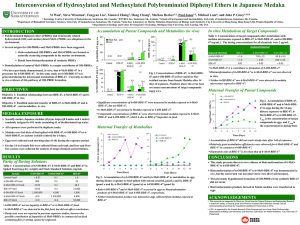
‑OH- Bioaccumulation, Biotransformation, and Toxicity of BDE-47, 6
advertisement

Article pubs.acs.org/est Bioaccumulation, Biotransformation, and Toxicity of BDE-47, 6‑OHBDE-47, and 6‑MeO-BDE-47 in Early Life-Stages of Zebrafish (Danio rerio) Hongling Liu,*,†,# Song Tang,‡,# Xinmei Zheng,† Yuting Zhu,† Zhiyuan Ma,† Chunsheng Liu,† Markus Hecker,‡,§ David M.V. Saunders,§ John P. Giesy,†,§,∥,⊥ Xiaowei Zhang,† and Hongxia Yu*,† † State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, Jiangsu 210023, China ‡ School of Environment and Sustainability, University of Saskatchewan, Saskatoon, Saskatchewan S7N 5B3, Canada § Toxicology Centre, University of Saskatchewan, Saskatoon, Saskatchewan S7N 5B3, Canada ∥ Department of Veterinary Biomedical Sciences, University of Saskatchewan, Saskatoon, Saskatchewan S7N 5B3, Canada ⊥ Department of Biology and Chemistry, City University of Hong Kong, Kowloon, Hong Kong SAR, China S Supporting Information * ABSTRACT: 2,2′,4,4′-Tetrabromodiphenyl ether (BDE-47), 6-hydroxy-tetrabromodiphenyl ether (6-OH-BDE-47), and 6methoxy-tetrabromodiphenyl ether (6-MeO-BDE-47) are the most detected congeners of polybrominated diphenyl ethers (PBDEs), OH-BDEs, and MeO-BDEs, respectively, in aquatic organisms. Although it has been demonstrated that BDE-47 can interfere with certain endocrine functions that are mediated through several nuclear hormone receptors (NRs), most of these findings were from mammalian cell lines exposed in vitro. In the present study, embryos and larvae of zebrafish were exposed to BDE-47, 6-OH-BDE-47, and 6-MeO-BDE-47 to compare their accumulation, biotransformation, and bioconcentration factors (BCF) from 4 to 120 hpf. In addition, effects on expression of genes associated with eight different pathways regulated by NRs were investigated at 120 hpf. 6-MeO-BDE-47 was most bioaccumulated and 6-OH-BDE-47, which was the most potent BDE, was least bioaccumulated. Moreover, the amount of 6-MeO-BDE-47, but not BDE-47, transformed to 6-OH-BDE-47 increased in a time-dependent manner, approximately 0.01%, 0.04%, and 0.08% at 48, 96, and 120 hpf, respectively. Expression of genes regulated by the aryl hydrocarbon receptor (AhR), estrogen receptor (ER), and mineralocorticoid receptor (MR) was affected in larvae exposed to 6-OH-BDE-47, whereas genes regulated by AhR, ER, and the glucocorticoid receptor (GR) were altered in larvae exposed to BDE-47. The greatest effect on expression of genes was observed in larvae exposed to 6-MeO-BDE-47. Specifically, 6-MeO-BDE-47 affected the expression of genes regulated by AhR, ER, AR, GR, and thyroid hormone receptor alpha (TRα). These pathways were mostly down-regulated at 2.5 μM. Taken together, these results demonstrate the importance of usage of an internal dose to assess the toxic effects of PBDEs. BDE-47 and its analogs elicited distinct effects on expression of genes of different hormone receptor-mediated pathways, which have expanded the knowledge of different mechanisms of endocrine disrupting effects in aquatic vertebrates. Because some of these homologues are natural products, assessments of risks of anthropogenic PBDE need to be made against the background of concentrations from naturally occurring products. Even though PBDEs are being phased out as flame retardants, the natural products remain. ■ INTRODUCTION environment, has raised concern about its potential adverse effects to ecosystems and human health.3−5 In addition to the synthetic BDE-47, its hydroxylated (OH−) or methoxylated (MeO−) forms, 6-OH-BDE-47 and 6-MeO-BDE-47, have been Polybrominated diphenyl ethers (PBDEs) have been extensively employed as flame retardants (FRs) in various consumer and commercial products for decades.1,2 As a result of the substantial production, long-term use, disposal, and recycling processes, these chemicals are now frequently found in the environment.3 The persistence, bioaccumulation potential, and toxic potency (PBT criteria) of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), one of the primary PBDEs found in the © 2015 American Chemical Society Received: Revised: Accepted: Published: 1823 August 6, 2014 December 24, 2014 January 7, 2015 January 7, 2015 DOI: 10.1021/es503833q Environ. Sci. Technol. 2015, 49, 1823−1833 Article Environmental Science & Technology suggested to be natural products of marine organisms6 and have been detected in a wide variety of freshwater and marine organisms including mollusks, mussels, shellfish, clam, fish, seal, dolphin, and whale.4,7−13 Moreover, it has been conclusively demonstrated that MeO-BDEs, and not PBDEs, are precursors of OH-BDEs.6,17−19 In zebrafish (Danio rerio), 6-MeO-BDE-47 can be transformed into 6-OH-BDE-47; however, BDE-47 cannot be transformed into 6-OH-BDE-47.20 In addition, interconversion between 6-MeO-BDE-47 and 6-OH-BDE-47 has been observed during dietary exposure of Japanese medaka (Oryzias latipes).17 To date, increasing evidence has shown that exposure to BDE-47 and its two natural analogs, 6-OH-BDE-47 and 6MeO-BDE-47, can elicit a number of adverse effects in aquatic organisms including disruption of the endocrine system,21,22 disruption of molting,23 developmental defects,20,24−26 and neurobehavioral toxicity.27−29 OH- and MeO-BDEs have been shown to exhibit greater toxic potencies than PBDEs for certain end-points such as estrogenicity and androgenicity.21,30 However, mechanisms of their toxicity are complex and have not been fully resolved.31 PBDEs, as well as OH- and MeOBDEs, are structural analogs to thyroid hormones, T3 and T4, as well as dioxin-like chemicals such as polychlorinated biphenyls (PCBs), dioxins (TCDD), and furans (PCDF).31 This raises the question of whether the adverse biological outcomes resulting from exposure to PBDEs and OH- or MeO-BDEs are due to their ability to simulate thyroid hormones or whether they elicit effects similar to those of the above dioxin-like chemicals. Nuclear receptors (NRs) are a superfamily of ligandactivated, transcription factors that act globally to regulate a broad range of biological processes, including development, reproduction, and metabolism.32,33 NRs mediate signaling by ligands such as endogenous hormones, lipids, and xenobiotics.34,35 Upon binding of a ligand to the ligand binding domain of several kinds of NRs, a complex array of cellular responses is initiated. Recently, several in vivo and in vitro studies have investigated effects of BDE-47, 6-OH-BDE-47, or 6-MeO-BDE-47 on certain NR mediated physiological pathways, in particular the pathways involving the thyroid hormone receptor (TR), estrogen receptor (ER), androgen receptor (AR), and aryl hydrocarbon receptor (AhR). For example, in adult fathead minnows (Pimephales promelas), dietary exposure to BDE-47 induced transcription of TRα in the brain of females, and decreased the transcription of TRβ in the brain of fish of both sexes.41 In porcine ovarian follicles, both BDE-47 and 6-OH-BDE-47 did not alter expression of AR mRNA or associated protein, but decreased expression of ERβ mRNA and protein following exposure to BDE-47 and increase both ERα and ERβ gene and protein expression following exposure to 6OH-BDE-47.42 In an AhR-responsive luciferase reporter assay, 6-OH-BDE-47 exhibited greater potency to induce AhR activity than that of 6-MeO-PBDEs and BDE-47.43 However, so far, most NR studies of BDE-47, 6-OH-BDE-47, and 6-MeO-BDE47 were completed by use of mammalian or cellular assays. The zebrafish represents an excellent vertebrate model organism in environmental toxicology studies,44−46 especially in context with investigating effects of endocrine disrupting chemicals (EDCs) on reproductive and developmental systems.47−49 Moreover, developmental profiling of zebrafish gene expression patterns has confirmed a high degree of conservation in NR expression patterns between zebrafish and other vertebrate models.50 Therefore, in the present study, zebrafish embryos and larvae were used to determine the timecourse of accumulation, biotransformation, and bioconcentration factors (BCFs) of BDE-47, 6-OH-BDE-47, and 6-MeOBDE-47. Additionally, in order to gain a more comprehensive understanding of the molecular mechanisms of the toxicity of BDE-47 and related OH- and MeO-analogs on the endocrine system, their effects on expression of genes associated with eight nuclear hormone receptor pathways, particularly ER, AR, AhR, TRα, peroxisome proliferator-activated receptor alpha (PPARα), glucocorticoid receptor (GR), mineralocorticoid receptor (MR), and pregnane x receptor (PxR), were investigated and compared. ■ MATERIALS AND METHODS Materials and Reagents. BDE-47 (98% purity) was purchased from Chem Service (West Chester, PA, USA). 6MeO-BDE-47 and 6-OH-BDE-47 were synthesized at City University of Hong Kong, and purities were more than 98% as described previously.51 13C-PCB-178, 13C-2′-OH-BDE-99, and 13 C-BDE-139 were purchased from Cambridge Isotope Laboratories (Andover, MA, USA). BDE-47, 6-OH-BDE-47, and 6-MeO-BDE-47 were dissolved in dimethyl sulfoxide (DMSO, Generay Biotech, Shanghai, China) to prepare stock solutions and then diluted with embryonic rearing water (60 mg/L instant ocean salt in aerated distilled water) to the desired test concentrations. Concentration of DMSO in final test solutions did not exceed 0.1%. RNAlater, RNA Stabilization Reagents, and RNeasy Mini Kit were purchased from QIAGEN (Hilden, Germany). Maxima First Strand cDNA Synthesis Kits were purchased from Fermentas (St Leon-Rot, Germany). SYBR Real time PCR Master Mix Plus Kits were purchased from Toyobo (Tokyo, Japan). Animals and Exposure Experiment. Adult (7 months old) AB strain zebrafish maintenance and culturing were performed as previously described.20 The eggs were examined under a stereomicroscope and only normally developed embryos were used for exposure experiments. Briefly, 20 embryos were randomly distributed into a 25 mL glass beaker containing 20 mL of exposure solution. Fish were exposed until 120 h post fertilization (hpf), by which time they had developed into free-swimming larvae and most organs had completed development.52 The control group received 0.1% DMSO (v/v) only. 100% of the exposure solutions were replaced by fresh exposure solution every 48 h. For 6-OH-BDE47, BDE-47, and 6-MeO-BDE-47 exposures, the experiments included two parts: First, zebrafish embryos were exposed to 6OH-BDE-47 (0, 0.008, 0.02, 0.05, 0.1, 0.5 μM), 6-MeO-BDE47 (0, 0.02, 0.1, 0.5, 2.5 μM), and BDE-47 (0, 0.02, 0.1, 0.5, 2.5 μM) from 4 to 120 hpf to study the morphologic toxicity of compounds as previously described.20 Second, on the basis of the results of acute toxicity test, three comparable exposure concentrations for each compound were chosen: 6-OH-BDE47 at 0.008, 0.02, and 0.05 μM, and at 0.1, 0.5, and 2.5 μM for both 6-MeO-BDE-47 and BDE-47 from 4 to 120 hpf to study the effects on expression of 63 genes involved in eight receptormediated pathways by q-RT-PCR. After exposure for 120 hpf, larvae were anesthetized with ethyl 3-aminobenzoate methanesulfonate (MS-222, Suzhou Xin Yong Biological Medicine Technology Co., Ltd., Jiangsu, China), and were preserved in RNAlater RNA Stabilization Reagents until total RNA isolation. Bioavailability Analysis and QA/QC. This experiment was designed to analyze bioaccumulation of the three chemicals in early life-stages of zebrafish. In each treatment, 600 zebrafish 1824 DOI: 10.1021/es503833q Environ. Sci. Technol. 2015, 49, 1823−1833 Article Environmental Science & Technology in R. A heatmap of gene expression results was implemented by “pheatmap” package version 0.7.7 in R. embryos were exposed to 6-OH-BDE-47, 6-MeO-BDE-47, or BDE-47 at 300 μg/L (0.6, 0.58, and 0.62 μM, respectively) from 4 to 120 hpf in glass beakers. Variation among concentrations of the three compounds in both the exposure medium and the embryos/larvae was determined. 80 embryos/ larvae and corresponding exposure solutions were collected at 12, 24, 48, 72, 96, and 120 hpf. Detailed protocols for extraction, clean up, and quantification, and quality assurance and quality control (QA/QC) are provided in previous studies20,53,54 and the Supporting Information, Methods and Table S1. Quantitative RT-PCR. Total RNA was isolated from zebrafish larvae using RNeasy Mini Kit. The concentration and quality of total RNA were determined in accordance with the procedures described in a previous study.45 First-strand cDNA synthesis and quantitative RT-PCR were performed using Maxima First Strand cDNA Synthesis and SYBR Realtime PCR Master Mix Plus Kits.20 Quantitative RT-PCR was performed by an Applied Biosystems Stepone Plus Real-time PCR System (Foster City, California, USA). The primers were either mined from previous literature55 or designed using Primer 3 (http://bioinfo.ut.ee/primer3-0.4.0/). Primer sequences are listed in the Supporting Information, Table S2. The housekeeping gene 18S small subunit rRNA (18S rRNA) was used as an internal control.56 The thermal cycle was set at 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Melting curves were derived during RT-PCR to validate that all cDNA samples amplified only a single product. Levels of expression of genes were normalized to 18S rRNA mRNA contents using the 2−ΔΔCt method. Each concentration was measured in triplicate or quadruplicate in a composite sample containing 20 larvae. Nuclear Receptor Pathway Analysis. For genes relating to AhR and ER pathways, the Agilent Literature Search application was used to construct a biological interaction network within the Cytoscape software v3.1.1 (Cytoscape consortium, San Diego, CA, USA).57−59 The gene networks of the other six NR pathways were retrieved by either WikiPathways (http://www.wikipathways.org)60 or SABioscience Gene Network Central (http://www.sabiosciences.com/ genenetwork/genenetworkcentral.php), and integrated with AhR and ER pathways as “associations” and visualized as one network by Cytoscape. Only genes of interest were shown in this pathway network. The resulting network genes (nodes) were colored by the Enhanced Graphics application within Cytoscape according to the significant fold changes of gene expressions in the respective treatments. Statistical Analysis. SPSS 12.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. A Kolmogorov−Smirnov test was used to verify the normality of the data, and the homogeneity of variances was analyzed by Levene’s test as previously described.20 If the data failed the Kolmogorov− Smirnov test, logarithmic transformation was performed and data was checked again for homogeneity of variances. A oneway analysis of variance (ANOVA) followed by LSD test was used to evaluate differences between the control and exposure groups. A value of p < 0.05 was considered statistically significant. To capture the likely nonlinearity in concentrations in exposure water or in zebrafish embryos−larvae across different time-points, generalized additive models (GAMs) were used by the “mgcv” package in R software version 3.10 (R Core Team, Vienna, Austria). Hierarchical cluster analysis for the gene expression was performed by the “complete” method ■ RESULTS Morphologic Effects of 6-OH-BDE-47, 6-MeO-BDE-47 and BDE-47. Among the three compounds, 6-OH-BDE-47 was the most potent to zebrafish embryos/larvae (Supporting Information, Figure S1B,C,D,F and Table S3). Exposure to all concentrations caused delayed development of embryos for up to 6−8 at 24 hpf. In embryos exposed to 0.5 μM 6-OH-BDE47, mortality significantly increased to 22.5 ± 4.1%, at 48 hpf. At 48 hpf, in groups exposed to concentrations greater than 0.1 μM, the embryos developed hypopigmentation. At 72 hpf, development was arrested in all embryos exposed to 0.5 μM 6OH-BDE-47 (Supporting Information, Figure S1B,C) whereas development of embryos at 12−18 hpf was not altered. Larvae exposed to 0.1 μM 6-OH-BDE-47 exhibited spinal curvatures (Supporting Information, Figure S1D), decreased heartbeats, and reduced body lengths (3516 ± 250 μm in 0.1 μM group and 4040 ± 55 μm in control). The LC50 values of 6-OH-BDE47 for teratogenic effects were 0.28 μM (0.21−0.38) at 72 hpf, 0.13 μM (0.11−0.16) at 96 hpf, and 0.09 μM (0.04−0.10) at 120 hpf. The most sensitive toxicological end-point was spinal curvature (Supporting Information, Figure S1F), which was manifested in a concentration-dependent manner with maximal effects resulting from exposure to 0.08 μM (0.07−0.09) 6-OHBDE-47 at 120 hpf. There were no significant differences in developmental alterations in individuals exposed to 6-MeO-BDE-47 and the control group until 96 hpf. The most sensitive toxicological end-points were concomitant spinal curvature and pericardial edema at 120 hpf at 2.5 μM (Supporting Information, Figure S1G). Although no statistically significant differences were observed within 96 hpf following exposure to BDE-47 up to 2.5 μM, concomitant spinal curvature and pericardial edema occurred in embryos at 120 hpf. The NOEC of spinal curvature and pericardial edema was 0.5 μM of BDE-47, although there were significant differences at 2.5 μM and the proportion of affected larvae was 27.6 ± 18.3% (Supporting Information, Figure S1H). In addition, at 120 hpf following exposure to 2.5 μM BDE-47, the body lengths of larvae (3751 ± 152 μm) were significantly reduced compared to those in the control group (4029 ± 201 μm). Accumulation by Zebrafish. Concentrations of BDE-47, 6-OH-BDE-47, or 6-MeO-BDE-47 were below their analytical method detection limits in the control group (Supporting Information Table S4). At 120 hpf, 100% mortality occurred following exposure to 300 μg/L 6-OH-BDE-47; therefore, no data is available for this time-point. gas chromatography−mass spectrometry (GC/MS) results indicated that the chemical concentrations in exposure solutions decreased and the doses in embryos and larvae increased in a time-dependent manner (Figure 1). Moreover, the concentrations of BDE-47 and 6MeO-BDE-47 were approximately 10- to 100-fold greater than concentrations of 6-OH-BDE-47 in zebrafish embryos and larvae that were exposed to the same concentrations of the three compounds (Figure 1). The three BDE congeners ranked as follows regarding their in vivo accumulation (values from greater to lesser potential): 6-MeO-BDE-47 > BDE-47 > 6OH-BDE-47. Calculated BCF values for 6-OH-BDE-47 were 4.07, 9.88, 21.7, 26.9, and 23.3 at 12, 24, 48, 72, and 96 hpf, respectively (Figure 2A). For 6-MeO-BDE-47, the BCF values were 17.2, 1825 DOI: 10.1021/es503833q Environ. Sci. Technol. 2015, 49, 1823−1833 Article Environmental Science & Technology concentration of 6-OH-BDE-47 (0.21 and 1.07 μg/g, wm vs 0.15 μg/g, wm) in larvae exposed to 0.05 μM of 6-OH-BDE-47 (Table 1). Transcriptional Responses of NR Pathways to 6-OHBDE-47. A hierarchical cluster analysis of gene expression data showed a dendrogram that highlighted five principal clusters (Figure 3A). Exposures to 2.5 μM 6-MeO-BDE-47 resulted in a unique clustering of gene expression data that revealed a significantly different gene expression profile from the other exposures (Figure 3A). Exposures to the same compound but at different concentrations generally clustered in the same group, especially for exposures to 0.008, 0.1, and 0.5 μM 6-OHBDE-47 (Figure 3A). Zebrafish embryos exposed to 6-OH-BDE-47 had significant alterations in the expression of genes associated with several NR pathways. The most significant effects occurred along the AhR pathway (Figures 3 and 4 and the Supporting Information, Table S5) with exposure to 0.008 μM 6-OH-BDE-47 causing a significant up-regulation in the expression of ahr1a, ahr1b, ahr2, and arnt2 by 2.32-, 1.71-, 1.62-, and 1.62-fold, respectively (p < 0.05), and a significant down-regulation of ahrra and cyp19b expression by 2.27- and 1.81-fold, respectively (p < 0.05). Exposure to 0.02 μM 6-OH-BDE-47 significantly induced expression of ahr1b and ahr2 by 1.63- and 1.67-fold, respectively, and reduced the expression of ahrra and cyp1a1 by 1.96- and 1.75-fold (p < 0.05). Exposure to 0.05 μM 6-OHBDE-47 significantly reduced the expression of cyp1a1, cyp1b1, arnt1la, ahrra, and cyp19b by 1.92-, 2.63-, 1.25-, 1.96-, and 2.23fold, respectively (p < 0.05). In addition to the ahr receptor, following exposure to 0.008 and 0.05 μM 6-OH-BDE-47, the expression of mr and er2b were significantly up-regulated by 1.51- and 1.83-fold, respectively (p < 0.05). Transcriptional Responses of NR Pathways to 6-MeOBDE-47. Some NR-mediated pathways such as AhR, ER, AR, TR, and GR were affected by exposure to 6-MeO-BDE-47, with the greatest effects occurring at the greatest concentration tested, 2.5 μM (Figures 3 and 4). Exposure to lesser concentrations of 6-MeO-BDE-47, 0.1 μM, also induced the expression of several genes in these pathways. Specifically, arnt2, dut, ugtlal, ctnnb1, pa2g4a, dap3, and rela were significantly induced by 2.08-, 1.67-, 2.23-, 1.56-, 1.78-, and 1.51-fold, respectively (p < 0.05). Exposure to 0.5 μM 6-MeOBDE-47 did not significantly alter the expressions of most genes, except for the down-regulation cyp1a1 and er2a by 9.09and 1.56-fold, respectively (p < 0.05). However, exposure to a greater concentration of 6-MeO-BDE-47, 2.5 μM, reduced the expression of most altered genes. Along the AhR pathway, ahr2 was down-regulated by 1.92-fold and associated genes such as cyp1a1, cyp1b1, cyp365a, and sp1 were also down-regulated by 50-, 100-, 3.03-, and 1.89-fold, respectively (p < 0.05). However, both ahrra and ahrrb were significantly induced by 3.16- and 5.71-fold, respectively (p < 0.05). In the ER pathway, er2a and ccnd1 were down-regulated by 1.43- and 1.85-fold, respectively, whereas er2b was up-regulated by 1.44-fold following exposure to 2.5 μM 6-MeO-BDE-47 (p < 0.05). In the AR pathway, ar, ctnnb1, pa2g4b, and ncoa1 were downregulated by 1.85-, 1.89-, 1.47-, and 1.67-fold, respectively (p < 0.05). Exposure to 2.5 μM 6-MeO-BDE-47 also decreased the expression of thra by 2.17-fold and TR associated genes such as ncor and fus were significantly down-regulated by 2.08- and 1.85-fold (p < 0.05). Also, following exposure to 2.5 μM 6MeO-BDE-47, the expression of gr and tgfb1 were downregulated by 2.04- and 1.72-fold, respectively (p < 0.05). Figure 1. Measured concentrations in exposure medium (μg/L) and in zebrafish embryos−larvae (mg/g, wm) after exposure to 300 μg/L of BDE-47 (0.62 μM), 6-MeO-BDE-47 (0.58 μM), or 6-OH-BDE-47 (0.6 μM) across time-points (hpf). Generalized additive model (GAM) plots between concentrations (after log transformation) in exposure water or in zebrafish embryos−larvae and time (hpf) are given. Shaded areas are the 95% confidence intervals for each GAM. The F-statistics, p-values, and adjusted R2 for the specific GAMs are given in each plot, whereas D shows the deviance explained. 42.6, 89.7, 390, 1207, and 935 at 12, 24, 48, 72, 96, and 120 hpf, respectively. For BDE-47, bioconcentration factor (BCF) values were 25.7, 68.0, 489, 750, 2489, and 2430 at 12, 24, 48, 72, 96, and 120 hpf, respectively. These results indicated that trends of BCF values after various durations of exposure were similar for 6-MeO-BDE-47 and BDE-47, reaching a maximum at 96 hpf, and showing a slight decrease at later timepoints. Biotransformation in Zebrafish. Exposure of zebrafish to 300 μg/L 6-MeO-BDE-47 resulted in increasing tissue concentrations of 6-OH-BDE-47 in their tissues were quantified in a time-dependent manner, with 0.02, 0.11, 0.12, and 0.25 μg/ g, wm (wet mass) at 48, 72, 96, and 120 hpf, respectively (Figure 2B). However, no transformation of BDE-47 into 6OH-BDE-47 or 6-MeO-BDE occurred. Also, 6-OH-BDE-47 was not transformed into either BDE-47 or 6-MeO- BDE-47. Based on these transformation ratios, the amounts of biotransformed 6-OH-BDE-47 were expected to be 0.04, 0.21, and 1.07 μg/g, wm after 120 hpf exposure to 0.1, 0.5, or 2.5 μM 6-MeO-BDE-47, respectively (Table 1). Amounts of biotransformed 6-OH-BDE-47 in larvae exposed to 0.5 and 2.5 μM of 6-MeO-BDE-47 were greater than the accumulated 1826 DOI: 10.1021/es503833q Environ. Sci. Technol. 2015, 49, 1823−1833 Article Environmental Science & Technology Figure 2. (A) Bioconcentration factors (BCF) calculated after exposure to 300 μg/L of BDE-47 (0.62 μM), 6-MeO-BDE-47 (0.58 μM), and 6-OHBDE-47 (0.6 μM) across time-points (hpf). BCF was calculated based on the ratio of measured concentrations in zebrafish embryos−larvae (μg/kg, wm) and measured concentrations in exposure medium (μg/L) at a specific time (hpf). (B) Measured concentrations of biotransformed 6-OHBDE-47 in zebrafish embryos−larvae (μg/g, wm) after exposure to 300 μg 6-MeO-BDE-47/L (0.58 μM) across several time-points (hpf). A linear regression between concentrations in zebrafish embryos−larvae and time (hpf) is given (R2 = 0.911; p < 0.05). Table 1. Estimated Internal Doses of BDE-47, 6-OH-BDE-47, and 6-MeO-BDE-47 Exposures in Zebrafish Larvae at 120 hpfa chemical nominal concentration (μM) BDE-47 (μg/g) 6-MeO-BDE-47 (μg/g) 6-OH-BDE-47 (μg/g) biotransformed 6-OH-BDE-47 (μg/g) BDE-47 0.1 9.05 0.5 45.27 6-MeO-BDE-47 2.5 226.33 6-OH-BDE-47 0.1 0.5 2.5 18.17 90.87 454.37 0.04 0.21 1.07 0.008 0.02 0.05 0.024 0.061 0.15 a The calculation of internal dose for each compound was based on the ratio of the exposure concentration at 300 μg/L and the measured concentration in larvae at 120 hpf. Transcriptional Responses of NR Pathways to BDE-47. Exposure to BDE-47 significantly affected the expression of receptors in the AhR, ER, and GR pathways (Figures 3 and 4). The number of altered genes increased in a concentration dependent manner. The expression of gr was significantly down-regulated by 1.28-fold following exposure to 0.5 μM BDE-47 (p < 0.05). When exposed to 2.5 μM, expressions of ahr1b, er2a, and er2b were significantly greater by 1.64-, 1.35-, and 1.40-fold, respectively (p < 0.05) than those of the respective genes controls. Expressions of genes along AhR and ER pathways, such as cyp1a1, cyp19a, cyp3a65, and ccnd1, were significantly less by factors of 5.88-, 2.88-, 2.04-, and 1.52-fold, respectively, relative to that of the controls (p < 0.05). then correlated with the concentrations of the compounds in the ambient media. Because chemicals need to be accumulated into organisms and distributed to target sites for the induction of toxic effects, usage of target site effect concentrations are postulated to best represent the hazards of a compound in vivo.61,62 However, for small-bodied organisms such as the zebrafish, effect concentrations at the target site are difficult to determine, particularly at earlier stages of development. Average body concentrations of contaminants in zebrafish embryos and larvae are subject to competitive dynamic processes, which include the ability of compounds to penetrate the chorion, in vivo biotransformation, distribution, and excretion. Hence, internal effect concentrations need to be determined. Moreover, the ratio of the internal concentration in a fish to the surrounding concentration at a steady state represents the compound’s BCF, which is an important metric for regulatory assessment of chemicals.63 ■ DISCUSSION Hazard assessment of contaminants is typically based on the exposure of aquatic organisms to chemical solutions for a defined exposure time and the adverse outcomes observed are 1827 DOI: 10.1021/es503833q Environ. Sci. Technol. 2015, 49, 1823−1833 Article Environmental Science & Technology Figure 3. Dendrogram displaying similarities of chemicals and doses based on effects on genes in nuclear receptor pathways for BDE-47, 6-MeOBDE-47, and 6-OH-BDE-47 in zebrafish larvae at 120 hpf. (A) The dendrogram of hierarchical cluster analysis was calculated using the average gene expression values (63 genes in total) of the three or four biological replicates per exposure. Samples names are composed by the name of exposure compound followed by the exposure concentration (μM). Different colors in the dendrogram denoted five clustering groups. (B) The heatmap of gene expression profiles was generated using the average gene expression values of the three or four biological replicates per exposure. The foldchanges of gene expression are given in the respective cells and genes involved in different receptor pathways are given different colors (see legend). ences in lipophilicity were considered to be the most important parameters for the different accumulation properties of the test chemicals. For compounds such as 6-OH-BDE-47, the hydroxyl group by making the compound more polar, result in greater excretion, and might also be an important parameter for the lesser in vivo concentration. Hence, our results confirmed that in aquatic exposure tests, it is not sufficient to evaluate the ecotoxicological risk of a compound based solely on the exposure concentration. In addition to accumulation in the body, the results of this study indicated that the amounts of 6MeO-BDE-47, but not BDE-47, that were transformed to 6OH-BDE-47 increased in a time-dependent manner, (approximately 0.01%, 0.04%, and 0.08% at 48, 96, and 120 hpf, respectively), which is indicative of an increasing metabolic capability of zebrafish embryos/larvae with increasing age. Photomicrographs demonstrated that exposures to 6-OHBDE-47, 6-MeO-BDE-47, and BDE-47 resulted in developmental abnormalities in zebrafish embryos and larvae. The embryo-toxic effects of BDE47, 6-OH-BDE47 and 6-MeOBDE47 have been investigated in a previous study with In the present study, accumulation, biotransformation, varied among BCF of BDE-47, 6-OH-BDE-47, and 6-MeO-BDE-47 during multiple developmental stages of zebrafish. The timepoints during which accumulation of BDE-47 and 6-MeOBDE-47 increased substantially coincide with the hatching period of zebrafish embryos (48 hpf). In the late developmental periods of the larvae (after 96 hpf), the bioaccumulation of BDE-47 and 6-MeO-BDE-47 reached a plateau, which might be due to an increase in metabolism and/or excretion activities as well as the rapid growth of larvae at this time that might dilute their body concentrations over this time period. In this study, the BCF of 6-OH-BDE-47 at 96 hpf was 23.3, which is similar to previously reported results,64 in which BCF values were calculated in liver of zebrafish after 96 h exposure to 100 nM 6OH-BDE-47. At all six durations of exposure, 6-OH-BDE-47 was the least accumulated into the body, though it had the greatest toxic potency. It is known that a compound with greater log Kow generally has greater bioaccumulation potential. Values of log Kow were 6.76, 7.17, and 6.59 for BDE-47, 6MeO-BDE-47, and 6-OH-BDE-47, respectively.65 Thus, differ1828 DOI: 10.1021/es503833q Environ. Sci. Technol. 2015, 49, 1823−1833 Article Environmental Science & Technology of this study (Supporting Information, Figure S1B,C,D).64 Furthermore, the lack of toxic effects of BDE47 or 6-MeOBDE47 at 2.5 μM until 120 hpf (Supporting Information, Figure S1G,H) is consistent with previous findings that showed that no toxicity was observed for both BDE47 and 6-MeOBDE47 in zebarfish at 72 hpf.64 Because disruptions of cellular molecules or processes are thought to precede adverse outcomes, changes to normal molecular processes might function as sensitive biomarkers to predict adverse biological outcomes.66 Moreover, alteration of NR mediated pathways has been shown to be associated with adverse endocrine and developmental effects that were linked with morphological deformities.20,55 For example, previous studies have demonstrated that BDE-47 can alter thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain of adult fathead minnows,67 and both 6OH-BDE-47 and 6-MeO-BDE-47 were shown to affect expression of TRα and TRβ genes in the TR pathway, which can result in teratogenic effects such as pericardial edema, developmental retardation, and curved spine in zebrafish embryos.20 Also, all BDE-47, TBBPA and BPA have been demonstrated to alter expression of genes along the hypothalamus-pituitary-thyroid (HPT) axis of zebrafish larvae as well as induce acute toxicity.68 Additionally, zebrafish has been used to investigate effects of EDCs on the expression of genes in six NR mediated pathways.55 In this study, two additional receptor pathways-AR and PxR were added, and the interactions of sixty-three genes involved in eight zebrafish receptor pathways were integrated. This pathway network might represent a novel tool for the examination of the molecular function of each individual receptor as well as for the study of their combinatorial regulatory network within NRs. The structures of the three compounds tested are similar. The cluster dendrogram showed that expression of genes in individuals exposed to various concentrations of 6-OH-BDE-47 clustered together. Nevertheless, clustering is a function of concentration for 6-MeO-BDE-47 and BDE-47. Patterns of expression of genes following exposures to 2.5 μM BDE-47 and 0.5 μM 6-MeO-BDE-47 were grouped into the same cluster, which indicates BDE-47 likely has fewer effects on zebrafish NR-mediated pathways than 6-MeO-BDE-47 at similar waterborne exposure concentrations. Exposure to 2.5 μM of the more bioaccumulative 6-MeO-BDE-47 resulted in a unique gene expression profile compared to BDE-47 and 6-OH-BDE47. In addition, 1.07 μg/g, wm of biotransformed 6-OH-BDE47 were detected in larvae exposed to 2.5 μM of 6-MeO-BDE47, which was much greater than the detected amount of 6OH-BDE-47, 0.15 μg/g, which resulted from exposure to 0.05 μM 6-OH-BDE-47. The greater body burden of 6-OH-BDE-47 resulting from exposure to 2.5 μM 6-MeO-BDE-47 might explain the significant and great effect on gene transcription of NR pathways observed in this exposure group. The effects that occurred in the greatest exposure group of 6-MeO-BDE-47, therefore, were attributed to the combined effects of biotransformed 6-OH-BDE-47 as well as 6-MeO-BDE-47. The clustering of the three compounds correlated well with their respective accumulation potency, indicating the great importance of the usage of internal dose to assess the dose− response relationship for studies of PBDEs, especially MeOPBDEs. Further analyses of endocrine pathways indicated general disruption of receptor pathways by all three BDEs congeners, which correlated well with the observed teratogenic effects in Figure 4. Interaction network of selected genes in nuclear steroid receptor pathways of zebrafish. Nodes represent single genes, edges either protein−protein or protein−DNA interactions. Statistically significant changes (p < 0.05) in gene expression following different concentrations of treatment of BDE-47, 6-MeO-BDE-47, and 6-OHBDE-47 at 120 hpf are given in the respective boxes (see legend). zebrafish embryos exposed from 3 to 72 hpf.64 Those authors showed that 6-OH-BDE-47 was the most toxic BDE-47 congener, inducing a range of developmental defects including pericardial edema, yolk sac deformations, lesser pigmentation, lessened heart rate, and delayed development at concentrations of 25−50 nM, which is consistent with the morphology findings 1829 DOI: 10.1021/es503833q Environ. Sci. Technol. 2015, 49, 1823−1833 Article Environmental Science & Technology regulated by 6-OH-BDE-47 exposure, indicating 6-OH-BDE-47 might be an agonist of zebrafish PxR. Reports on the effect of PBDEs on PPARα, MR, and GR are limited. PPARα plays an important role in lipid homeostasis, inflammation, adipogenesis, reproduction, and carcinogenesis.78 In this study, none of three compounds significantly affected the expression of PPARα in zebrafish. However, treatment with a PBDE mixture, BDE-71 and BDE-47 caused increases in PPARγ transcript levels at day 8 in 3T3-L1 mouse embryo fibroblast cells.79 GR and MR are essential for regulation of multiple physiological functions, such as glucose metabolism, mineral balance, and behavior.80 In this study, exposure to 2.5 μM 6-MeO-BDE-47 or 0.5 μM BDE-47 caused downregulation of GR, whereas 0.008 μM 6-OH-BDE-47 increased MR expression, which suggests that GR or MR signaling pathways might be involved in the endocrine disrupting effects induced by PBDEs. Altogether, the results of present study, which compared the toxicities of BDE-47 with its OH- and MeO- analogs in zebrafish via multiple quantitative approaches, ranging from in vivo toxicity tests, bioaccumulation and biotransformation, to the molecular analysis of response patterns of genes along NR pathways, highlight the importance of the usage of internal dose to evaluate the toxic effects for PBDEs, and the use of early lifestages of zebrafish as an efficient and reliable vertebrate model to assess toxicological effects of endocrine disruptors. Our data also elucidated several molecular aspects of BDE-47, 6-OHBDE-47, and 6-MeO-BDE-47 induced toxicities. Specifically, the data provided valuable insights into the early interaction of these compounds with steroid hormone receptor pathways, which provided novel clues for their in vivo mechanisms of subsequent endocrine disruption and developmental toxicities. zebrafish. Specifically, 6-OH-BDE-47 altered the expression of AhR, ER, and MR receptor-mediated pathways, whereas AhR, ER, and GR were the primary pathways altered by BDE-47. Yet, exposure to the more bioaccumulative 6-MeO-BDE-47 affected AhR, ER, AR, GR, and TRα pathways. Molecular structures of OH-BDEs closely resemble those of thyroid hormones (THs) and 6-OH-BDE-47 can disrupt normal thyroid homeostasis and functions as either an agonist or an antagonist.31,69 For example, expression of genes along the hypothalamuspituitary-thyroid (HPT) axis that is responsible for regulation of metabolism and early life-stage development was affected by BDE-47 and its OH- or MeO- forms in zebrafish embryos− larvae.20,68 However, in the present experiment, expression of thra was not significantly altered following exposure to 6-OHBDE-47, which contradicted previous findings showing thra was reduced in zebrafish exposed to 200 μg/L 6-OH-BDE-47.20 This difference might be due to the almost 10 times greater concentrations used in the previous study. However, exposure to 2.5 μM 6-MeO-BDE-47 significantly decreased the expression of thra, nocr, and f us in TRα pathway. Because significant quantities of biotransformed 6-OH-BDE-47 were found in vivo, 6-MeO-BDE-47 may exert adverse effects indirectly via transformation into 6-OH-BDE-47, which then can bind directly to TH targeted genes by mimicking THs. Apart from the TRα pathway, recent studies have also focused on disruption of the AhR and ER pathways by PBDEs. Cross talk between ER- and AhR-signaling pathways in fish has been hypothesized previously.72,73 The pathway analyses conducted in this study also suggest interactions between these pathways as visualized in the constructed networks. All three compounds altered AhR and ER pathways in zebrafish. 6OH-BDE-47 significantly increased expression of er2b in zebrafish, which is consistent with previous in vitro findings that both ERα and ERβ gene and protein expression were induced by 6-OH-BDE-47.42 Exposure to 2.5 μM 6-MeO-BDE47 significantly reduced expression of er2a but induced er2b indicating the compound might cause endocrine disrupting effects through interfering with the ER signaling pathway.21 In addition, 6-OH-BDE-47 increased the expression of several AhR receptors including ahr1a, ahr1b, and ahr2 in vivo, while 6MeO-BDE-47 and BDE-47 only affected ahr2 and ahr1a transcription, respectively. These results have confirmed that OH-BDEs can induce greater dioxin-like activity than corresponding MeO-BDEs and parent PBDEs in vitro.43,74 AR and PxR pathways have been previously shown to be affected by PBDEs in vitro.21,75−77 In the MDA-kb2 human cell line AR receptor binding assay, all three compounds exhibited potent antiandrogenicity, with potencies ranking as follows: 6OH-BDE-47 (IC50 = 0.34 μM) > BDE47 (IC50 = 3.83 μM) > 6-MeO-BDE-47 (IC50 = 41.8 μM).21,76 However, in zebrafish, both BDE-47 and 6-OH-BDE-47 did not significantly alter AR expression, which is consistent with a study in porcine ovarian follicular cells, showing BDE-47 and its OH- metabolites had no effect on the expression of AR mRNA and protein expression.42 The PxR, a steroid and xenobiotic nuclear receptor (SXR), can be activated by BDE-47 in mice.75 Nevertheless, in zebrafish, BDE-47 significantly down-regulated PxR associated genes of cyp3a65, hnf4a, and ugtlal, but increased pou1f1. The incongruities between these results could be due to differences between species and lesser concentrations used in our experiments. Furthermore, PxR associated genes cyp24a1 and hnf4a were significantly up- ■ ASSOCIATED CONTENT * Supporting Information S Further details on the analytical methods and additional tables and figures as noted in the text. This material is available free of charge via the Internet at http://pubs.acs.org/. ■ AUTHOR INFORMATION Corresponding Authors *Dr. Hongling Liu. Tel: +86-25-89680356. Fax: +86-2589680356. E-mail: hlliu@nju.edu.cn. *Dr. Hongxia Yu. Tel: +86-25-89680356. Fax: +86-2589680356. E-mail: yuhx@nju.edu.cn. Author Contributions # These authors contributed equally to this work. Notes The authors declare no competing financial interest. ■ ACKNOWLEDGMENTS We thank Dr. Richard A. Erickson (Upper Midwest Environmental Sciences Center, U.S. Geological Survey) for providing the expertise in statistical analyses. This work was funded by National Natural Science Foundation (No. 21377053 and 20977047) and Major National Science and Technology Projects (No. 2012ZX07506-001 and 2012ZX07501-003-02) of China. J.P.G. and M.H. were supported by the Canada Research Chair Program. J.P.G. was supported by the Program of 2012 “Great Level Foreign Experts” (#GDW20123200120) funded by the State Administration of Foreign Experts Affairs, China to Nanjing University, and the Einstein Professor 1830 DOI: 10.1021/es503833q Environ. Sci. Technol. 2015, 49, 1823−1833 Article Environmental Science & Technology (16) Malmvarn, A.; Zebuhr, Y.; Kautsky, L.; Bergman, K.; Asplund, L. Hydroxylated and methoxylated polybrominated diphenyl ethers and polybrominated dibenzo-p-dioxins in red alga and cyanobacteria living in the Baltic Sea. Chemosphere 2008, 72 (6), 910−916. (17) Wan, Y.; Liu, F.; Wiseman, S.; Zhang, X.; Chang, H.; Hecker, M.; Jones, P. D.; Lam, M. H.; Giesy, J. P. Interconversion of hydroxylated and methoxylated polybrominated diphenyl ethers in Japanese medaka. Environ. Sci. Technol. 2010, 44 (22), 8729−8735. (18) Wan, Y.; Wiseman, S.; Chang, H.; Zhang, X.; Jones, P. D.; Hecker, M.; Kannan, K.; Tanabe, S.; Hu, J.; Lam, M. H.; Giesy, J. P. Origin of hydroxylated brominated diphenyl ethers: Natural compounds or man-made flame retardants? Environ. Sci. Technol. 2009, 43 (19), 7536−7542. (19) Liu, F.; Wiseman, S.; Wan, Y.; Doering, J. A.; Hecker, M.; Lam, M. H.; Giesy, J. P. Multi-species comparison of the mechanism of biotransformation of MeO-BDEs to OH-BDEs in fish. Aquat. Toxicol. 2012, 114−115, 182−188. (20) Zheng, X.; Zhu, Y.; Liu, C.; Liu, H.; Giesy, J. P.; Hecker, M.; Lam, M. H.; Yu, H. Accumulation and biotransformation of BDE-47 by zebrafish larvae and teratogenicity and expression of genes along the hypothalamus-pituitary-thyroid axis. Environ. Sci. Technol. 2012, 46 (23), 12943−12951. (21) Hu, W.; Liu, H.; Sun, H.; Shen, O.; Wang, X.; Lam, M. H.; Giesy, J. P.; Zhang, X.; Yu, H. Endocrine effects of methoxylated brominated diphenyl ethers in three in vitro models. Mar. Pollut. Bull. 2011, 62 (11), 2356−2361. (22) Chan, W. K.; Chan, K. M. Disruption of the hypothalamicpituitary-thyroid axis in zebrafish embryo-larvae following waterborne exposure to BDE-47, TBBPA and BPA. Aquat. Toxicol. 2012, 108, 106−111. (23) Davies, R.; Zou, E. Polybrominated diphenyl ethers disrupt molting in neonatal Daphnia magna. Ecotoxicology 2012, 21 (5), 1371−1380. (24) Dong, W.; Macaulay, L. J.; Kwok, K. W. H.; Hinton, D. E.; Stapleton, H. M. Using whole mount in situ hybridization to examine thyroid hormone deiodinase expression in embryonic and larval zebrafish: A tool for examining OH-BDE toxicity to early life stages. Aquat. Toxicol. 2013, 132, 190−199. (25) Usenko, C. Y.; Hopkins, D. C.; Trumble, S. J.; Bruce, E. D. Hydroxylated PBDEs induce developmental arrest in zebrafish. Toxicol. Appl. Pharmacol. 2012, 262 (1), 43−51. (26) Kallqvist, T.; Grung, M.; Tollefsen, K. E. Chronic toxicity of 2,4,2′,4′-tetrabromodiphenyl ether on the marine alga Skeletonema costatum and the crustacean Daphnia magna. Environ. Toxicol. Chem. 2006, 25 (6), 1657−1662. (27) Zhao, J.; Xu, T.; Yin, D. Q. Locomotor activity changes on zebrafish larvae with different 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) embryonic exposure modes. Chemosphere 2014, 94, 53− 61. (28) Chen, X.; Huang, C.; Wang, X.; Chen, J.; Bai, C.; Chen, Y.; Chen, X.; Dong, Q.; Yang, D. BDE-47 disrupts axonal growth and motor behavior in developing zebrafish. Aquat. Toxicol. 2012, 120− 121, 35−44. (29) Chou, C. T.; Hsiao, Y. C.; Ko, F. C.; Cheng, J. O.; Cheng, Y. M.; Chen, T. H. Chronic exposure of 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) alters locomotion behavior in juvenile zebrafish (Danio rerio). Aquat. Toxicol. 2010, 98 (4), 388−395. (30) Hamers, T.; Kamstra, J. H.; Sonneveld, E.; Murk, A. J.; Visser, T. J.; Van Velzen, M. J.; Brouwer, A.; Bergman, A. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47). Mol. Nutr. Food Res. 2008, 52 (2), 284−298. (31) Ren, X. M.; Guo, L. H. Molecular toxicology of polybrominated diphenyl ethers: Nuclear hormone receptor mediated pathways. Environ. Sci. Process Impact. 2013, 15 (4), 702−708. (32) Castrillo, A.; Tontonoz, P. Nuclear receptors in macrophage biology: At the crossroads of lipid metabolism and inflammation. Annu. Rev. Cell Dev. Biol. 2004, 20, 455−480. Program of the Chinese Academy of Sciences. He was also supported by a Visiting Distinguished Professorship in the Department of Biology and Chemistry and State Key Laboratory in Marine Pollution at City University of Hong Kong. ■ REFERENCES (1) Birnbaum, L. S.; Staskal, D. F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004, 112 (1), 9−17. (2) Qu, W. Y.; Bi, X. H.; Sheng, G. Y.; Lu, S. Y.; Fu, H.; Yuan, J.; Li, L. P. Exposure to polybrominated diphenyl ethers among workers at an electronic waste dismantling region in Guangdong, China. Environ. Int. 2007, 33 (8), 1029−1034. (3) Chen, D.; Letcher, R. J.; Burgess, N. M.; Champoux, L.; Elliott, J. E.; Hebert, C. E.; Martin, P.; Wayland, M.; Chip Weseloh, D. V.; Wilson, L. Flame retardants in eggs of four gull species (Laridae) from breeding sites spanning Atlantic to Pacific Canada. Environ. Pollut. 2012, 168, 1−9. (4) Valters, K.; Li, H.; Alaee, M.; D’Sa, I.; Marsh, G.; Bergman, A.; Letcher, R. J. Polybrominated diphenyl ethers and hydroxylated and methoxylated brominated and chlorinated analogues in the plasma of fish from the Detroit River. Environ. Sci. Technol. 2005, 39 (15), 5612− 5619. (5) Haraguchi, K.; Kotaki, Y.; Relox, J. R., Jr.; Romero, M. L.; Terada, R. Monitoring of naturally produced brominated phenoxyphenols and phenoxyanisoles in aquatic plants from the Philippines. J. Agric. Food Chem. 2010, 58 (23), 12385−12391. (6) Wiseman, S. B.; Wan, Y.; Chang, H.; Zhang, X. W.; Hecker, M.; Jones, P. D.; Giesy, J. P. Polybrominated diphenyl ethers and their hydroxylated/methoxylated analogs: Environmental sources, metabolic relationships, and relative toxicities. Mar. Pollut. Bull. 2011, 63 (5−12), 179−188. (7) Rotander, A.; van Bavel, B.; Riget, F.; Auethunsson, G. A.; Polder, A.; Gabrielsen, G. W.; Vikingsson, G.; Mikkelsen, B.; Dam, M. Methoxylated polybrominated diphenyl ethers (MeO-PBDEs) are major contributors to the persistent organobromine load in sub-Arctic and Arctic marine mammals, 1986−2009. Sci. Total Environ. 2012, 416, 482−489. (8) Yogui, G. T.; Sericano, J. L. Polybrominated diphenyl ether flame retardants in the U.S. marine environment: A review. Environ. Int. 2009, 35 (3), 655−666. (9) Liu, X.; Jiao, Y.; Lin, C.; Sun, K.; Zhao, Y. PBDEs, hydroxylated PBDEs and methoxylated PBDEs in bivalves from Beijing markets. Chemosphere 2014, 110, 97−103. (10) Sun, J.; Liu, J.; Liu, Y.; Jiang, G. Hydroxylated and methoxylated polybrominated diphenyl ethers in mollusks from Chinese coastal areas. Chemosphere 2013, 92 (3), 322−328. (11) Yin, G.; Asplund, L.; Qiu, Y.; Zhou, Y.; Wang, H.; Yao, Z.; Jiang, J.; Bergman, A. Chlorinated and brominated organic pollutants in shellfish from the Yellow Sea and East China SeaEnviron. Sci. Pollut. Res. Int. 2014, DOI: 10.1007/s11356-014-3198-8. (12) Wang, H. S.; Du, J.; Ho, K. L.; Leung, H. M.; Lam, M. H. W.; Giesy, J. P.; Wong, C. K. C.; Wong, M. H. Exposure of Hong Kong residents to PBDEs and their structural analogues through market fish consumption. J. Hazard. Mater. 2011, 192 (1), 374−380. (13) McKinney, M. A.; De Guise, S.; Martineau, D.; Beland, P.; Lebeuf, M.; Letcher, R. J. Organohalogen contaminants and metabolites in beluga whale (Delphinapterus leucas) liver from two Canadian populations. Environ. Toxicol. Chem. 2006, 25 (5), 1246− 1257. (14) Malmvarn, A.; Marsh, G.; Kautsky, L.; Athanasiadou, M.; Bergman, A.; Asplund, L. Hydroxylated and methoxylated brominated diphenyl ethers in the red algae Ceramium tenuicorne and blue mussels from the Baltic Sea. Environ. Sci. Technol. 2005, 39 (9), 2990−2997. (15) Bowden, B. F.; Towerzey, L.; Junk, P. C. A new brominated diphenyl ether from the marine sponge Dysidea herbacea. Aust. J. Chem. 2000, 53 (4), 299−301. 1831 DOI: 10.1021/es503833q Environ. Sci. Technol. 2015, 49, 1823−1833 Article Environmental Science & Technology and potential polybrominated diphenyl ether metabolites. Eur. J. Org. Chem. 2003, 14, 2566−2576. (52) Amsterdam, A.; Nissen, R. M.; Sun, Z.; Swindell, E. C.; Farrington, S.; Hopkins, N. Identification of 315 genes essential for early zebrafish development. Proc. Natl. Acad. Sci. U. S. A. 2004, 101 (35), 12792−12797. (53) Wen, Q.; Liu, H. L.; Zhu, Y. T.; Zheng, X. M.; Su, G. Y.; Zhang, X. W.; Yu, H. X.; Giesy, J. P.; Lam, M. H. Maternal transfer, distribution, and metabolism of BDE-47 and its related hydroxylated, methoxylated analogs in zebrafish (Danio rerio). Chemosphere 2014, 120C, 31−36. (54) Wen, Q.; Liu, H. L.; Su, G. Y.; Wei, S.; Feng, J. F.; Yu, H. X. Determination of polybrominated diphenyl ethers and their derivates in zebrafish eggs. Chin. J. Anal. Chem. 2012, 40 (11), 1698−1702. (55) Liu, C.; Wang, Q.; Liang, K.; Liu, J.; Zhou, B.; Zhang, X.; Liu, H.; Giesy, J. P.; Yu, H. Effects of tris(1,3-dichloro-2-propyl) phosphate and triphenyl phosphate on receptor-associated mRNA expression in zebrafish embryos/larvae. Aquat. Toxicol. 2013, 128−129, 147−157. (56) Tang, S.; Allagadda, V.; Chibli, H.; Nadeau, J. L.; Mayer, G. D. Comparison of cytotoxicity and expression of metal regulatory genes in zebrafish (Danio rerio) liver cells exposed to cadmium sulfate, zinc sulfate and quantum dots. Metallomics 2013, 5 (10), 1411−1422. (57) Cline, M. S.; Smoot, M.; Cerami, E.; Kuchinsky, A.; Landys, N.; Workman, C.; Christmas, R.; Avila-Campilo, I.; Creech, M.; Gross, B.; Hanspers, K.; Isserlin, R.; Kelley, R.; Killcoyne, S.; Lotia, S.; Maere, S.; Morris, J.; Ono, K.; Pavlovic, V.; Pico, A. R.; Vailaya, A.; Wang, P.-L.; Adler, A.; Conklin, B. R.; Hood, L.; Kuiper, M.; Sander, C.; Schmulevich, I.; Schwikowski, B.; Warner, G. J.; Ideker, T.; Bader, G. D. Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2007, 2 (10), 2366−2382. (58) Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N. S.; Wang, J. T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13 (11), 2498−2504. (59) Saito, R.; Smoot, M. E.; Ono, K.; Ruscheinski, J.; Wang, P. L.; Lotia, S.; Pico, A. R.; Bader, G. D.; Ideker, T. A travel guide to Cytoscape plugins. Nat. Methods 2012, 9 (11), 1069−1076. (60) Pico, A. R.; Kelder, T.; van Iersel, M. P.; Hanspers, K.; Conklin, B. R.; Evelo, C. WikiPathways: pathway editing for the people. PLoS Biol. 2008, 6 (7), e184. (61) Escher, B. I.; Hermens, J. L. M. Modes of action in ecotoxicology: Their role in body burdens, species sensitivity, QSARs, and mixture effects. Environ. Sci. Technol. 2002, 36 (20), 4201−4217. (62) Escher, B. I.; Hermens, J. L. M. Internal exposure: Linking bioavailability to effects. Environ. Sci. Technol. 2004, 38 (23), 455A− 462A. (63) Adolfsson-Erici, M.; Akerman, G.; McLachlan, M. S. Internal benchmarking improves precision and reduces animal requirements for determination of fish bioconcentration factors. Environ. Sci. Technol. 2012, 46 (15), 8205−8211. (64) van Boxtel, A. L.; Kamstra, J. H.; Cenijn, P. H.; Pieterse, B.; Wagner, J. M.; Antink, M.; Krab, K.; van der Burg, B.; Marsh, G.; Brouwer, A.; Legler, J. Microarray analysis reveals a mechanism of phenolic polybrominated diphenylether toxicity in zebrafish. Environ. Sci. Technol. 2008, 42 (5), 1773−1779. (65) Yu, Y.; Yang, W.; Gao, Z.; Lam, M. H. W.; Liu, X.; Wang, L.; Yu, H. RP-HPLC measurement and quantitative structure-property relationship analysis of the noctanol-water partitioning coefficients of selected metabolites of polybrominated dyphenyl ethers. Environ. Chem. 2008, 5 (5), 332−339. (66) Aardema, M. J.; MacGregor, J. T. Toxicology and genetic toxicology in the new era of “toxicogenomics”: Impact of “-omics” technologies. Mutat. Res. 2002, 499 (1), 13−25. (67) Lema, S. C.; Dickey, J. T.; Schultz, I. R.; Swanson, P. Dietary exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain. Environ. Health Perspect. 2008, 116 (12), 1694−1699. (33) Chinenov, Y.; Gupte, R.; Rogatsky, I. Nuclear receptors in inflammation control: Repression by GR and beyond. Mol. Cell. Endocrinol. 2013, 380 (1−2), 55−64. (34) Knoedler, J. R.; Denver, R. J. Kruppel-like factors are effectors of nuclear receptor signaling. Gen. Comp. Endrocrinol. 2014, 203, 49−59. (35) Omiecinski, C. J.; Vanden Heuvel, J. P.; Perdew, G. H.; Peters, J. M. Xenobiotic metabolism, disposition, and regulation by receptors: From biochemical phenomenon to predictors of major toxicities. Toxicol. Sci. 2011, 120 (Suppl 1), S49−S75. (36) Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81 (3), 1269−1304. (37) Jennings, P.; Limonciel, A.; Felice, L.; Leonard, M. O. An overview of transcriptional regulation in response to toxicological insult. Arch. Toxicol. 2013, 87 (1), 49−72. (38) Jacobs, M. N.; Dickins, M.; Lewis, D. F. Homology modelling of the nuclear receptors: Human oestrogen receptorbeta (hERbeta), the human pregnane-X-receptor (PXR), the Ah receptor (AhR) and the constitutive androstane receptor (CAR) ligand binding domains from the human oestrogen receptor alpha (hERalpha) crystal structure, and the human peroxisome proliferator activated receptor alpha (PPARalpha) ligand binding domain from the human PPARgamma crystal structure. J. Steroid Biochem. Mol. Biol. 2003, 84 (2−3), 117−132. (39) Prossnitz, E. R.; Arterburn, J. B.; Smith, H. O.; Oprea, T. I.; Sklar, L. A.; Hathaway, H. J. Estrogen signaling through the transmembrane G protein-coupled receptor GrPR30. Annu. Rev. Physiol. 2008, 70, 165−190. (40) Norman, A. W.; Mizwicki, M. T.; Norman, D. P. Steroidhormone rapid actions, membrane receptors and a conformational ensemble model. Nat. Rev. Drug Discovery 2004, 3 (1), 27−41. (41) Lema, S. C.; Dickey, J. T.; Schultz, I. R.; Swanson, P. Dietary Exposure to 2,2′,4,4′-Tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain. Environ. Health Perspect. 2008, 116 (12), 1694− 1699. (42) Karpeta, A.; Ptak, A.; Gregoraszczuk, E. L. Different action of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) and its hydroxylated metabolites on ERalpha and ERbeta gene and protein expression. Toxicol. Lett. 2014, 229 (1), 250−256. (43) Su, G.; Xia, J.; Liu, H.; Lam, M. H.; Yu, H.; Giesy, J. P.; Zhang, X. Dioxin-like potency of HO- and MeO- analogues of PBDEs’ the potential risk through consumption of fish from Eastern China. Environ. Sci. Technol. 2012, 46 (19), 10781−10788. (44) Stegeman, J. J.; Goldstone, J. V.; Hahn, M. E. Perspectives on zebrafish as a model in environmental toxicology. Fish Physiol. 2010, 29, 367−439. (45) Tang, S.; Cai, Q.; Chibli, H.; Allagadda, V.; Nadeau, J. L.; Mayer, G. D. Cadmium sulfate and CdTe-quantum dots alter DNA repair in zebrafish (Danio rerio) liver cells. Toxicol. Appl. Pharmacol. 2013, 272 (2), 443−452. (46) Dai, Y. J.; Jia, Y. F.; Chen, N.; Bian, W. P.; Li, Q. K.; Ma, Y. B.; Chen, Y. L.; Pei, D. S. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 2014, 33 (1), 11−17. (47) Baker, M. E.; Hardiman, G. Transcriptional analysis of endocrine disruption using zebrafish and massively parallel sequencing. J. Mol. Endocrinol. 2014, 52 (3), R241−R256. (48) Scholz, S.; Fischer, S.; Gundel, U.; Kuster, E.; Luckenbach, T.; Voelker, D. The zebrafish embryo model in environmental risk assessmentApplications beyond acute toxicity testing. Environ. Sci. Pollut. Res. Int. 2008, 15 (5), 394−404. (49) Scholz, S.; Mayer, I. Molecular biomarkers of endocrine disruption in small model fish. Mol. Cell. Endocrinol. 2008, 293 (1−2), 57−70. (50) Bertrand, S.; Thisse, B.; Tavares, R.; Sachs, L.; Chaumot, A.; Bardet, P. L.; Escriva, H.; Duffraisse, M.; Marchand, O.; Safi, R.; Thisse, C.; Laudet, V. Unexpected novel relational links uncovered by extensive developmental profiling of nuclear receptor expression. PLoS Genet. 2007, 3 (11), 2085−2100. (51) Marsh, G.; Stenutz, R.; Bergman, A. Synthesis of hydroxylated and methoxylated polybrominated diphenyl ethers - Natural products 1832 DOI: 10.1021/es503833q Environ. Sci. Technol. 2015, 49, 1823−1833 Article Environmental Science & Technology (68) Chan, W. K.; Chan, K. M. Disruption of the hypothalamicpituitary-thyroid axis in zebrafish embryo-larvae following waterborne exposure to BDE-47, TBBPA and BPA. Aquat. Toxicol. 2012, 108, 106−111. (69) Ren, X. M.; Guo, L. H.; Gao, Y.; Zhang, B. T.; Wan, B. Hydroxylated polybrominated diphenyl ethers exhibit different activities on thyroid hormone receptors depending on their degree of bromination. Toxicol. Appl. Pharmacol. 2013, 268 (3), 256−63. (70) Zhou, H.; Wu, H.; Liao, C.; Diao, X.; Zhen, J.; Chen, L.; Xue, Q. Toxicology mechanism of the persistent organic pollutants (POPs) in fish through AhR pathway. Toxicol. Mech. Methods 2010, 20 (6), 279− 286. (71) Shibata, H.; Spencer, T. E.; Onate, S. A.; Jenster, G.; Tsai, S. Y.; Tsai, M. J.; O’Malley, B. W. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog. Horm. Res. 1997, 52, 141−164 discussion 164-5. (72) Gjernes, M. H.; Schlenk, D.; Arukwe, A. Estrogen receptorhijacking by dioxin-like 3,3′4,4′,5-pentachlorobiphenyl (PCB126) in salmon hepatocytes involves both receptor activation and receptor protein stability. Aquat. Toxicol. 2012, 124−125, 197−208. (73) Grans, J.; Wassmur, B.; Celander, M. C. One-way inhibiting cross-talk between arylhydrocarbon receptor (AhR) and estrogen receptor (ER) signaling in primary cultures of rainbow trout hepatocytes. Aquat. Toxicol. 2010, 100 (3), 263−270. (74) Kojima, H.; Takeuchi, S.; Uramaru, N.; Sugihara, K.; Yoshida, T.; Kitamura, S. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using Chinese hamster ovary cells. Environ. Health Perspect. 2009, 117 (8), 1210−1218. (75) Pacyniak, E. K.; Cheng, X.; Cunningham, M. L.; Crofton, K.; Klaassen, C. D.; Guo, G. L. The flame retardants, polybrominated diphenyl ethers, are pregnane X receptor activators. Toxicol. Sci. 2007, 97 (1), 94−102. (76) Liu, H.; Hu, W.; Sun, H.; Shen, O.; Wang, X.; Lam, M. H.; Giesy, J. P.; Zhang, X.; Yu, H. In vitro profiling of endocrine disrupting potency of 2,2′,4,4′-tetrabromodiphenyl ether (BDE47) and related hydroxylated analogs (HO-PBDEs). Mar. Pollut. Bull. 2011, 63 (5− 12), 287−296. (77) Fery, Y.; Buschauer, I.; Salzig, C.; Lang, P.; Schrenk, D. Technical pentabromodiphenyl ether and hexabromocyclododecane as activators of the pregnane-X-receptor (PXR). Toxicology 2009, 264 (1−2), 45−51. (78) Abbott, B. D. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Reprod. Toxicol. 2009, 27 (3−4), 246−257. (79) Tung, E. W.; Boudreau, A.; Wade, M. G.; Atlas, E. Induction of adipocyte differentiation by polybrominated diphenyl ethers (PBDEs) in 3T3-L1 cells. PLoS One 2014, 9 (4), e94583. (80) Greenwood, A. K.; Butler, P. C.; White, R. B.; DeMarco, U.; Pearce, D.; Fernald, R. D. Multiple corticosteroid receptors in a teleost fish: Distinct sequences, expression patterns, and transcriptional activities. Endocrinology 2003, 144 (10), 4226−4236. 1833 DOI: 10.1021/es503833q Environ. Sci. Technol. 2015, 49, 1823−1833 Supporting Information Bioaccumulation, biotransformation and toxicity of BDE-47, 6-OH-BDE-47 and 6-MeO-BDE-47 in early life-stages of zebrafish (Danio rerio) Hongling Liu1#*, Song Tang2#, Xinmei Zheng1, Yuting Zhu1, Zhiyuan Ma1, Chunsheng Liu1, Markus Hecker2,3, David M.V. Saunders3, John P. Giesy1,3,4,5, Xiaowei Zhang1, Hongxia Yu1* 1 State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, Jiangsu 210023, China 2 School of Environment and Sustainability, University of Saskatchewan, Saskatoon, SK S7N 5B3, Canada 3 Toxicology Centre, University of Saskatchewan, Saskatoon, SK S7N 5B3, Canada 4 Department of Veterinary Biomedical Sciences, University of Saskatchewan, Saskatoon, SK S7N 5B3, Canada 5 Department of Biology and Chemistry, City University of Hong Kong, Kowloon, Hong Kong, SAR, China # These authors contributed equally to this work. * Correspondence to: Drs. Hongling Liu and Hongxia Yu, School of the Environment, Nanjing University, Nanjing, Jiangsu 210023, China Tel: +86-25-89680356; Fax: +86-25-89680356; Email: hlliu@nju.edu.cn (Dr. Hongling Liu) and yuhx@nju.edu.cn (Dr. Hongxia Yu) A summary of 13 pages including 1 figure and 5 tables S1 Chemical Analysis Procedures Bioavailability analysis This experiment was designed to determine bioaccumulation of the three chemicals in early life-stages of zebrafish. In each treatment, 600 zebrafish embryos were exposed to 6-OH-BDE-47, 6-MeO-BDE-47 or BDE-47 at 300 μg/L (0.6, 0.58, and 0.62 μM, respectively) from 4 to 120 hpf in glass beakers (Diameter 150 mm). Concentration of each of the three compounds was quantified in both exposure solution and embryos/larvae. 80 embryos/larvae and corresponding exposure solutions were collected at 0, 12, 24, 48, 72, 96, and 120 hpf. Detailed protocols for extraction, clean up, identification and quantification, and quality assurance and quality control (QA/QC) are provided in previous publications.1-3 In brief, samples of exposure solutions were collected and diluted to 5 mL with MilliQ water, and 1 mL hydrochloric acid (2 M HCl) were added. After vortex mixing, samples were extracted with 6 mL n-hexane-methyl tert butyl ether mixture (MTBE) (1:1, v/v) three times and were dried under nitrogen. Dried extracts were dissolved in 480 μL of derivatization solvent (acetonitrile: methanol: water: pyridine = 5:2:2:1, v/v/v/v), and 40 μL methylchloroformate (MCF) was added. Samples were then incubated at 25 oC with vortex for 1 h, after which 1.4 mL MilliQ water was added and extracted three times with 6 mL n-hexane. The extracts were concentrated, and 100 μL of 13C-PCB-178 was added as the internal injection standard and made up to 100 μL prior to GC/MS analysis. Embryos and larvae from each exposure were composited, rinsed with MilliQ water, gently dried, and weighed. Internal dose and potential biotransformation of individual OH-PBDEs, MeO-PBDEs and PBDEs were also determined. Approximately 0.1 g sample was homogenized and transferred into amber serum bottles, and spiked surrogate recovery standard (13C-2’-OH-BDE-99 and 13C-BDE-139), and 2 mL MilliQ water, 50 µL HCl at 2 M, and 2 mL 3-propanol was added. Then they were extracted three times with 10 mL of n-hexane/MTBE (1:1,v/v). Extracts were concentrated by rotary evaporation and dried under nitrogen. After, the derivation of PBDEs, MeO-PBDEs and OH-PBDEs and purification was followed by the previous method.1-3 Concentrations of 12 PBDEs, 12 OH-PBDEs and 12 MeO-PBDEs were determined by use of a TSQ Quantum GC/MS (Thermo Scientific, USA) coupled with an Agilent DB-XLB column (15 m × 0.25 mm × 0.25 μm, J&W Scientific, USA) in 3 separate runs. Identification of specific PBDEs, S2 OH-PBDEs and MeO-PBDEs was performed by comparing relative retention times versus internal standard and product ions in SRM mode with the standards. Time-courses of bioconcentration factors (BCF) for each chemical were also calculated based on measured concentrations in zebrafish embryos and larvae divided by the determined concentration in water. QA/QC QA/QC was conducted by the analysis of procedural blanks (RSD<22.6%, for 6 replicates) as previously described3 and the obtained recoveries of standard compounds were ranged 93%-146%, 67%-113% and 103%-133% for PBDEs, OH-PBDEs and MeO-PBDEs, respectively. Limit of detection (LOD) of each compound was defined as three times the SD of the laboratory blanks. For congeners not detected in the blanks, we set the LOD at the instrumental limit of quantification (LOQ). Method LODs ranged 9.2×10-2-2.9×101 ng/g wet mass (wm) for individual PBDEs, OH-PBDEs and MeO-PBDEs congeners. Concentrations less than the method LOD were assumed to be not detected. PBDEs, OH-PBDEs and MeO-PBDEs were all quantified in samples extracts relative to 13 C-PCB-178. Recoveries of surrogate standards were 62.1%-104.7%, 82.9%-117.9%, and 44.9%-74.9% for BDE-47 (13C-BDE-139), 6-MeO-BDE-47 (13C-BDE-139), and 6-OH-BDE-47 (13C-2’-OH-BDE-99), respectively. Water Quality of Exposure Solutions During the exposure, zebrafish embryos/larvae were maintained at 28 oC in egg water (60 mg/L ocean salts) and the water quality parameters were measured every day including pH (7.1-7.6), DO (95% saturation), hardness (15-18 mg/L as CaCO3) and ammonia (0-3 mg/L). Temperature, hardness, pH, oxygen concentration, and ammonia in all treatments were similar from start and end of the exposures. S3 Figure S1. Effects of exposures to 6-OH-BDE-47, 6-MeO-BDE-47 and BDE-47 on morphologies of several development stages of zebrafish embryos and larvae. A) normal zebrafish at 72 hpf; B) edema caused by 0.5 μM 6-OH-BDE-47 at 72 hpf; C) development arrested by 0.5 μM 6-OH-BDE-47 at 72 hpf; D) spinal curvature caused by 0.1 μM 6-OH-BDE-47 at 72 hpf; E) normal zebrafish at 120 hpf; F) severe edema and spinal curvature caused by 0.05 μM 6-OH-BDE-47 at 120 hpf, LC50=0.09 μM; G) spinal curvature caused by 2.5 μM 6-MeO-BDE-47 at 120 hpf, NOEC=0.5 μM; H) spinal curvature caused by 2.5 μM BDE-47 at 120 hpf, NOEC=0.5 μM. S4 Table S1. Results of repeatability, recovery and detection limit with spiked procedural blanks. Repeatability Compounds RSD Detection Limit Recovery BDE-17 BDE-28 BDE-71 BDE-47 BDE-66 BDE-100 BDE-99 BDE-85 BDE-154 BDE-138 BDE-183 BDE-190 6'-MeO-BDE-17 2'-MeO-BDE-28 2'-MeO-BDE-68 6-MeO-BDE-47 2'-MeO-BDE-47 4'-MeO-BDE-49 4’-MeO-BDE-90 6-MeO-BDE-90 3-MeO-BDE-100 2-MeO-BDE-123 6-MeO-BDE-85 6-MeO-BDE-137 15.2% 14.6% 10.0% 9.5% 9.0% 10.1% 10.8% 11.9% 12.2% 12.9% 13.4% 12.4% 11.9% 11.7% 10.2% 9.1% 9.9% 10.2% 9.6% 11.4% 10.5% 11.4% 10.1% 12.1% 119.6% 144.0% 114.6% 119.1% 129.2% 101.9% 105.6% 116.0% 146.0% 93.0% 101.1% 109.5% 113.5% 109.7% 116.4% 119.6% 120.5% 127.3% 106.0% 112.6% 133.0% 116.5% 107.7% 103.0% Instrument (ng/mL) 2.7 4.5×10-1 9.2×10-2 7.8×10-1 1.2 2.3 5.1×10-1 6.7×10-1 2.6 2.9 4.1 4.2 5.4×10-1 5.5×10-1 4.9 8.0 6.2 6.5 1.9×101 4.5 9.8 8.9 1.0×101 1.6×101 3'-OH-BDE-7 21.2% 21.5% 18.2% 22.6% 16.7% 11.6% 20.8% 20.9% 17.0% 15.4% 21.6% 17.3% 89% 91.9% 92.6% 81% 103.2% 88.9% 71.6% 67% 74.6% 112.8% 87% 91.4% 2.2×101 1.4×101 1.1×101 2.2×101 9.7 6.6 1.5×101 2.9×101 9.9 1.7×101 2.5×101 1.6×101 2'-OH-BDE-7 2'-OH-BDE-17 2'-OH-BDE-25 2'-OH-BDE-28 2-OH-BDE-47 2'-OH-BDE-68 2'-OH-BDE-66 2-OH-BDE-90 2-OH-BDE-85 4'-OH-BDE-49 2-OH-BDE-137 S5 Method (ng/g) 2.7 4.5×10-1 9.2×10-2 7.8×10-1 1.2 2.3 5.1×10-1 6.7×10-1 2.6 2.9 4.1 4.2 5.4×10-1 5.5×10-1 4.9 8.0 6.2 6.5 1.9×101 4.5 9.8 8.9 1.0×101 1.6×101 2.2×101 1.4×101 1.1×101 2.2×101 9.7 6.6 1.5×101 2.9×101 9.9 1.7×101 2.5×101 1.6×101 Table S2. Primer sequences of NR related genes for qRT-PCR. Genes 11βhsd 18s abcb311 adrb2a adrb2b ahr1b ahr2 ahrra ahrrb aip ar arnt2 arntl1a arntl1b c1d ccnd1 ctnnb1 cyp1a1 cyp1b1 cyp24a1 cyp3a65 dap3 dut egfr er1 er2a er2b flh fus gr hdac3 hnf4a hpse hsp90aa1 il6 il8 lpl mr ncoa1 ncoa2 ncoa3 ncoa4 Forward Sequence (5’-3’) tggtgaagtatgccatcgaa ttgttggtgttgttgctggt agcgtgtctcttttgggaga gctgatctggtcatgggatt cccgattacaagctgatggt ggagagcacttgaggaaacg atctccatgggcaaaacaag gctgctgatgtttggactga acctgggatttcatcagacg ccatcacttgaagcctccat acattctggaggccattgag gaatggtctcggtccgtcta tctcctgggggaaagaagat ctcgctgaatgccatagaca acggagagctgacagaccat tgacttgccttgacttgtcg atcctgtccaacctgacctg cctgggcggttgtctatcta gctcagctcggtaacactcc aagacgtggaaggaccacac ctgtgcatcatggaccaaac tcgaccgttcatgtaaacca tacagacgctggatgagacg aacgcaaataatggcaggac ggtccagtgtggtgtcctct agcttgtgcacatgatcagc ttgtgttctccagcatgagc ctcttccaaatgccacgaat ccaatatgcaggagcaggat agaccttggtccccttcact agccatgaaggtgtccattc gccgacactacagagcatca cggcagtctgaacagatgaa ggatctggtgatcctgctgt tcctggtgaacgacatcaaa gtcgctgcattgaaacagaa ctggccttctcaccaaacat tttgagggaccagacaaacc tgagagcctctgttggaggt agagcctgtcagtcccaaga aactcacctgcccacaaatc gacaactgcggaaaagaagc Reverse Sequence (5’-3’) gcaaagctttttgagccatc ggatgctcaacaggggttcat atagcacagctagggccaga atgtgatggcgatgtaacga tatgagcaaccccactgtca ggatccagatcgtcctttga tccctcttgtgtcgataccc gacgctgtgttcacgtcact gctgtacagatgagccgtca tgcatgtgctccaacttctc acgtgcaagttacggaaacc agctggtcacctgcagtctt ccatcgctgcttcatcatta cccgagacgactgtattggt gccgacatcagatccagttt gaaaaagcagggagcacttg tctctgcatcctggtgtctg tgaggaatggtgaagggaag cgttagacacgaaccggaat ctctgttgtggcagcgtaaa ggtgaaggatggtgagagga ctggatgctgagacacctga aatgcagcaacacaaacagc tctccagaaccacagtgcag cacacgaccagactccgtaa gctttcatccctgctgagac ccacatatggggaaggaatg gggcacgcagtagttatggt cttccccgtctctctgtctg cgcctttaatcatgggagaa agaagctgcttgcaggactc aggtgttcctggaccagatg aacacgggacaaatccacat tccagaacgggcatatcttc tcatcacgctggagaagttg cttaacccatggagcagagg gcctttgaatcccaatgcta cacactttggctgtcgaaga ctctgaccctggtttggtgt ggtcgtagccaccatcagtt agaggcctgttgctggtcta ctggggatttggcagagtta S6 Gene ID AY578180 NM_200713 NM_001006594 NM_001102652 NM_001089471 NM_001024816 NM_131264 NM_001035265 NM_001033920 NM_214712 NM_001083123 NM_131674 NM_131577 NM_178300 NM_001007059 NM_131025 NM_131059 AF210727 AY727864 NM_001089458 AY452279 NM_001098737 NM_001006005 AY332223 NM_152959 NM_180966 NM_174862 DQ118096 NM_201083 EF567112 NM_200990 NM_194368 NM_001045005 NM_131328 JN698962 XM_001342570 NM_131127 EF567113 XM_686652 NM_131777 XM_687846 NM_201129 ncor ncor2 pa2g4a pa2g4b pgr pou1f1 pparg ppargc1a pparα pxr rela sp1 tgfb1 thra ube2i ugt1a1 vtg1 vtg2 vtg4 vtg5 agggtaaggagcagagcaca ttgaaccagtttcaccacca cgggaaaaggacatgaagaa caaagacaccaccacgtttg caacaggtggttgtggacag cggagctttgtggagaagag tgccgcatacacaagaagag aatcaggattcggtgtggag gattcaaatcttgccgtggt ccagctaccagagccttgac tataagccacacccacacga tcctccattaatcggtcgag aactactgcatggggtcctg caatgtaccatttcgcgttg tggaaagagggaagatgtgg attggagaaggctcccaagt ctgcgtgaagttgtcatgct tactttgggcactgatgcaa ctacaaggtggaggctctgc agctaatgctctgcccgtta gcaaaactggttcaggtggt tgacaatggctgagttgctc aagccgtcaacatgaactcc gtgccaccattacgcttttt atttggagatgtccgctttg ttggtcatgaaggagctgtg atgtggttcacgtcactgga ttggatgcttcattgccata tcgtcgctgagagactgaga tggtcctccataaccagagc gaatgggttgttttgcgtct tgtgtgtgagcacaaaacga ggacaattgctccaccttgt gctcctgctctgtgttttcc cgaatgaagtgaaggggtgt ggaaaggatccgtgagcata gaccagcattgcccataact agacttcgtgaagcccaaga ggaggacaaatcaccagcat gttcagcctcaaacagcaca S7 EF016488 NM_001007032 NM_001002170 NM_212641 NM_001166335 NM_212851 NM_131467 AY998087 NM_001161333 DQ069792 AY163839 NM_212662 AY178450 NM_131396 NM_131351 NM_001037428 NM_001044897 AY729644 NM_001045294 NM_001025189 Table S3. Toxicity of 6-OH-BDE-47, 6-MeO-BDE-47, and BDE-47 to early life-stages of zebrafish. Chemicals 6-OH-BDE-47 6-MeO-BDE-47 BDE-47 Duration of development (hpf) 48 72 72 72 96 120 120 120 120 120 Toxicity Endpoints Hypopigmentation Spinal curvatures, slow heartbeats, and reduced body lengths Arrested development LC50 LC50 LC50 NOEC Spinal curvatures and pericardial edema NOEC Spinal curvatures, pericardial edema, and reduced body lengths Concentration or Mean (95% CI) (μM) ≥0.1 0.1 0.5 0.28 (0.21-0.38) 0.13 (0.11-0.16) 0.09 (0.04-0.10) 0.5 2.5 0.5 2.5 Table S4. Measured concentrations of BDE-47, 6-OH-BDE-47 and 6-MeO-BDE-47 in exposure solutions (ng/g) with a nominal concentration of 300 μg/L at 0 hpf. Chemical Control BDE-47 6-MeO-BDE-47 6-OH-BDE-47 BDE-17 BDE-28 BDE-71 BDE-47 BDE-66 BDE-100 BDE-99 BDE-85 BDE-154 BDE-138 n.d n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. 22.39 27.02 n.d. 227.56 n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. S8 BDE-183 BDE-190 6'-MeO-BDE-17 2'-MeO-BDE-28 2'-MeO-BDE-68 6-MeO-BDE-47 6-MeO-BDE-90 3-MeO-BDE-100 4-MeO-BDE-90 2-MeO-BDE-123 6-MeO-BDE-85 6-MeO-BDE-137 5-MeO-BDE-47 4'-MeO-BDE-49 3'-OH-BDE-7 2'-OH-BDE-17 2'-OH-BDE-25 2'-OH-BDE-28 2'-OH-BDE-7 6-OH-BDE-47 2'-OH-BDE-68 2'-OH-BDE-66 6-OH-BDE-90 6-OH-BDE-85 4'-OH-BDE-49 6-OH-BDE-137 n.d. n.d. n.d. n.d. n.d. n.d. n.d.. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. S9 n.d. n.d. n.d. n.d. n.d. 493.19 n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. 282.73 n.d. n.d. n.d. n.d. n.d. n.d. S10 Table S5. Fold-change of gene expressions in NR pathways, * p<0.05 indicates significant difference between exposure groups and the control. NRs AhR AhR AhR AhR AhR AhR AhR AhR AhR AhR AhR AhR AhR AR AR AR AR AR AR AR ER ER ER Genes cyp1a1 ahr1a ahr1b ahr2 ahrra ahrrb aip arnt2 arntl1a arntl1b cyp1b1 ncor2 sp1 ar ctnnb1 ncoa1 ncoa2 ncoa4 pa2g4a pa2g4b brca2 ccnd1 cyp19b 0 1.01 1.02 1.01 1.02 1.03 1.05 1.01 1.01 1.01 1.01 1.25 1.00 1.03 1.00 1.00 1.00 1.03 1.00 1.00 1.01 1.01 1.00 1.00 6-OH-BDE-47 (μM) 0.008 0.02 -1.16 -1.75* 2.32* 1.54 1.71* 1.63* 1.62* 1.67* -2.27* -1.96* 1.16 -1.35 1.13 -1.14 1.62* 1.40 -1.04 -1.18 1.15 1.11 -1.03 -1.52 1.03 1.53 -1.16 1.67 1.35 1.20 1.22* 1.24* 1.18 1.22 1.33 1.68 1.20* 1.39* 1.02 1.01 -1.15 -1.18 -1.25 -1.10 1.09 -1.03 -1.81* -1.20 0.05 -1.92* 1.69 1.18 1.11 -1.64* -1.96 -1.06 -1.01 -1.25* -1.99 -2.63* 1.21 -1.39 -1.15 -1.11 -1.14 1.14 1.44* 1.91 1.40 1.00 -1.41* -2.23* 0 1.01 1.02 1.01 1.00 1.00 1.00 1.04 1.01 1.01 1.00 1.24 1.02 1.03 1.00 1.00 1.00 1.01 1.00 1.00 1.01 1.01 1.00 1.00 6-MeO-BDE-47 (μM) 0.1 0.5 2.5 -1.54* -9.09* -50.00* 1.49 -1.21 1.20 1.16 1.48 1.23 -1.02 -1.20 -1.92* 1.50 2.18 3.16* 1.50 2.15 5.71** -1.20 -1.30 -1.00 2.08* -1.18 -1.75 1.14 -1.19 -1.10 1.35 -1.47 -1.49 -1.05 -1.75 -100.0* 1.30 -1.36 -2.61 1.00 -1.41 -1.89* 1.08 -1.23 -1.85* 1.40* 1.01 -1.89* 1.11 1.04 -1.67* 1.31 1.07 -1.67 1.25 1.05 -1.25 1.56* 1.06 -1.33 1.14 -1.08 -1.47* 1.02 1.03 -1.09 1.15 -1.12 -1.85* -1.10 2.43 1.07 S11 0 1.03 1.01 1.00 1.00 1.01 1.06 1.02 1.01 1.01 1.00 1.27 1.00 1.06 1.00 1.00 1.00 1.01 1.00 1.00 1.01 1.08 1.05 1.01 BDE-47 (μM) 0.1 0.5 -1.49 -1.72 1.10 1.03 1.29 1.53 -1.05 1.13 1.25 1.18 1.13 1.04 -1.24 1.06 1.22 1.51 1.49 1.91 1.15 1.07 1.17 1.44 1.07 -1.05 1.04 1.40 1.60 1.08 1.13 -1.02 -1.02 1.30 1.04 1.11 1.00 -1.03 1.04 -1.06 -1.03 -1.15 -1.77* -1.54 -1.35 -1.28 1.39 -1.14 2.5 -5.88* -1.75 1.64* -1.09 1.38 -1.28 -1.28 -1.05 -1.28 -1.22 -6.25 -1.49 -2.50 -1.20 -1.45* 1.07 -1.12 1.02 -1.23* -1.27* 1.22 -1.52* -2.28* ER ER ER ER ER ER ER ER ER GR GR GR GR GR MR MR MR MR MR MR MR PPARα PPARα PPARα PPARα PPARα er1 er2a er2b ncoa3 pgr vtg1 vtg2 vtg4 vtg5 dap3 gr hsp90aa1 rela tgfb1 11βhsd3 adrb2a adrb2b egfr hpse mr ube2i dut il6 il8 lpl ppara 1.16 1.00 1.02 1.07 1.05 1.02 1.01 1.01 1.04 1.01 1.00 1.00 1.01 1.00 1.01 1.01 1.01 1.01 1.02 1.02 1.00 1.01 1.00 1.02 1.03 1.00 1.29 1.68 1.47 1.55 -1.36 -1.31 -1.22 -1.67 -1.39 1.42 1.12 -1.25 1.21 1.03 -1.35 1.00 -1.32 1.27 -1.28 1.51* -1.06 -1.20 -1.20 -2.50* 1.39 -1.13 1.25 1.60 1.64 1.09 -1.12 -1.30 -1.21 -1.20 -1.34 1.28 -1.02 -1.06 1.19 1.04 -1.30 -1.01 -1.25 1.39 -1.06 1.42 -1.11 -1.08 1.56 -1.85* -1.32 -1.02 1.59 1.23 1.83* 1.37 1.13 -1.02 1.10 -1.09 -1.13 2.18* -1.30 1.49 1.34 3.27 -1.27 -1.12 -1.25 1.69 -1.16 -1.15 -1.22* -1.27 2.29 -1.67 -1.51 -1.00 1.04 1.00 1.01 1.05 1.07 1.02 1.02 1.02 1.00 1.01 1.00 1.00 1.01 1.01 1.01 1.00 1.00 1.00 1.02 1.03 1.00 1.00 1.00 1.00 1.00 1.00 -1.24 -1.05 -1.16 -1.09 1.03 -1.35 -1.17 2.23 -3.85 1.78* 1.14 1.65 1.51* 1.09 1.40 -1.14 -1.14 1.47 -1.08 1.26 1.00 1.67* -2.17 2.04 -1.10 1.15 S12 1.22 -1.56* -1.05 1.08 1.02 -1.08 1.15 3.55 -5.26 -1.20 1.18 -1.04 1.03 -1.35 1.00 -1.01 1.02 1.07 -1.11 1.19 -1.11 1.02 1.01 2.47 1.28 1.26 1.88 -1.43* 1.44* -1.75 -1.08 -1.14 1.19 1.06 -3.85 1.28 -2.04* 1.37 -1.96 -1.72* -1.30 -1.05 1.22 -2.05 -1.45* -1.89 -1.32* 1.30 2.30 2.21 1.47 -1.18 1.02 1.00 1.00 1.02 1.08 1.12 1.01 1.00 1.00 1.03 1.00 1.00 1.01 1.00 1.01 1.01 1.01 1.01 1.01 1.02 1.00 1.00 1.00 1.00 1.03 1.01 1.51 1.43* 1.27 1.23 -1.25 -1.33 1.44 -7.69* 1.07 1.14 -1.05 -1.07 1.03 1.09 -1.15 1.13 1.48* -1.15 1.12 1.05 1.10 1.08 2.06 1.33 1.11 1.10 1.69 1.30 1.11 1.31 1.04 -1.36 1.46 -1.19 1.13 1.15 -1.28* -1.17 -1.10 -1.12 -1.35* 1.05 1.31 -1.14 -1.00 1.09 1.07 1.07 1.72 1.49 1.02 1.40 1.38 1.35* 1.40* -1.07 1.66 1.03 1.36 1.30 -1.29 1.15 -1.14 -1.01 -1.02 -1.08 -1.49* 1.17 1.36 -1.11 -1.09 -1.09 -1.25* 1.14 -1.56 1.13 -1.79 -1.08 PPARα PPARα PxR PxR PxR PxR PxR PxR PxR TR TR TR TR TR pparg ppargcla abcb3l1 cyp24a1 cyp3a65 hnf4a pou1f1 pxr ugtlal c1d fus hdac3 ncor thra 1.02 1.00 1.03 1.00 1.00 1.00 1.02 1.02 1.02 1.01 1.01 1.08 1.00 1.00 -1.17 1.24 -1.08 1.01 1.08 1.34* -1.06 1.12 1.05 -1.49* 1.10 1.04 1.14 1.27 -1.22 1.18 1.12 1.07 1.05 1.38* 1.14 1.23 1.08 -1.23 1.22 1.52 -1.12 1.37 1.24 -1.05 1.04 2.05* 1.22 1.33* 1.16 -1.07 1.12 -1.08 -1.37 1.04 -1.45* 1.05 1.01 1.00 1.05 1.04 1.00 1.00 1.01 1.02 1.00 1.00 1.00 1.00 1.00 1.00 1.50 2.02 -1.19 -1.22 1.12 1.19 -1.02 1.58 2.23* 1.00 1.11 -1.37 1.24 1.42 -1.01 1.01 -1.15 -1.28 -1.23 1.15 1.17 -1.20 1.05 1.06 -1.16 -1.34 -1.19 -1.01 -2.26 -1.27 -1.02 -3.13* -3.03* -1.56 1.21 -1.23 -3.13 -1.09 -1.85* -1.33 -2.08* -2.17* 1.01 1.00 1.02 1.01 1.01 1.00 1.01 1.01 1.00 1.00 1.00 1.00 1.00 1.00 -1.06 1.07 1.53 1.69 1.18 1.08 1.31 -1.03 1.15 -1.04 1.33 1.05 1.17 -1.05 -1.23 -1.16 1.25 1.42 1.03 -1.06 1.19 -1.18 -1.25 -1.03 1.13 1.30 1.61 1.13 -1.20 -1.79* 1.04 -1.61 -2.04* -1.56* 1.53* -1.43 -2.70* 1.13 -1.11 1.69 1.06 -1.22 References 1. Wen, Q.; Liu, H. L.; Zhu, Y. T.; Zheng, X. M.; Su, G. Y.; Zhang, X. W.; Yu, H. X.; Giesy, J. P.; Lam, M. H., Maternal transfer, distribution, and metabolism of BDE-47 and its related hydroxylated, methoxylated analogs in zebrafish (Danio rerio). Chemosphere 2014, 120C, 31-36. 2. Wen, Q.; Liu, H. L.; Su, G. Y.; Wei, S.; Feng, J. F.; Yu, H. X., Determination of Polybrominated Diphenyl Ethers and Their Derivates in Zebrafish Eggs. Chine. J Anal. Chem. 2012, 40, (11), 1698-1702. 3. Zheng, X.; Zhu, Y.; Liu, C.; Liu, H.; Giesy, J. P.; Hecker, M.; Lam, M. H.; Yu, H., Accumulation and biotransformation of BDE-47 by zebrafish larvae and teratogenicity and expression of genes along the hypothalamus-pituitary-thyroid axis. Environ. Sci. Technol. 2012, 46, (23), 12943-12951. S13