity of PCP 2.1.1. Chemicals Pentachlorophenol was purchased from Sigma-Aldrich (98%; St. Louis, MO, USA) and was dissolved in dimethyl sulfoxide (DMSO) to make a stock solution, which was stored at −20 °C. Concentrations of DMSO in treatments did not exceed 0.05% (v/v). 2.1.2. Selection of test species Test species were selected based on the following criteria: (1) native species in China; (2) species found in Tai Lake; (3) ability to be raised in the laboratory; and (4) sufficient numbers to repeat the experiment. The nine (9) species (Table S1) used in the present study Y. Chen et al. / Science of the Total Environment 541 (2016) 65–73 were from 4 phyla, including green algae (Scenedesmus obliquus), oligochaete (Limnodrilus hoffmeisteri), water flea (Daphnia magna), shrimp (Neocaridina denticulate), tadpole of wood frog (Rana chensinensis), yellow catfish (Pelteobagrus fulvidraco), silver carp (Hypophthalmichthysmolitrix), bighead carp (Hypophthalmichthys nobilis), and grass carp (C. idellus). S. obliquus and D. magna were cultured and maintained following previously published methods (Xing et al., 2012a, 2012b). L. hoffmeisteri were collected from the field a year prior to initiating toxicity tests. Water was changed every other day and animals were fed with soybean powder and lettuce. N. denticulate, R. chensinensis and fish were purchased from a local market in Nanjing, China. All individuals of the same species were from the same collected location. N. denticulate was acclimated in the laboratory for at least 14 days and was fed with D. magna. Mortalities for all test animals during acclimation were less than 10%. Healthy young N. denticulate of similar mass (approximately 0.16 g) and length (2– 3 cm) were selected for the experiment. R. chensinensis tadpoles (20 days posthatching) were maintained at room temperature for 7 days. Tadpoles of wood frog were fed twice a day and water was changed daily. Mortality was less than 10% during acclimation. Tadpoles were not fed the day before exposure. Individuals with snout-to-vent length of 1.2–1.4 cm were randomly assigned to treatments. Juvenile fishes were also acclimated for at least 14 days and housing conditions were similar to those for other species. Only health larvae (3–4 cm length) were used in studies. Fish were not fed the day before initiating exposure. For all organisms, tap water aerated for at least 3 days was used. Dissolved oxygen (DO), pH, conductivity, alkalinity (CaCO3), and hardness (CaCO3) were 6.07 ± 0.24 mg/L, 8.12 ± 0.11, 319 ± 9.10 μs/cm, 95.48 ± 4.64 mg/L, and 126 ± 4.95 mg/L, respectively. 2.1.3. Acute toxicity testing For each species tested there was a control, a solvent control (DMSO) and six concentrations of PCP. There were three replicates per treatment and each replicated had ten organisms. Organisms were not fed during the experiment and water was changed daily. Temperature during the experiment was 24 ± 1 °C for D. magna and 20 ± 1 °C for other species. The initial density of S. obliquus was approximately 105 cells/mL in 50 ml Erlenmeyer flasks containing 20 mL sterilized WC medium. The exposure lasted for 24 h (Suter and Cormier, 2008). Biomass was measured after 24 h and the average rate of growth was calculated as described previously (Xing et al., 2012b). D. magna and all fish were exposed to PCP for 48 h and 96 h, respectively. 2.1.4. Toxicity data collection and selection Information on toxicity of PCP to aquatic organisms, published prior to April 2014, was obtained from AQUIRE (http://www.epa.gov/ ecotox), CNKI China, Weipu database or other published literature. Studies from previous literature were selected if the test species was native to Tai Lake and if information on pH and exposure duration was provided. Data were also selected according to criteria by Stephan et al. (1985) with the duration of exposure was 96 h for fish, 48 h for D. magna, and 24 h for algae. Endpoints for acute toxicity were 50% median lethal concentration (LC50) or 50% median effective concentration (EC50). To increase the number of species with toxicity data available for the analysis, acute studies using other exposure durations, such as 72 h for fish and 24 h for the water flea, were also included. For chronic toxicity data, exposures of 96 h or 72 h were selected for algae (due to rapid cell division rate of algae (CCME, 2007)), 21 d for D. magna, and 14 days or longer for fish. Compared to the no observed effect concentration (NOEC) and lowest observed effect concentration (LOEC) values which largely depend on experimental designs and statistical analyses, maximum acceptable toxicity concentration (MATC, the geometric average of NOEC and LOEC) and 10% effective concentration (EC10) derived from concentration effect curves are more reliable and therefore were given priority in WQC derivation. If the MATC or EC10 were not available, the NOEC or LOEC was used to 67 derive the chronic criteria (Xing et al., 2012a, 2012b). Chronic data for D. magna were calculated from previous studies on multi-generational toxicity data of PCP (Chen et al., 2014; Stephan et al., 1985). Toxicity data that had no corresponding pH value reported were eliminated. All the acute and chronic toxicity data screened are shown in Table S2 and S3. 2.2. Historical and current species compositions of Tai Lake Information on historic and current aquatic species assemblage in Tai Lake was obtained from the open literature, CNKI China and Weipu. Databases, and survey reports published by Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Suzhou Environmental Monitoring Station and Jiangsu Environmental Monitoring Center (Chen and Liu, 2003; Fan, 1996; Gu et al., 2005; Lei et al., 2008; Ni and Zhu, 2005; Sun and Huang, 1993; Zheng et al., 2007). Historical data on species from various ecological communities in Tai Lake are summarized in the supporting information (Tables S4-8). 2.3. Tai Lake toxicity tests To assess potential effects of physicochemical characteristics of Tai Lake and presence of other toxicants in Tai Lake on toxicity of PCP, acute toxicity tests with Tai Lake water were conducted with the 9 aquatic species used in the previous laboratory toxicity test. Samples were collected during April to December 2013 from West Tai Lake, the part of Tai Lake that was most polluted. Twenty-liter water samples were collected from each of the four sites, including Huangnian, Fenshui, Dapu, and Chendong (Fig. 1, Table S10). All samples were collected at a depth of 0.5 m, transported at 4 °C in amber jars to the Laboratory of Pollution Control and Resource Reuse at Nanjing University. An additional five hundred-milliliter water samples from each sampling site were also obtained for water chemistry analyses. Water quality parameters including pH, temperature, and dissolved oxygen were measured with YSI multiple water quality parameters (6600 V24, Ecosense, Ohio, USA) in situ. Average temperature was 16–17 °C and pH was approximately 6.0–7.0. DO was 7.56 ± 2.15 mg/L. Water samples from each site were homogenized by mixing, filtered (0.45 μm) and stored in a refrigerator at 4 °C for further determination of metal ions using inductively coupled plasma mass spectrometry, ICP-MS (NexION™ 300X ICP-MS, PerkinElmer, USA). After acute toxicities were determined for site waters, a water-effect ratio (WER) was calculated for each site. The WER is defined as the ratio of the toxic potency of PCP in Tai Lake water samples to that in laboratory water. 2.4. Data analysis 2.4.1. Acute toxicity and derivation of chronic data The LC50, EC50 values with 95% confidence intervals were estimated using Trimmed Spearman Karber method (TSK) Program Version 1.5. The EC10 of intrinsic rate of population of growth was calculated with Graphpad Prism (GraphPad Prism Development Core Team, http:// www.graphpad.com/scientific-software/prism/). 2.4.2. Data processing and WQC development Toxicity of PCP is affected by pH (Bello et al., 2013; Kishino and Kobayashi, 1995; Martí et al., 2011; Sinclair et al., 1999). Due to the hydroxyl on the benzene ring PCP can exist as the ion or protonated neutral form. The protonated neutral form is more likely to cross cell membranes and accumulate in organisms, and as a result has greater toxic potency. Toxicity and pH are related through the pKa, which determines the relative proportions of PCP that are in the ionic and neutral forms. In order to consider effects of pH on toxic potency of PCP, water quality criteria used by U.S. EPA have been expressed as functions of pH (USEPA, 2009). In the present study, toxicity data were 68 Y. Chen et al. / Science of the Total Environment 541 (2016) 65–73 Fig. 1. Map of Tai Lake in China and the four sites where water samples were collected for in situ toxicity testing. standardized to the standard pH according to USEPA (http://water.epa. gov/scitech/swguidance/standards/criteria/current/index.cfm#altable), which is 7.8, by use of a previously developed relationship to predict toxicity of PCP as a function of pH (R2 = 0.3310, P = 0.00020) (Eq. (1)) (Xing et al., 2012b). △lnEC50 =LC50 ¼ 0:7330 △pH ð1Þ After correction for pH (Tables S11 ~ 12), species sensitivity distributions (SSDs) were developed for acute and chronic toxicity. In order to be protective of the healthier and more integrated ecological community in Tai Lake during the 1980s, in addition to the conventional SSD method, a weighted SSD (WSSD) methodology was considered when calculating a site-specific WQC for PCP (Shi et al., 2014; Xing et al., 2012a). To obtain a large number of species for chronic SSD analysis, the Acute-Chronic ratio (ACR) method was used for estimating chronic toxicity. Since the ACRs for a majority of species were within a factor of ten, the final ACR was calculated as the geometric mean of all the ACRs available (Stephan et al., 1985). The SSD method uses all acute and chronic toxicity data available to generate a cumulative probability distribution of sensitivity using the log-normal model. Hazard concentrations that are protective of 95% species (HC5) were calculated. Due to uncertainties in calculating the HC5, the final WQC was calculated by dividing the HC5 values by a factor of 2 (Shi et al., 2014; Stephan et al., 1985). For the SSD method, the cumulative probability was calculated (Eq. (2)). Pi ¼ Ri =ðN þ 1Þ ð2Þ Where: Pi was the cumulative probability of species, Ri was the rank of specie i, N was the number of toxicity data screened. In order to take the species composition into consideration, weighted SSDs were also developed (Eq. (3)). 8 Sp1 > > > > < Sp2 X ¼ Sp3 > > ⋮ > > : SPk SI1 SV1 SI2 SV2 SI3 SV3 ⋮ ⋮ SIl SVm 9 > > > > = > > > > ; ð3Þ Where k, l and m were the number of toxicity data for plants, invertebrates and vertebrates representing the first, second and third trophic levels, respectively (Forbes and Calow, 2002). Spi (i = 1,2,…,k) was the sample of plants, SIi (i = 1,2,…,l) the sample of invertebrates, and SVi (i = 1,2,…,m) the sample of vertebrates. The probability of one species of plant, invertebrate and vertebrate was calculated by Eqs. (4), (5) and (6), respectively. PPi ¼ Np =ðN kÞ ð4Þ PIi ¼ NI =ðN lÞ ð5Þ PVi ¼ NV =ðN mÞ ð6Þ In which, PPi, PIi and PVi were probabilities of individual plants, invertebrates and vertebrates, respectively. Values for Np, NI and NV were the number of plants, invertebrates and vertebrates in Tai Lake; N was the number of total species. The resistant green algae and cyanobacteria were included in the plant taxonomic level. The cumulative probability was calculated by adding the probabilities of species for which the toxicity data were ranked. Since the relative sensitivities of the species that have disappeared from Tai Lake are unknown, a conservative estimation method was applied (Wang et al., 2014). This method has been shown to be appropriate and that it delivers output specifically for the ecosystems of interest. Briefly, p% Hazardous Concentration (HCp) can be derived that relates to a probability of a fraction p(%) of species that would exceed the threshold for a given degree of effect, and p% depends on the number (N) of species in Tai Lake. 3. Results and discussion 3.1. Development of WQC based on current acute and chronic toxicity data 3.1.1. Acute and chronic toxicity data collection Acute toxicities were determined for nine species native to Tai Lake, China (Table 1). The LC50 for H. molitrix, P. fulvidraco, H. nobilis, and C. idellus were less than 400 μg/L (22–368 μg/L), indicating PCP is extremely toxic to fish according to the toxicity classification standards (Jin et al., 2012b; Zhang et al., 2007). The LC50 was 450.08 μg/L for Y. Chen et al. / Science of the Total Environment 541 (2016) 65–73 69 Table 1 Acute toxicity of pentachlorophenol to nine native aquatic species at Tai Lake, China, at pH = 8.10. Species Silver carp (Hypophthalmichthysmolitrix) Yellow catfish (Pelteobagrus fulvidraco) Shrimp (Neocaridina denticulate) Bighead carp (Hypophthalmichthys nobilis) Grass carp (Ctenopharynodon idellus) Water flea (Daphnia magna) Wood frog (Rana chensinensis) Oligochaete (Limnodrilus hoffmeisteri) Green algae (Scenedesmus obliquus) Duration Endpoint 4 days 4 days 4 days 4 days 4 days 2 days 4 days 4 days 1 days LC50 LC50 LC50 LC50 LC50 EC50 LC50 LC50 EC50 D. magna, which is also relative sensitive to PCP. The EC50 and LC50 of PCP for S. obliquus and L. hoffmeisteri were 1861.58 and 884.18 μg/L, respectively, consistent with previously studies in which an EC50 of 930 μg/L (pH = 7.5) for S. obliquus and a LC50 of 500 μg/L (pH = 7.0) for L. hoffmeisteri were reported (Chapman et al., 1982; Xing et al., 2012b). Chronic data for D. magna, an EC10 value of 4.79 μg/L, based on intrinsic growth rate of population (Chen et al., 2014), was calculated from previous studies on multi-generational toxicity data of PCP. There were 63 acute toxicity values for 30 aquatic species, and 19 chronic toxicity values for 16 species (Fig. 2). At pH = 7.80, acute toxicity ranged from 17.00 (H. smolitrix) to 29,622.83 μg/L (Chlorella kessleri) with a median of 242.36 μg/L (n = 30), and chronic toxicity ranged from 3.79 (D. magna) to 7470.48 μg/L (C. kessleri) with a median of 124.45 μg/L (n = 16). The most acutely sensitive species were H. smolitrix, P. fulvidraco, Carassius auratus, I. punctatus (Fig. 3a) while the most chronically sensitive species were D. magna, Macrobrachium superbum, Mylopharyngodon piceus, Plagiognathops microlepis (Fig. 3b). The difference in numbers of relatively more sensitive species between acute and chronic toxicity was due to a lack of chronic toxicity data and the fact that there is usually a lack of chronic toxicity data for sensitive species with acute toxicity data available. The ACR of each species with chronic data was calculated and the final acute-chronic ratio (FACR) was 3.75 (Table S12). For species with no chronic toxicity data, toxicity data were generated using the FACR (Table S13). Based on ACR method, the most chronically sensitive species were D. magna, H. smolitrix, M. superbum and P. fulvidraco. As indicated above, H. molitrix and P. fulvidraco were also the most acutely sensitive species (Fig. 3a). 3.1.2. Development of WQC for PCP and comparison among methods for deriving chronic WQC Toxicity values adjusted for pH were used for the construction of SSDs. Two SSDs were derived for chronic toxicity using screened Fig. 2. Aquatic toxicity data of pentachlorophenol included for derivation of species sensitivity distributions. n is the number of data points and the line in the boxplot indicates the median toxicity value. Geometric mean was used when there was more than one toxicity value for a species. Concentration (95% CI) (μg/L) 21.63 (18.64–26.63) 98.54 (85.22–111.85) 151.80 (135.82–173.11) 181.10 (165.12–197.08) 367.52 (351.54–386.16) 450.08 (412.80–476.71) 548.62 (503.34–599.22) 884.18 (761.68–1028.00) 1861.58 (1720.43–3542.06) experimental data only (n = 16) or experimental data and ACRderived data combined (n = 30) (Fig. 3). The HC5 of the SSD for acute and chronic toxicity were 36.22 and 3.48 μg/L, respectively. To protect 95% of species, the CMC was 18.11 μg/L and the CCC was 1.74 μg/L. The chronic HC5 based on both screened and ACR predicted toxicity values was 4.66 μg/L (n = 30), the corresponding CCC was 2.33 μg/L, 1.5-fold greater than the CCC derived with screened data only. The CMC obtained in this study was similar to the CMC (19 μg/L, freshwater, at pH = 7.8) used by the USEPA, while the CCC, derived in this study was almost 8.6-fold less than the CCC (15 μg/L, freshwater, at pH = 7.8) used by the USEPA. This result suggests that the USEPA chronic water criteria would be less protective than the more site-specific WQC developed with endemic species for Tai Lake. However, this result is likely due to variation that would be observed among empirical results. 3.2. Development of WQC based on historical species composition 3.2.1. Historical changes in species composition Numbers of species of phytoplankton, macrophytes, zooplankton, benthic animals and fishes in the last century and the early 21st century were collected and compared (Tables S4–S8). In the 1980s, there were seven phyla of phytoplankton, three phyla of macrophytes, three phyla of zooplankton, five phyla of benthic animals and fourteen orders of fishes. In recent years, the number of phyla was eight, three and nine for phytoplankton, benthic animals, and fish, respectively (Cai et al., 2010; Chen and Liu, 2003; Gu et al., 2005). Numbers of species of phytoplankton have increased, with a majority increase in cyanobacteria. This is likely due to increasing pollution from rapid human population growth and industrial development around Tai Lake (Fan, 1996). Proportions of plants, invertebrates and vertebrates were 38%, 40% and 22%, respectively, in 1980s, and 67%, 21%, and 12%, respectively in the early 21st (Fig. 4). The number of relatively more sensitive fish decreased significantly from 107 to 60 (Fig. S1), such as Cynoglossus purpureomaculatus, Fugu obscurus, Opsariichthys uncirostris bidens, and Distoechodon tumirostris (Table S9). Lots of migratory fishes (e.g., Acipenser sinensis, Tenualosa reevesii, Myxocyprinus asiaticus, Ochetobibus elongates, Luciobrama macrocephalalus, Elopichthys bambusa, Coreius heterokon, Rhinogobio typus Bleeker, Saurogobio dumerili, Cobitis sinensis, Leiocssis longirostris, Pseudobagrus taeniatus, etc.) almost disappeared. Numbers of species of Chlorophyta, Aulacoseira and Cyanophyta have increased greatly, especially for Chlorophyta, with an almost four-fold increase than that in 1987. Cyanobacteria became the dominant taxa since 1998 (Qian et al., 2008). However, there were reductions in numbers of species for invertebrates and fishes. Numbers of species of Mollusca, Arthropoda and Cypriniformes have decreased by nearly half. Invertebrates belonging to Aschelminthes, and some fish like Mugiliformes have disappeared. Numbers of species of invertebrates and fishes have decreased from 190 to 111, 107 to 60, respectively (Fan, 1996). Which might due to the increasing development of agriculture and industries and urbanization that has resulted in pollution of Tai Lake. 70 Y. Chen et al. / Science of the Total Environment 541 (2016) 65–73 Fig. 3. Species sensitivity distributions (SSDs) for acute (a) and chronic toxicity (b and c) of pentachlorophenol. Chronic toxicity values in (b) are experimental data whereas values in (c) include both experimentally and acute-chronic ratio (ACR) derived data. Values in red (a and b) are the data obtained in the present study, while those in red (c) are data calculated by the ACR method. 3.2.2. Weighted SSD based on 1980s and current species composition in Tai Lake Considering species diversity, the aquatic ecological community in the 1980s would be considered “healthier” than the current community. The theoretical basis of SSD is that the data on sensitivities of individual species to toxicants are distributed as a random sample from the entire population of possible sensitivities and are used to estimate descriptive parameters of the SSD. Generally, the SSD method has been found to be useful to predict the response of the entire community to toxicants (Maltby et al., 2005; Schroer et al., 2004), but there are still some issues regarding the application of SSDs (Forbes and Calow, 2002). As mentioned previously, the screened toxicity data include acute values from 30 species and chronic values from 16 species. The most sensitive species were L. hoffineisteri, fish, and crustaceans. The more sensitive species included H. molitrix, P. fulvidraco, Carassius auratus, I. punctatus, D. magna, M. superbum, M. piceus, and P. microlepis, which all belong to invertebrates and fishes. Since 1980, the total numbers of species of Fig. 4. Proportions of taxonomic groups (plants, invertebrates and vertebrates, in 1980s and 21st century in Tai Lake, China.) vertebrates and fishes decreased from 297 to 171, respectively (Fig. S1) and several species including C. purpureomaculatus, F. obscurus, O. uncirostris bidens, and D. tumirostris have disappeared (Table S9). The elimination of these sensitive species had an impact on the structure and function of the community. Since sensitive species that have disappeared from Tai Lake were not available, the WQC derived with toxicity data obtained in this study may not be adequate to protect the healthier community of 1980s. To generate the most appropriate criteria, HCp was calculated based on the concept that if the threshold for effect was equal to the effect concentration for the most sensitive species. The chance that a species would be affected would be 1/N (number of species considered in targeted basin.). However, the probability of adversely affecting functioning of the ecosystem would remain unknown (Wang et al., 2014). Since more tolerant plants or algae account for a large proportions of the species present currently, only the total number of invertebrates and vertebrates were considered when deriving the HCp (P = 1/297 and 1/171). Water quality criteria that would promote restoration of the more diverse community that was present in the 1980s (297 species) compared to that for the current community (171 species) are considered more protective. By considering the species compositions present in the 1980s and current the species compositions, weighted methods were used with SSDs to estimate HC5 or HCp as the cutoff point (Table 2). For weighted SSDs, HCp was used as the cutoff point, and the CMC and CCC for acute and chronic toxicity were 10.99 and 0.38 μg/L, respectively, by considering the number of invertebrates and vertebrates in 1980s. While, the CMC and CCC for acute and chronic toxicity were 32.81 and 4.48 μg/L, respectively, when using the number of invertebrates and vertebrates in the current community. The acute and the chronic values derived by the weighted SSDs combined with HCp considering that species compositions of the 1980s were slightly more conservative than values obtained with the conventional SSD (HC5/2, 18.11 and 1.74 μg/L) (Table 2, Fig. 5). The acute value generated by the weighted SSD was Y. Chen et al. / Science of the Total Environment 541 (2016) 65–73 71 Table 2 Comparison of water quality criteria derived in this study and current national standard. Method Cutoff point Acute value with 95% CI (μg/L) Chronic value with 95% CI (μg/L) Current national standard (μg/L) 0.05 0.05 P1 0.05 P2 36.22 (27.99–48.19) 48.64 (39.36–61.23) 10.99 (−) 104.23 (83.95–131.52) 32.81 (24.77–50.58) 3.48 (−) 4.72 (−) 0.38 (−) 21.38 (11.80–42.66) 4.48 (−) 9 Unweighted SSD Weighted SSDa Weighted SSDb a b 1 2 Weighted SSD based on species composition in 1980s. Weighted SSD based on current species composition. Cutoff point selected by considering the number of species in 1980s. Cutoff point selected by considering the current number of species. 3-fold lower than the value obtained with the unweighted method, while the chronic value was approximately 7-fold lower than that obtained with unweighted method. Criteria considering number of species (sensitive invertebrates and vertebrates), with HCp as the cutoff point resulted in the most conservative WQC (Table 2). As we know, using models built for two species in the same taxonomic level, when predicted toxicity values were compared with the actual values, even for species in the same kingdom, differences were greater than 10-fold for 29% of data points studied (Raimondo et al., 2010). In order to decrease the uncertainty, further taxonomic level classification should be performed to improve the values estimated by use of the SSD, because for species in the same family, predicted values were with 5fold of actual value for 91% of data points for species in the same family (Raimondo et al., 2010). Whereas, when community structure were considered, uncertainty was reduced and the developed WQC are more protective (Versteeg et al., 1999). Additionally, while taking into account the relative proportions of species and their respective sensitivities, the weighted SSD method with cutoff point HCp is likely to be more protective of the most sensitive species compared to the conventional SSD with cutoff point HC5 (Wang et al., 2014). From a conservation perspective, the weighted SSD method with cutoff point HCp is deemed to be more appropriate to protect ecosystem integrity since it would not be possible to determine if the most sensitive species are keystone species upon which ecosystem function might be dependent. 3.3. Toxicity in water of Tai Lake 3.3.1. Water effect ratio (WER) Water from the Dapu site was the most toxic to P. fulvidraco, N. denticulate, H. nobilis, D. magna, R. chensinensis, L. hoffmeisteri, and S. obliquus compared with the other three sites (Table S15). Because toxicity of PCP is affected by pH, water effect ratios (WER) were calculated after correcting original toxicity data for pH (Table 3). The WER was 4.72, 5.81, 5.98 and 7.21 for Dapu, Huangnian, Chendong, and Fenshui, respectively. The range of WER values among locations was relatively small so the most conservative value of 4.72 observed at Dapu was chosen to be protective. Based on surveys conducted in July 2013 and December 2013, four physicochemical parameters (Temperature, pH, turbidity and Chl a) (Table S16) and concentrations of 13 elements (Cr, Pb, V, and Zn et al) (Table S17) of the water samples from all sampling sites were summarized, respectively. Concentrations of Cr, Pb, V, and Zn (Table S16) were the greatest at Dapu (Table S17), which might result into a relatively small WER value at Dapu. Therefore, in order to derive the WQC conservatively, the WER of Dapu (4.72) was applied to derive site-specific WQC considering real water effect. The fact that the WER was greater than 1.0 suggests that there were factors in water from Tai Lake that reduced the exposure (i.e., bioavailability) and/or toxicity of PCP to test organisms. This is not uncommon since standard laboratory testing procedures is likely to generate more conservative toxicity estimates due to procedures such as continuous exposure and use of clean water in which there lacks ligands that can bind to toxicants to reduce bioavailability. 3.3.2. Development of Tai Lake site-specific WQC Using a WER of 4.72, when pH = 7.8, to restore the aquatic species composition in 1980s, the CMC and CCC values were 51.87 and 1.79 μg/L, respectively. Considering the relationship between LC50/ EC50 and WQC (Xing et al., 2012a), the WQC were derived as a function of pH. Eqs. (7)–(8) (Eqs. (7) and (8) were equations for acute and chronic criteria as a function of pH): CMC ðμg=LÞ ¼ exp:ð0:7330 pH 1:769Þ ð7Þ CCC ðμg=LÞ ¼ exp:ð0:7330 pH 5:135Þ ð8Þ Site-specific WQC derived during this study were compared to the current threshold value of 9 μg/L specified by water quality standard (WQS) for surface water source for drinking water (ChinaEPA. GB38382002, 2002) (Table 4, Fig. 6). The current national criteria of 9 μg/L, Fig. 5. Acute (a) and chronic (b) unweighted (orange) and weighted (green) pentachlorophenol species sensitivity distributions (SSDs) for aquatic organisms in Tai Lake, China. The SSDs were derived using the 1980s species composition data. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) 72 Y. Chen et al. / Science of the Total Environment 541 (2016) 65–73 Table 3 Acute toxicity of pentachlorophenol during in situ exposures of nine aquatic species at pH = 7.80. Sites Speciesa In situ EC50/LC50 (μg/L) EC50/LC50 (μg/L) Water effect ratio (WER) Final WER Chendong D. magna L. hoffmeisteri R. chensinensis S. obliquus H. molitrix C. idellus H. nobilis P. fulvidraco N. denticulate D. magna L. hoffmeisteri R. chensinensis S. obliquus H. molitrix C. idellus H. nobilis P. fulvidraco N. denticulate D. magna L. hoffmeisteri R. chensinensis S. obliquus H. molitrix C. idellus H. nobilis P. fulvidraco N. denticulate D. magna L. hoffmeisteri R. chensinensis S. obliquus H. molitrix C. idellus H. nobilis P. fulvidraco N. denticulate 1640.19 5703.22 1470.04 12,774.40 120.66 2081.39 904.95 482.64 444.93 1360.43 5116.84 1121.85 8177.80 138.13 1657.54 546.23 295.09 433.22 2295.05 7181.03 1767.27 14,394.05 282.53 866.44 1406.39 539.95 596.46 2119.78 7032.76 1691.23 11,999.31 131.98 1370.58 558.39 380.72 568.54 210.02 792.79 433.91 1841.21 17.00 290.68 143.00 77.94 120.06 210.02 792.79 433.91 1841.21 17.00 290.68 143.00 77.94 120.06 210.02 792.79 433.91 1841.21 17.00 290.68 143.00 77.94 120.06 210.02 792.79 433.91 1841.21 17.00 290.68 143.00 77.94 120.06 7.81 7.19 3.39 6.94 7.10 7.16 6.33 6.19 3.71 6.48 6.45 2.59 4.44 8.13 5.70 3.82 3.79 3.61 10.93 9.06 4.07 7.82 16.62 2.98 9.83 6.93 4.97 10.09 8.87 3.90 6.52 7.76 4.72 3.90 4.89 4.74 5.98 Dapu Fenshui Huannian Fig. 6. Comparison among water quality criteria (WQC) derived from the present study, the current water quality standard (WQS) in China and WQC provided by U.S. EPA. 4.72 water_sanitation_health/en/). Unlike the criteria of PCP in US EPA, all of these general criteria values do not take into account the effect of pH on toxicity. Our results indicate that species compositions and the physicochemical properties of the local basins should be considered in the derivation of WQC so that site-specific water standards can be obtained to address the protection goals for a particular site. 7.21 4. Conclusions 5.81 a Endpoints for S. obliquus, D. magna and other species are 24 h EC50, 48 h EC50 and 96 h EC50s, respectively. which directly referred to the guideline value of World Health Organization (WHO) (9 μg/L) (http://www.who.int/water_sanitation_health/ en/), would not be sufficient to protect the biotic community present in Tai Lake during the 1980s (1.79 μg/L). Due to slightly acidic conditions, the current Chinese WQS would be under-protective of the adverse effects of PCP on a more integrated community in the 1980s. According to directive of European Union, the annual average of environmental quality standards for PCP in surface waters was 0.4 μg/L, and the maximum allowable concentration was 1 μg/L (EP and EU, 2008), which were stricter than the criteria used by US EPA and the criteria derived for Tai Lake at a pH of 7.8 from the current study. As for the trigger values of PCP for freshwater in Australian and New Zealand, the 95% protection level trigger value of PCP was 10 μg/L (ANZECC and ARMCANZ, 2000). And the guideline value given by WHO, as mentioned above, was 9 μg/L (http://www.who.int/ In conclusion, without considering the species composition and sitespecific water effect in Tai Lake, the acute water quality criteria of PCP in Tai Lake was determined to be 18.11 μg/L, and the chronic criteria derived with only experimental data only and experimental and ACRderived data combined were 1.74 and 2.33 μg/L, respectively. In order to restore the aquatic ecological community of the 1980s, SSDs taking the species composition in the 1980s into consideration were generated. The acute and chronic criteria derived from these SSDs were 10.99 and 0.38 μg/L, respectively. Finally, a WER of 4.72 was obtained which resulted in a final acute criterion of 51.87 μg/L and a final chronic criterion of 1.79 μg/L. Our results suggest that the use of laboratory and in situ toxicity data with further consideration of site-specific species composition generate most appropriate WQC for Tai Lake and the current national criteria of 9 μg/L would not be sufficient to protect the biotic community present in Tai Lake. Acknowledgements This work has been co-financially supported by theNational Natural Science Foundation (No. 21377053) and Major National Science and Technology Projects (No. 2012ZX07506-001 and 2012ZX07501-00302) of China. Dr. Giesy was supported by the program of 2012 “High Level Foreign Experts” (#GDW20123200120) funded by the State Administration of Foreign Experts Affairs, the P.R. China to Nanjing University and the Einstein Professor Program of the Chinese Academy of Sciences. He was also supported by the Canada Research Chair program, a Visiting Distinguished Professorship in the Department of Biology and Chemistry and State Key Laboratory in Marine Pollution, City University of Hong Kong. In addition, the authors would like to thank Allen Burton for his help in English. Table 4 Water quality criteria (μg/L) for pentachlorophenol based on species compositions in Tai Lake during 1980s at different pH values. a a pH=6.00 pH=6.50 pH=7.50 pH=8.00 pH=8.50 pH=9.00 CMC/bCCC CMC/CCC CMC/CCC CMC/CCC CMC/CCC CMC/CCC CMC/CCC 13.86/0.48 20.00/0.69 28.85/0.99 41.62/1.44 60.04/2.07 86.62/2.99 124.96/4.31 pH=7 .00 CMC = criteria maximum concentration, bCCC = criteria continuous concentration. The data in red were less than current water quality criteria 9 μg/L in China. Y. Chen et al. / Science of the Total Environment 541 (2016) 65–73 Appendix A. Supplementary data Supplementary data to this article can be found online at http://dx. doi.org/10.1016/j.scitotenv.2015.09.006. References ANZECC, ARMCANZ, 2000. Australian and New Zealand guidelines for fresh and marine water quality vol. 1. Bello, D., Trasar, C., Leirós, M., 2013. Modification of enzymatic activity in soils of contrasting pH contaminated with 2, 4-dichlorophenol and 2, 4, 5-trichlorophenol. Soil Biol. Biochem. 56, 80–86. Cai, Y., Gong, Z., Qin, B., 2010. Community structure and diversity of macrozoobenthos in Lake Taihu,a large shallow eutrophic lake in China. Biodivers. Sci. 18, 50–59. Carlson, A., 1984. Guidelines for Deriving Numerical Aquatic Site-specific Water Quality Criteria By Modifying National Criteria. Environmental Research Laboratory, Office of Research and Development, US Environmental Protection Agency. CCME, 2007. A Protocol for the Derivation of Water Quality Guidelines for the Protection of Aquatic Life. Canadian Council of Ministers of the Environment, Winnipeg, Manitoba. Chapman, P., Farrel, M., Brinkhurst, R., 1982. Relative tolerances of selected aquatic oligochaetes to combinations of pollutants and environmental factors. Aquat. Toxicol. 2, 69–78. Chen, L., Liu, Y., 2003. Ecological succession and sustainable development in Taihu Lake. J. East China Normal Univ. (Nat. Sci.) 99–106. Chen, Y., Huang, J., Xing, L., Liu, H., Giesy, J., Yu, H., et al., 2014. Effects of multigenerational exposures of D. magna to environmentally relevant concentrations of pentachlorophenol. Environ. Sci. Pollut. Res. 21, 234–243. ChinaEPA. GB3838-2002, 2002. Environmental Quality Standard for Surface Water (in Chinese). China State Environmental Protection Administration, Beijing. EP, EU, 2008. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008. Off. J. Eur. Union. Fan, C., 1996. Historical evolution of water ecological setting in Taihu Lake. J. Lake Sci. 8, 297–304. Forbes, V., Calow, P., 2002. Species sensitivity distributions revisited: a critical appraisal. Hum. Ecol. Risk. Assess. 8, 473–492. Gao, J., Liu, L., Liu, X., Zhou, H., Huang, S., Wang, Z., 2008. Levels and spatial distribution of chlorophenols - 2,4-dichlorophenol, 2,4,6-trichlorophenol, and pentachlorophenol in surface water of China. Chemosphere 71, 1181–1187. Gu, X., Zhang, S., Bai, X., Hu, W., Hu, Y., Wang, X., 2005. Evolution of community structure of aquatic macrophytes in east Taihu Lake and its wetlands. Acta Ecol. Sin. 25, 1541–1548. Hodin, F., Boren, H., Grimvall, A., 1991. Formation of chlorophenols and related compounds in natural and technical chlorination processes. Water Sci. Technol. 24, 403–410. Jin, X., Zha, J., Xu, Y., Giesy, J., Wang, Z., 2012a. Derivation of predicted no effect concentrations (PNEC) for 2, 4, 6-trichlorophenol based on Chinese resident species. Chemosphere 86, 17–23. Jin, X., Zha, J., Xu, Y., Giesy, J., Wang, Z., 2012b. Toxicity of pentachlorophenol to native aquatic species in the Yangtze River. Environ. Sci. Pollut. Res. 19, 609–618. Kishino, T., Kobayashi, K., 1995. Relation between toxicity and accumulation of chlorophenols at various pH, and their absorption mechanism in fish. Water Res. 29, 431–442. Lei, Z., Xu, D., Gu, J., Liu, Z., 2008. Distribution characteristics of aquatic macrophytes and their effects on nutrients of water and sediment in Taihu Lake. J. Agro-Environ. Sci. 27, 698–704. Luo, Q., Zha, J., Lei, B., Xu, Y., Wang, Z., 2009. Review of the aquatic ecotoxicology and water quality criteria of three chlorophenols. Acta Sci. Circumst. 29, 2241–2249. Maltby, L., Blake, N., Brock, T.C.M., 2005. Insecticide species sensitivity distributions: importance of test species selection and relevance to aquatic ecosystems. Environ. Toxicol. Chem. 24, 379–388. Mari, M., Borrajo, M., Schuhmacher, M., 2007. Monitoring PCDD/Fs and other organic substances in workers of a hazardous waste incinerator: a case study. Chemosphere 67, 574–581. Martí, E., Sierra, J., Cáliz, J., 2011. Ecotoxicity of chlorophenolic compounds depending on soil characteristics. Sci. Total Environ. 409, 2707–2716. 73 Ni, Y., Zhu, C., 2005. Fishes of the Taihu Lake. Shanghai Scientific and Technical Publishers, Shanghai. Qian, K., Chen, Y., Song, X., 2008. Long-term development of phytoplankton dominant species related to eutrophicarion in Lake Taihu. Ecol. Sci. 27, 65–70. Qu, L., Ming, X., Zou, H., 2004. Determination of chlorophenols in drinking water and water resource by solidphase microextraction and gas chromatogryphy. Environ. Pollut. Control 26, 154–157. Raimondo, S., Jackson, C., Barron, M., 2010. Influence of taxonomic relatedness and chemical mode of action in acute interspecies estimation models for aquatic species. Environ. Sci. Technol. 44, 7711–7716. Schroer, A.F.M., Belgers, D., Brock, T.C.M., Maund, S.J., Van den Brink, P.J., 2004. Acute toxicity of the pyrethroid insecticide lambda- cyhalothrin to invertebrates of lenthic freshwater ecosystems. Arch. Environ. Contam. Toxicol. 46, 324–335. Shi, R., Yang, C., Su, R., Jin, J., Chen, Y., Liu, H., et al., 2014. Weighted species sensitivity distribution method to derive site-specific quality criteria for copper in Tai Lake, China. Environ. Sci. Pollut. Res. 21, 12968–12978. Sinclair, G., Paton, G., Meharg, A., 1999. Lux-biosensor assessment of pH effects on microbial sorption and toxicity of chlorophenols. FEMS Microbiol. Lett. 174, 273–278. Stephan, C., Mount, D., Hansen, D., Gentile, J., Chapman, G., Brungs, W., 1985. Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses. National Technical Information Service, Springfield, VA, USA. Sun, S., Huang, Y., 1993. Taihu Lake. Ocean Press, Beijing (In Chinese). Suter, G., Cormier, S., 2008. What is meant by risk-based environmental quality criteria? Integr. Environ. Assess. Manag. 4, 486–489. USEPA, 2002. National recommended water quality criteria. Office of Science and Technology, US Environmental Protection Agency. USEPA, 2004. The incidence and severity of sediment contamination in surface waters of United States. Vol. 1: National Sediment Quality Survey. Office of Science and Technology, US Environmental Protection Agency. USEPA, 2009. National recommended water quality criteria. Office of Science and Technology, US Environmental Protection Agency. Versteeg, D., Belanger, S., Carr, G., 1999. Understanding single-species and model ecosystem sensitivity: data-based comparison. Environ. Toxicol. Chem. 18, 1329–1346. Wang, Y., Zhang, L., Meng, F., Zhou, Y., Jin, X., Giesy, J., et al., 2014. Improvement on species sensitivity distribution methods for deriving site-specific water quality criteria. Environ. Sci. Pollut. Res. Wu, F., Meng, W., Song, Y., Liu, Z., Jin, X., Zheng, B., 2008. Research progress in lake water quality criteria in China. Acta Sci. Circumst. 28, 2385–2392. Xing, L., Liu, H., Zhang, X., Hecker, M., Giesy, J., Yu, H., 2012a. pH-dependent aquatic criteria for 2, 4-dichlorophenol, 2, 4, 6-trichlorophenol and pentachlorophenol. Sci. Total Environ. 441, 125–131. Xing, L., Sun, J., Liu, H., Yu, H., 2012b. Combined toxicity of three chlorophenols 2,4-dichlorophenol, 2,4,6-trichlorophenol and pentachlorophenol to Daphnia magna. J. Environ. Monit. 14, 1677–1683. Yin, D., Jin, H., Yu, L., Hu, S., 2002. Deriving freshwater quality criteria for 2,4-dichlorophenol for protection of aquatic life in China. Environ. Pollut. 122, 217–222. Yin, D., Hu, S., Jin, H., Yu, L., 2003. Deriving freshwater quality criteria for 2,4,6trichlorophenol for protection of aquatic life in China. Chemosphere 52, 67–73. Yin, D., Zhu, H., Wu, Z., 2008. Research on molecular ecological toxicity of typical chlorophenols. Chinese Academic Journal Electronic Publishing House, pp. 36–37. Zhang, Y., Yan, J., Zhao, Q., Zheng, Q., Du, Y., Li, J., 2007. Test method of fish acute toxicity for dangerous chemical products. Zheng, M., Zhu, L., 2005. Toxicity effects of pentachlorophenol on Brachydanio rerio. Chin. J. Appl. Ecol. 16, 1967–1971. Zheng, M., Zhang, B., Bao, Z., Yang, H., Xu, B., 2000. analysis of pentachlorophenol from water, sediments, and fish bile of Dongting Lake in China. Bull. Environ. Contam. Toxicol. 64, 16–19. Zheng, B., Tian, Z., Zhang, L., Zheng, F., 2007. The characteristics of the Hydrobios' distribution and the analysis of water quality along the west shore of Taihu Lake. Acta Ecol. Sin. 27, 4214–4223. Zheng, W., Yu, H., Wang, X., Qu, W., 2012. Systematic review of pentachlorophenol occurrence in the environment and in humans in China: not a negligible health risk due to the re-emergence of schistosomiasis. Environ. Int. 42, 105–116. Zhou, W., Fu, D., Sun, Z., 1990. List for priority pollutants in waters. Environ. Monit. China 6, 1–3. Supplementary data Table S1 Average life expectancy of tested species Species Silver carp (Hypophthalmichthy smolitrix) Yellow catfish (Pelteobagrus fulvidraco) Shrimp (Neocaridina denticulate) Bighead carp (Hypophthalmichthys nobilis) Grass carp (Ctenopharynodon idellus) Water flea (Daphnia magna) Wood frog (Rana chensinensis) Oligochaete (Limnodrilus hoffmeisteri) Average Life Expectancy 7-8 Years 2-4 Years 12-15 months 7-8 Years 7-8 Years 68.40±9.82 Days 7-8 Years 80 Days 1 Sources https://en.wikipedia.org/ https://en.wikipedia.org/ https://en.wikipedia.org/ https://en.wikipedia.org/ https://en.wikipedia.org/ (Zhuang and Liang, 1986) https://en.wikipedia.org/ https://en.wikipedia.org/ Table S2. Acute toxicities, expressed a median lethal concentrations of PCP to aquatic organisms. Species Endpoint Effect L. hoffmeisteri LC50 MORT L. hoffmeisteri LC50 Ceriodaphnia reticulata Exposure Concentration method pH 4 R, U 7.00 500.00 (Chapman et al., 1982) MORT 4 S, U 8.12 884.18 This study LC50 MORT 2 F, M 7.30 150.00 (Hedtke et al., 1986) Ceriodaphnia dubia LC50 MORT 2 F, M 8.00 307.00 (Hedtke et al., 1986) Daphnia carinata LC50 MORT 2 R, U 7.90 570.00 (Hickey, 1989) D. magna LC50 MORT 2 S, M 8.58 145.00 (Spehar et al., 1985) D. magna LC50 MORT 2 S, U 6.50 55.00 (Oikari et al., 1992) D. magna LC50 MORT 2 S, U 6.50 38.00 (Oikari et al., 1992) D. magna LC50 MORT 2 S, U 6.50 55.00 (Oikari et al., 1992) D. magna LC50 MORT 2 S, M 8.00 860.00 (Kim et al., 2006) D. magna LC50 IMBL 2 S, U 7.10 62.00 (Xing et al., 2012) D. magna LC50 IMBL 2 S, U 8.00 346.00 (Xing et al., 2012) D. magna EC50 IMBL 2 S, U 8.80 1232.00 (Xing et al., 2012) D. magna EC50 IMBL 2 S, U 8.12 450.08 This study Simocephalus vetulus LC50 MORT 2 F, M 7.30 160.00 (Hedtke et al., 1986) Simocephalus vetulus LC50 MORT 2 F, M 7.70 250.00 (Hedtke et al., 1986) Simocephalus vetulus LC50 MORT 2 F, M 8.00 255.00 (Hedtke et al., 1986) Simocephalus vetulus LC50 MORT 2 F, M 8.30 364.00 (Hedtke et al., 1986) Mesocyclops leuckarti LC50 MORT 2 S, U 7.30 138.00 (Willis, 1999) Mesocyclops leuckarti LC50 MORT 2 S, U 7.30 173.00 (Willis, 1999) Chironomus riparius LC50 MORT 1 S, U 9.00 1948.00 (Warwick and Wadleigh, 1986) Chironomus riparius LC50 MORT 1 S, U 4.00 384.00 (Warwick and Wadleigh, 1986) (d) 2 (μg/L) Reference Species Endpoint Effect Chironomus riparius LC50 MORT Macrobrachium superbum LC50 N. denticulate Exposure Concentration Reference method pH 1 S, U 6.00 465.00 (Warwick and Wadleigh, 1986) MORT 4 R, M 7.24 140.00 (Jin et al., 2012) LC50 MORT 4 S, U 8.12 151.80 This study Corbicula fluminea LC50 MORT 4 R, M 7.24 230.00 (Jin et al., 2012) Lymnaea acuminata LC50 MORT 2 R, U 7.90 228.00 (Gupta and Durve, 1984) Viviparus bengalensis LC50 MORT 2 S, U 7.90 1570.00 (Gupta and Durve, 1984) Brachionus calyciflorus EC50 HTCH 2 S, U 7.50 1310.00 (Radix et al., 2000) Lemna minor LC50 MORT 3 S, U 5.10 190.00 (Blackman et al., 1955) Soirodela polyrhiz LC50 GGRO 4 R, M 7.24 1120.00 (Jin et al., 2012) Chlamydomonas reinhardtii EC50 PGRT 4 F, U 7.00 410.00 (Schafer et al., 1994) Chlorella kessleri EC50 ABND 4 S, U 8.00 34300.00 (Mostafa and Helling, 2002) S. obliquus EC50 GGRO 1 S, U 6.50 261.00 (Xing et al., 2012) S. obliquus EC50 GGRO 1 S, U 7.50 927.00 (Xing et al., 2012) S. obliquus EC50 GGRO 1 S, U 9.00 24063.00 (Xing et al., 2012) S. obliquus EC50 MORT 1 S, U 8.12 1861.58 This study Carassius auratus LC50 MORT 4 S, U 7.30 23.00 (Inglis and Davis) Carassius auratus LC50 MORT 4 S, U 7.70 49.00 (Inglis and Davis) Carassius auratus LC50 MORT 4 S, U 7.50 55.00 (Inglis and Davis) Carassius auratus LC50 MORT 4 S, U 7.30 56.00 (Inglis and Davis) Carassius auratus LC50 MORT 4 F, M 7.84 200.00 (Thurston et al., 1985) Carassius auratus LC50 MORT 4 F, M 7.94 328.00 (Thurston et al., 1985) C. idellus LC50 MORT 4 S, U 8.12 367.52 This study Erythroculter ilishaeformis LC50 MORT 4 R, M 7.24 130.00 (Jin et al., 2012) (d) 3 (μg/L) Species Endpoint Effect H. smolitrix LC50 MORT H. nobilis LC50 Mylopharyngodon piceus Exposure Concentration Reference method pH 4 S, U 8.12 21.63 This study MORT 4 S, U 8.12 181.10 This study LC50 MORT 4 R, M 7.24 95.00 (Jin et al., 2012) Plagiognathops microlepis LC50 MORT 4 R, M 7.24 90.00 (Jin et al., 2012) Oncorhynchus mykiss LC50 MORT 4 S, NR 7.40 115.00 (Mayer and Ellersieck, 1986) Oncorhynchus mykiss LC50 MORT 4 S, NR 7.40 34.00 (Mayer and Ellersieck, 1986) Oncorhynchus mykiss LC50 MORT 4 S, NR 7.40 52.00 (Mayer and Ellersieck, 1986) Oncorhynchus mykiss LC50 MORT 4 S, NR 7.40 121.00 (Mayer and Ellersieck, 1986) Oncorhynchus mykiss LC50 MORT 4 F, U 7.40 66.00 (Dominguez and Chapman, 1984) Oncorhynchus mykiss LC50 MORT 4 F, M 7.85 115.00 (Thurston et al., 1985) Oncorhynchus mykiss LC50 MORT 4 S, U 8.00 160.00 (Sappington et al., 2001) Oncorhynchus mykiss LC50 MORT 4 R, NR 6.22 153.00 (1991) Oncorhynchus mykiss LC50 MORT 4 S, U 8.00 160.00 (Dwyer et al., 2005) Ictalurus punctatus LC50 MORT 4 S, NR 7.40 66.00 (Mayer and Ellersieck, 1986) Ictalurus punctatus LC50 MORT 4 S, NR 7.40 68.00 (Mayer and Ellersieck, 1986) Ictalurus punctatus LC50 MORT 4 F, M 7.71 132.00 (Thurston et al., 1985) P. fulvidraco LC50 MORT 4 S, U 8.12 98.54 This study R. chensinensis LC50 GGRO 4 S, U 8.12 548.62 This study (d) 4 (μg/L) Table S3. Chronic toxicities, expressed as the maximum allowable toxicant concentration (MATC) or EC10 during chronic exposure of aquatic organisms to PCP. Species Endpoint Effect Exposure (d) Method pH Concentration (μg/L) Reference Ceriodaphnia dubia MATC PROG 14 R, U 7.90 158.00 (Hickey, 1989) Daphnia carinata MATC PROG 14 R, U 7.90 354.00 (Hickey, 1989) D. magna EC10 PGRT 21 S, U 8.12 4.79 This study Simocephalus vetulus MATC PROG 14 R, U 7.90 71.00 (Hickey, 1989) Mesocyclops leuckarti MATC MORT 6 S, U 7.30 104.00 (Willis, 1999) Macrobrachium superbum MATC SURV 21 R, M 7.24 12.00 (Jin et al., 2012) Corbicula fluminea MATC SURV 21 R, M 7.24 70.00 (Jin et al., 2012) Brachionus calyciflorus EC10 HTCH 2 S, M 7.50 600.00 (Radix et al., 2000) Soirodela polyrhiz MATC CHLO 10 R, M 7.24 350.00 (Jin et al., 2012) Chlamydomonas reinhardtii NOEC PGRT 10 F, M 7.00 360.00 (Schafer et al., 1994) Chlorella kessleri EC10 ABND 2 S, U 8.00 8650.00 (Mostafa and Helling, 2002) S. obliquus EC10 GGRO 3 S, U 6.50 174.00 (Xing et al., 2012) S.obliquus EC10 GGRO 3 S, U 7.50 301.00 (Xing et al., 2012) S. obliquus EC10 GGRO 3 S, U 9.00 12440.00 (Xing et al., 2012) Erythroculter ilishaeformis MATC GGRO 28 R, M 7.24 25.00 (Jin et al., 2012) Mylopharyngodon piceus MATC GGRO 28 R, M 7.24 14.00 (Jin et al., 2012) Plagiognathops microlepis MATC GGRO 28 R, M 7.24 14.00 (Jin et al., 2012) Oncorhynchus mykiss EC10 DBMS 30 F, M 8.30 60.00 (Besser et al., 2005) Oncorhynchus mykiss MATC DWGT 30 F, M 8.30 51.00 (Besser et al., 2005) Note: * Results from the present study; F-flow through; R-renewal; S-static; NR-not reported; U-not measured; M-measured; LC50-lethal concentration; EC10/EC50-effect concentration for 10% and 50% of the population; MATC-maximum acceptable toxic concentration; NOEC-no observed effect concentration; MORT-mortality; IMBL-immobility; GGRO-general growth;GGRT-growth rate;SURV-survival;WGHT-weight;CHLO-chlorophyll;HTCH-hatchability;ABND-abundance;PGRT-population growth rate; DWGT-dry weight;DBMS-dry biomass 5 Table S4. Number of taxa of phytoplankton in various families occurring in Tai Lake a---from historical survey. Phyla 1960 1980 1987 2010 Chlorophyta 48 54 39 131 Aulacoseira 18 25 25 75 Cyanophyta 15 17 19 20 Chrysophyta 1 3 5 3 Xanthophyta 2 3 0 2 Cryptomonas 0 2 3 3 Pyrrophyta 2 4 3 8 Euglenophyta 5 6 3 36 Total 91 114 97 278 a Note: (Ni and Zhu, 2005; Sun and Huang, 1993; Xu and Zhang, 2012) 6 Table S5. Number of species of aquatic macrophytes in several families found to be occurring in Tai Lake during historical surveys b. Phyla 1960 1981 1996 2002 Pteridophyta 4 4 4 4 Dicotyledonous 24 31 31 31 Monocotyledonous 38 31 39 40 Total 66 66 74 75 b Note: (Liu et al., 2007; Nanjing Institute of Geography, 1965; Sun and Huang, 1993; Zhang and Qian, 1999) 7 Table S6. Number of taxa of zooplankton of several families found in Tai Lake during historical surveys c. Phyla 1980 1987 2007 Protozoa 41 22 13 Trochelminthes 42 30 34 Arthropoda 39 27 24 Total 122 79 71 c Note: (Ni and Zhu, 2005; Sun and Huang, 1993; Zheng et al., 2007) 8 Table S7. Number of taxa in several families of benthic invertebrates identified during historical surveys of Tai Lake d. Phyla 1980 1987 2008 Coelenterata 1 1 0 Aschelminthes 11 0 0 Annelida 7 8 14 Mollusca 24 23 12 Arthropoda 25 27 14 Total 68 59 40 Note: d (Cai et al., 2010; Chen and Liu, 2003; Sun and Huang, 1993) 9 Table S8. Number of fishes in several families as determined during historical surveys of Tai Lake e. Order 1981 2006 Gadiformes 1 0 Anguilliformes 1 1 Clupeiformes 2 1 Cypriniformes 65 37 Siluriformes 10 4 Osmeriformes 4 4 Mugiliformes 2 0 Beloniformes 1 1 Cyprinodontiformes 1 1 Symbranchir 1 1 Scorpaeniformes 1 0 Perciformes 16 10 Pleuronectiformes 1 0 Tetraodontiformes 1 0 Total 107 60 Note: e (Chen and Liu, 2003; Sun and Huang, 1993; Zhu, 2004; Zhu and Liu, 2007) 10 Table S9. Fish species changed from 1980 to 2006 as determined during historical surveys of Tai Lake f. Species found in 1986 Species found in 2006 Acipenser sinensis Macrura reetesii Coilia ectenes Coilia ectenes Coilia ectenes taihuensis Coilia brachygnathus Coilia brachygnathus Reganisalanx brachyrostratis Protosalanx hyalocranius Protosalanx hyalocranius Neosalanx tanghahkeii taihuensis Neosalanx tanghahkeii taihuensis Neosalanx oligodontis Neosalanx oligodontis Anguilla japonica Cyprinus carpio Cyprinus carpio Carassius auratus Carassius auratus Pseudorasbora parrva Pseudorasbora parrva Sarcocheilichtys siensis Sarcocheilichtys siensis Sarcocheilichtys nigripinnis Sarcocheilichtys nigripinnis Abbottina rivularis Abbottina rivularis Abbottina fukiensis Saurogobio dabryi Saurogobio dumerili Squalidus noteas Paracanthobrama guichenoti Paracanthobrama guichenoti 11 Rhinogobio typus Coreius heterodon Gnathopogon argentatus Gnathopogon sihuensis Gnathopogon wolteisorffi Hemibarbus maculatus Hemibarbus maculatus Hemibarbus labeo Hemibarbus labeo Aphyocypris chinensis Elopichthys bambusa Elopichthys bambusa Opsariichthys uncirostris bidens Squaliobarbus curriculus Squaliobarbus curriculus Ochetobibus elongatus Mylopharyngodon piceus Mylopharyngodon piceus Ctenopharyngodon idellus Ctenopharyngodon idellus Toxabramis swinhonis Toxabramis swinhonis Parabramis pekinensis Pseudobrama simoni Megalobrama amblycephala Megalobrama amblycephala Megalobrame terminalis Hemicculter leuciclus Hemicculter leuciclus Hemiculter bleekeri Culter erythropterus Culter erythropterus Erythroculter ilishaeformis Erythroculter ilishaeformis 12 Erythroculter mongolicus Erythroculter mongolicus Erythroculter dabryi Erythroculter Parapelecus argenteus Parapelecus engraulis Distoechodon tumirostris Acanthobrama simoni Plagiognathops microlepis Xenocypris davidi Xenocypris argentea Acheilognathus Acheilognathus gracilis Acanthorhodeus macropterus Acanthorhodeus macropterus Acanthorhodeus barbatutus Acanthorhodeus barbatutus Acanthorhodeus tonkinensis Acanthorhodeus tonkinensis Acanthorhodeus taenianalis Acanthorhodeus chankaensis Pararsilurus fangi Rhodeus sinensis Rhodeus ocellatus Rhodeus light Rhodeus light Aristichthys nobilis Aristichthys nobilis Hypohthalmichthys molitrix Hypohthalmichthys molitrix 13 Sinilabeo decorus tungting Myxocypriuus asiaticus Cobitis taenia Cobitis taenia Botia xeaiithi Botia wui Paramisgurnus dabryanus Misgurnus mizolepis Pararhychobdella sinemsis Misgurnus anguillicaudatus Misgurnus anguillicaudatus Pseudobagrus fulvidraco Pseudobagrus fulvidraco Pseudobagrus eupogon Pseudobagrus nitidus Leiocassis longirostris Leiocassis ussuriensis LeiocassisL albomargintus LeiocassisL taeniatus LeiocassisL adiposalis Silurus asotus Silurus asotus Oryzias latipes Hemirhamphus intermedius Hemirhamphus intermedius Mugil cephalus Mugil soiuy Monopterus albus Monopterus albus 14 Siniperca scherzeri Siniperca chuatsi Siniperca chuatsi Siniperca kneri Lateolabrax japonicas Lateolabrax japonicus Hypseleotris swinhnonis Hypseleotris swinhnonis Odontobutis obscurus Odontobutis obscurus Odontamblyopus rubicundus Ctenogobius giurinus Ctenogobius giurinus Ctenogobius cliffordpopei Taenioides cirratus Macropodus chinensis Macropodus chinensis Caltionymus olidus Ophicephalus argus Ophicephalus argus Channa asiatica Mastacembelus aculeatus Trachidermus fasciatus Cynoglessus graclfls Cynoglossus trigrammus Cynoglossus purpureomaculatus Fugu obscurus Fugu ocellatus Total 107 60 Note: f (Zhu, 2004; Zhu and Liu, 2007) 15 Table S10. Information of sample sites in the most contaminated area in West Tai Lake. Coordinates Location Sample ID Sampling Date N E Huangnian HN0408 31°31.158’ 120°00.978’ April 8, 2013 Huangnian HN0726 31°31.158’ 120°00.978’ July 26, 2013 Huangnian HN1201 31°31.158’ 120°00.978’ December 1, 2013 Fenshui FS0408 31°29.513’ 120°01.573’ April 8, 2013 Fenshui FS0726 31°29.513’ 120°01.573’ July 26, 2013 Fenshui FS1201 31°29.513’ 120°01.573’ December 1, 2013 Chendong CD0408 31°19.345’ 119°55.342’ April 8, 2013 Chendong CD0726 31°19.345’ 119°55.342’ July 26, 2013 Chendong CD1201 31°19.345’ 119°55.342’ December 1, 2013 Dapu DP0408 31°19.074’ 119°55.237’ April 8, 2013 Dapu DP0726 31°19.074’ 119°55.237’ July 26, 2013 Dapu DP1201 31°19.074’ 119°55.237’ December 1, 2013 16 Table S11. Acute toxicities of PCP to aquatic species inhabiting Tai Lake (pH = 7.8). Species Concentration (μg/L) H. smolitrix 17.00 P. fulvidraco 77.94 Carassius auratus 90.70 Ictalurus punctatus 104.39 N. denticulate 120.06 Oncorhynchus mykiss 124.63 Plagiognathops microlepis 135.68 H. nobilis 143.00 Mylopharyngodon piceus 143.22 Erythroculter ilishaeformis 195.98 D. magna 210.02 Macrobrachium superbum 211.06 Lymnaea acuminata 211.89 Ceriodaphnia reticulata 216.40 Mesocyclops leuckarti 222.91 Simocephalus vetulus 242.36 Ceriodaphnia dubia 265.14 Ctenopharynodon idellus 290.68 Corbicula fluminea 346.73 R. chensinensis 433.91 Daphnia carinata 529.71 Chlamydomonas reinhardtii 736.98 Limnodrilus hoffmeisteri 792.79 Lemna minor 1374.88 Viviparus bengalensis 1459.04 Brachionus calyciflorus 1632.20 Soirodela polyrhiz 1688.45 S. obliquus 1841.21 Chironomus riparius 2060.72 Chlorella kessleri 29622.83 17 Table S12. Chronic toxicities of PCP to aquatic species inhabiting Tai Lake (pH = 7.8). Concentration (μg/L) Species D. magna 3.79 Macrobrachium superbum 18.09 Mylopharyngodon piceus 21.11 Plagiognathops microlepis 21.11 Oncorhynchus mykiss 38.34 Erythroculter ilishaeformis 37.69 Simocephalus vetulus 65.98 Corbicula fluminea 105.53 Ceriodaphnia dubia 146.83 Mesocyclops leuckarti 150.04 Daphnia carinata 328.98 Soirodela polyrhiz 527.64 Chlamydomonas reinhardtii 647.10 Brachionus calyciflorus 747.57 S. obliquus 955.92 Chlorella kessleri 7470.48 18 Table S13. Acute-Chronic Ratios (ACR) of all species available. Species Acute Concentration (μg/L) Chronic Concentration (μg/L) Acute-Chronic Ratios Final ACR Oncorhynchus mykiss Plagiognathops microlepis Mylopharyngodon piceus Erythroculter ilishaeformis Daphnia magna Macrobrachium superbum Mesocyclops leuckarti Simocephalus vetulus Ceriodaphnia dubia Corbicula fluminea Daphnia carinata Chlamydomonas reinhardtii Brachionus calyciflorus Soirodela polyrhiz Scenedesmus obliquus Chlorella kessleri 124.63 135.68 143.22 195.98 210.02 211.06 222.91 242.36 265.14 346.73 529.71 736.98 1632.20 1688.45 1841.21 29622.83 38.34 21.11 21.11 37.69 3.79 18.09 150.04 65.98 146.83 105.53 328.98 647.10 747.57 527.64 955.92 7470.48 3.25 6.43 6.79 5.20 55.43 11.67 1.49 3.67 1.81 3.29 1.61 1.14 2.18 3.20 1.93 3.97 3.75 19 Table S14. Chronic toxicities of PCP with ACR method. Species Daphnia magna Hypophthalmichthy smolitrix Macrobrachium superbum Pelteobagrus fulvidraco Mylopharyngodon piceus Plagiognathops microlepis Carassius auratus Ictalurus punctatus Neocaridina denticulate Erythroculter ilishaeformis Hypophthalmichthys nobilis Oncorhynchus mykiss Lymnaea acuminata Ceriodaphnia reticulata Simocephalus vetulus Ctenopharynodon idellus Corbicula fluminea Rana chensinensis Ceriodaphnia dubia Mesocyclops leuckarti Limnodrilus hoffmeisteri Daphnia carinata Lemna minor Viviparus bengalensis Soirodela polyrhiz Chironomus riparius Chlamydomonas reinhardtii Brachionus calyciflorus Scenedesmus obliquus Chlorella kessleri 20 Concentrations (μg/L) 3.79 4.56 18.09 20.78 21.11 21.11 24.19 27.84 32.02 37.69 38.20 38.34 56.50 57.71 65.98 77.51 105.53 115.71 146.83 150.04 211.41 328.98 366.64 389.08 527.64 549.53 647.10 747.57 955.92 7470.48 Table S15. Acute toxicity of pentachlorophenol to nine aquatic species during in situ incubations to determine the water effects ratio. Species H. smolitrix P. fulvidraco N. denticulate H. nobilis C. idellus D. magna Water Duration Endpoints pH Concentration (95% C.I.) (μg/L) Aerated water 4d LC50 8.1 21.63 (18.64-26.63) Huangnian 4d LC50 6.9 69.24 (63.92-74.57) Fenshui 4d LC50 6.6 119.84 (103.86-135.82) Dapu 4d LC50 6.6 58.59 (50.60-66.58) Chendong 4d LC50 6.4 42.61 (34.62-53.26) Aerated water 4d LC50 8.1 98.54 (85.22-111.85) Huangnian 4d LC50 6.9 199.74 (170.44-231.70) Fenshui 4d LC50 6.6 229.04 (199.74-260.99) Dapu 4d LC50 6.6 125.17 (111.85-141.15) Chendong 4d LC50 6.4 170.44 (151.80-189.09) Aerated water 4d LC50 8.1 151.80 (135.82-173.11) Huangnian 4d LC50 6.9 298.28 (263.66-340.89) Fenshui 4d LC50 6.6 253.00 (223.71-287.63) Dapu 4d LC50 6.6 183.76 (154.47-218.38) Chendong 4d LC50 6.4 157.13 (141.15-175.77) Aerated water 4d LC50 8.1 181.10 (165.12-197.08) Huangnian 4d LC50 6.9 292.95 (258.33-330.24) Fenshui 4d LC50 6.6 596.56 (529.98-673.79) Dapu 4d LC50 6.6 231.70 (199.74-268.98) Chendong 4d LC50 6.4 319.58 (287.63-356.87) Aerated water 4d LC50 8.12 367.52 (351.54-386.16) Huangnian 4d LC50 6.92 719.06 (673.79-767.00) Fenshui 4d LC50 6.63 367.52 (351.54-386.16) Dapu 4d LC50 6.6 703.08 (652.48-759.01) Chendong 4d LC50 6.4 735.04 (703.08-772.33) Aerated water 2d LC50 8.1 450.08 (412.80-476.71) 21 R. chensinensis L. hoffmeisteri S. obliquus Huangnian 2d LC50 6.4 737.71 (636.50-857.55) Fenshui 2d LC50 6.3 764.34 (663.14-881.52) Dapu 2d LC50 6.9 713.74 (612.54-830.92) Chendong 2d LC50 6.5 641.83 (537.97-769.66) Aerated water 4d LC50 8.1 548.62 (503.34-599.22) Huangnian 4d LC50 6.4 588.57 (561.94-615.20) Fenshui 4d LC50 6.3 588.57 (561.94-615.20) Dapu 4d LC50 6.9 588.57 (561.94-615.20) Chendong 4d LC50 6.5 575.25 (540.63-612.54) Aerated water 4d LC50 8.1 884.18 (761.68-1028.00) Huangnian 4d LC50 6.4 2447.48 (2154.53-2780.38) Fenshui 4d LC50 6.3 2391.55 (2122.57-2695.16) Dapu 4d LC50 6.9 2684.51 (2442.15-2950.83) Chendong 4d LC50 6.5 2231.76 (1925.49-2585.97) Aerated water 1d EC50 8.1 1861.58 (1720.43-3542.06) Huangnian 1d EC50 6.4 4175.90 (3424.88-5089.38) Fenshui 1d EC50 6.3 4793.76 (4490.16-5116.01) Dapu 1d EC50 6.9 4290.42 (4002.79-4599.35) Chendong 1d EC50 6.5 4998.83 (4700.55-5315.75) 22 Table S16. The physical and chemical characteristics of samples of water. Temp Turbidity Chl a (NTU+) (ug/L) pH Sample (℃) HN0408 17.06 6.4 38.4 3.9 HN0726 30.75 6.9 38.6 9.9 HN1201 10.30 6.7 58.2 5.7 FS0408 16.95 6.3 1.3 10.5 FS0726 27.68 6.6 53.0 1.1 FS1201 10.20 6.7 170.9 9.1 CD0408 15.73 6.5 217.5 95.4 CD0726 30.92 6.4 44.9 12.1 CD1201 9.10 6.9 38.4 13.3 DP0408 17.13 6.9 15.6 45.1 DP0726 31.56 6.6 41.4 11.3 DP1201 9.21 6.8 37.2 10.3 23 Table S17. Concentrations (μg/L) of inorganic elements in samples of water. Sample Cr Mn Cu Ni Pb V Fe Co Zn As Se Ag Ba HN0726 0.37 2.02 0.94 0.9 0.07 0.09 15.27 0.02 1.71 0.04 0.02 0.01 0.1 HN1201 0.19 0.17 0.83 0.86 0.04 0.03 3.84 0.01 1.47 0.01 0.03 0.01 0.84 CD0726 0.23 2.14 0.83 0.86 0.08 0.1 19.77 0.02 1.64 0.03 0.03 0.01 1.28 CD1201 0.18 0.16 0.79 0.81 0.04 0.04 3.95 0.01 1.5 0.01 0.02 0.01 0.92 FS0726 0.26 1.35 0.84 0.83 0.05 0.05 8.53 0.01 1.545 0.02 0.02 0.01 0.6 FS1201 0.19 0.16 0.82 0.83 0.04 0.03 3.78 0.01 1.51 0.01 0.03 0.01 0.74 DP0726 0.38 1.91 0.87 1.02 0.09 0.1 18.82 0.03 3.06 0.03 0.02 0.01 1.13 DP1201 0.20 0.20 0.81 0.84 0.05 0.05 5.17 0.01 1.72 0.02 0.03 0.01 1.25 24 Figure S1. Species numbers of three taxonomic groups in Tai Lake 25 Reference: Toxicokinetic studies of chlorinated phenols and polycyclic aromatic hydrocarbons in rainbow trout (Oncorhynchus mykiss), 1991. Besser J, Wang N, Dwyer F. Assessing contaminant sensitivity of endangered and threatened aquatic species: Part II. Chronic toxicity of copper and pentachlorophenol to two endangered species and two surrogate species. Arch Environ Contam Toxicol 2005; 48: 155-165. Blackman G, Parke M, Garton G. The physiological activity of substituted phenols. I. Relationships between chemical structure and physiological activity. Arch Biochem Biophys 1955; 54: 45-54. Cai Y, Gong Z, Qin B. Community structure and diversity of macrozoobenthos in Lake Taihu,a large shallow eutrophic lake in China. Biodiversity Science 2010; 18: 50-59. Chapman P, Farrel M, Brinkhurst R. Relative tolerances of selected aquatic oligochaetes to combinations of pollutants and environmental factors. Aquat Toxicol 1982; 2: 69-78. Chen L, Liu Y. Ecological succession and sustainable development in Taihu Lake. Journal of East China Normal University(Natural Science) 2003: 99-106. Dominguez S, Chapman G. Effect of pentachlorophenol on the growth and mortality of embryonic and juvenile steelhead trout. Arch Environ Contam Toxicol 1984; 13: 739-743. Dwyer F, Mayer F, Sappington L. Assessing contaminant sensitivity of endangered and threatened aquatic species: Part I. Acute toxicity of five chemicals. Arch Environ Contam Toxicol 2005; 48: 143-154. Gupta P, Durve V. Evaluation of the toxicity of sodium pentachlorophenate, pentachlorophenol and phenol to the snail Viviparus bengalensis. Archiv für Hydrobiologie 1984; 101: 469-475. Hedtke S, West C, Allen K. Toxicity of pentachlorophenol to aquatic organisms under naturally varying and controlled environmental conditions. Environ Toxicol Chem 1986; 5: 531-542. Hickey C. Sensitivity of four New Zealand cladoceran species and Daphnia magna to aquatic toxicants. New Zealand Journal of Marine and Freshwater Research 1989; 23: 131-137. Inglis A, Davis E. Effects of water hardness on the toxicity of several organic and inorganic herbicides to fish. Federal Government Series; 1972. Jin X, Zha J, Xu Y, Giesy J, Wang z. Toxicity of pentachlorophenol to native aquatic species in the Yangtze River. Environ Sci Pollut Res 2012; 19: 609-618. Kim K, Lee Y, Kim S. Combined toxicity of copper and phenol derivatives to Daphnia magna: Effect of complexation reaction. Environ Int 2006; 32: 487-492. Liu W, Hu W, Chen Y. Temporal and spatial variation of aquatic macrophytes in west Taihu Lake. Acta Ecologica Sinica 2007; 27: 159-170. Mayer F, Ellersieck M. Manual of acute toxicity: interpretation and data base for 410 chemicals and 66 species of freshwater animals. Washington, DC: US Department of the Interior, Fish and Wildlife Service, 1986. 26 Mostafa F, Helling C. Impact of four pesticides on the growth and metabolic activities of two photosynthetic algae. J. Environ. Sci. Health., Part B 2002; 37: 417-444. Nanjing Institute of Geography AS. Investigation of Lake Taihu. Science Press, Beijing, 1965. Ni Y, Zhu C. Fishes of the Taihu Lake. Shanghai Scientific and Technical Publishers, Shanghai, 2005. Oikari A, Kukkonen J, Virtanen V. Acute toxicity of chemicals to Daphnia magna in humic waters. Sci Total Environ 1992; 117: 367-377. Radix P, Léonard M, Papantoniou C. Comparison of four chronic toxicity tests using algae, bacteria, and invertebrates assessed with sixteen chemicals. Ecotox Environ Safe 2000; 47: 186-194. Sappington L, Mayer F, Dwyer F. Contaminant sensitivity of threatened and endangered fishes compared to standard surrogate species. Environ Toxicol Chem 2001; 20: 2869-2876. Schafer H, Hettler H, Fritsche U. Biotests using unicellular algae and ciliates for predicting long-term effects of toxicants. Ecotox Environ Safe 1994; 27: 64-71. Spehar R, Nelson H, Swanson M. Pentachlorophenol toxicity to amphipods and fathead minnows at different test pH values. Environ Toxicol Chem 1985; 4: 389-397. Sun S, Huang Y. Taihu Lake. Ocean Press(In Chinese), Beijing, 1993. Thurston R, Gilfoil T, Meyn E. Comparative toxicity of ten organic chemicals to ten common aquatic species. Water Res 1985; 19: 1145-1155. Warwick F, Wadleigh R. Effects of pH on the acute toxicity and uptake of C14 pentachlorophenol in the midge, Chironomus riparius. Ecotox Environ Safe 1986; 11: 1-8. Willis K. Acute and chronic bioassays with New Zealand freshwater copepods using pentachlorophenol. Environ Toxicol Chem 1999; 18: 2580-2586. Xing L, Liu H, Zhang X, Hecker M, Giesy J, Yu H. pH-dependent aquatic criteria for 2, 4-dichlorophenol, 2, 4, 6-trichlorophenol and pentachlorophenol. Sci Total Environ 2012; 441: 125-131. Xu H, Zhang Y. Spatial and Temporal Variation in the Composition of Phytoplankton Species in Taihu Lake. . Environmental Monitoring and Forewarning. 2012; 4: 38-41. Zhang S, Qian J. Succession of hydrophytic vegetation and swampy tendency in the East Taihu Lake. Journal of Plant Resources and Environment 1999; 8: 1-6. Zheng B, Tian Z, Zhang L, Zheng F. The characteristics of the Hydrobios' distribution and the analysis of water quality along the west shore of Taihu Lake. Acta Ecologica Sinica 2007; 27: 4214-4223. Zhu S. Ichthyological Survey of Lake Taihu during 2002-2003. Journal of Lake Sciences 2004; 16: 120-124. Zhu S, Liu Z. Changes of the fish fauna and fish yield analysis in Lake Taihu. Journal of Lake Sciences 2007; 19: 664-669. Zhuang D, Liang Y. Growth, Reproduction and Population Growth Form of Daphnia Magna Straus. Acta Ecologica Sinica 1986; 10: 24-31. 27