Removal of Phosphate from Eutrophic Lakes through Adsorption by
advertisement
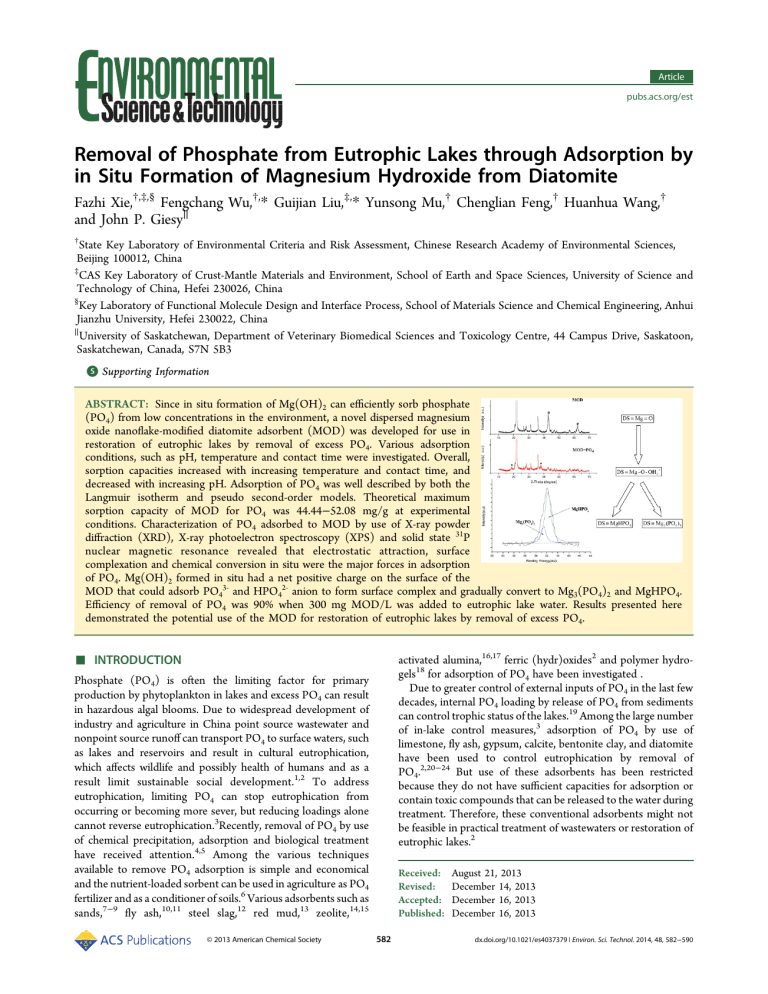
Article pubs.acs.org/est Removal of Phosphate from Eutrophic Lakes through Adsorption by in Situ Formation of Magnesium Hydroxide from Diatomite Fazhi Xie,†,‡,§ Fengchang Wu,†,* Guijian Liu,‡,* Yunsong Mu,† Chenglian Feng,† Huanhua Wang,† and John P. Giesy∥ † State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China ‡ CAS Key Laboratory of Crust-Mantle Materials and Environment, School of Earth and Space Sciences, University of Science and Technology of China, Hefei 230026, China § Key Laboratory of Functional Molecule Design and Interface Process, School of Materials Science and Chemical Engineering, Anhui Jianzhu University, Hefei 230022, China ∥ University of Saskatchewan, Department of Veterinary Biomedical Sciences and Toxicology Centre, 44 Campus Drive, Saskatoon, Saskatchewan, Canada, S7N 5B3 S Supporting Information * ABSTRACT: Since in situ formation of Mg(OH)2 can efficiently sorb phosphate (PO4) from low concentrations in the environment, a novel dispersed magnesium oxide nanoflake-modified diatomite adsorbent (MOD) was developed for use in restoration of eutrophic lakes by removal of excess PO4. Various adsorption conditions, such as pH, temperature and contact time were investigated. Overall, sorption capacities increased with increasing temperature and contact time, and decreased with increasing pH. Adsorption of PO4 was well described by both the Langmuir isotherm and pseudo second-order models. Theoretical maximum sorption capacity of MOD for PO4 was 44.44−52.08 mg/g at experimental conditions. Characterization of PO4 adsorbed to MOD by use of X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and solid state 31P nuclear magnetic resonance revealed that electrostatic attraction, surface complexation and chemical conversion in situ were the major forces in adsorption of PO4. Mg(OH)2 formed in situ had a net positive charge on the surface of the MOD that could adsorb PO43‑ and HPO42‑ anion to form surface complex and gradually convert to Mg3(PO4)2 and MgHPO4. Efficiency of removal of PO4 was 90% when 300 mg MOD/L was added to eutrophic lake water. Results presented here demonstrated the potential use of the MOD for restoration of eutrophic lakes by removal of excess PO4. ■ activated alumina,16,17 ferric (hydr)oxides2 and polymer hydrogels18 for adsorption of PO4 have been investigated . Due to greater control of external inputs of PO4 in the last few decades, internal PO4 loading by release of PO4 from sediments can control trophic status of the lakes.19 Among the large number of in-lake control measures,3 adsorption of PO4 by use of limestone, fly ash, gypsum, calcite, bentonite clay, and diatomite have been used to control eutrophication by removal of PO4.2,20−24 But use of these adsorbents has been restricted because they do not have sufficient capacities for adsorption or contain toxic compounds that can be released to the water during treatment. Therefore, these conventional adsorbents might not be feasible in practical treatment of wastewaters or restoration of eutrophic lakes.2 INTRODUCTION Phosphate (PO4) is often the limiting factor for primary production by phytoplankton in lakes and excess PO4 can result in hazardous algal blooms. Due to widespread development of industry and agriculture in China point source wastewater and nonpoint source runoff can transport PO4 to surface waters, such as lakes and reservoirs and result in cultural eutrophication, which affects wildlife and possibly health of humans and as a result limit sustainable social development.1,2 To address eutrophication, limiting PO4 can stop eutrophication from occurring or becoming more sever, but reducing loadings alone cannot reverse eutrophication.3Recently, removal of PO4 by use of chemical precipitation, adsorption and biological treatment have received attention.4,5 Among the various techniques available to remove PO4 adsorption is simple and economical and the nutrient-loaded sorbent can be used in agriculture as PO4 fertilizer and as a conditioner of soils.6 Various adsorbents such as sands,7−9 fly ash,10,11 steel slag,12 red mud,13 zeolite,14,15 © 2013 American Chemical Society Received: Revised: Accepted: Published: 582 August 21, 2013 December 14, 2013 December 16, 2013 December 16, 2013 dx.doi.org/10.1021/es4037379 | Environ. Sci. Technol. 2014, 48, 582−590 Environmental Science & Technology Article washed with deionized water until free from Cl−. The residue was dried at 323 K for 24 h and then stored at room temperature. The material produced was magnesium hydroxide modified diatomite (MHD). MOD was obtained by calcination of MHD at 723 K.33 Characterization of the Adsorbent. The zero point of charge (pHzpc) of adsorbents (raw diatomite, MHD and MOD) were measured by using mass titrations.36 Surface morphology was determined by use of a JEOL JSM-6700F scanning electron microscope (SEM). X-ray diffraction analysis (XRD) to investigate crystalline structures was conducted by use of a Rigaku TTR-III X-ray diffraction system equipped with Cu Kα radiation (λ = 0.15406 nm). Chemical composition of PO4 adsorbed to MOD was probed using X-ray photoelectron spectroscopy (XPS, ESCAL AB250) with Al Kα radiation (1487 eV) at a power of 300 W under a vacuum of 1.0 × 10−5 Pa. Data were analyzed by use of XPSpeak 41 software. N2 adsorption− desorption analysis was performed on each sorbent by ASAP2020 M+C system (Micromeritics). Surface areas measured by use of the Brunauer−Emmentt−Teller (BET) method. The solid state 31P nuclear magnetic resonance spectrum was collected on a Bruker Avance III 400 spectrometer operating at 161.984 MHz, by use of a Doty Scientific 4 mm H/ X/Y MAS probe. Effect of pH on Sorption of PO4. Adsorbents (0.20 mg) (raw diatomite, MHD and MOD) and 40 mL of 50 mg/L standard KH2PO4 solutions were added to 50 mL centrifuge tubes with initial pH adjusted to 5.0−10.0 with dilute NaOH and HCl, and shaken until equilibrated (12h) at a temperature of 298 K. Supernatant was filter using a 0.45 μm filter and concentrations of PO4 in supernatant were determined using the method of Murphy and Riley.37 The limit of quantification of PO4 was 0.01 mg/L and the limit of detection was 1.5 μg/L. Sorption capacity was calculated (eq 1). Diatomite is a natural material formed from the remains of diatoms, which grew and were historically deposited in sediments of seas or lakes.25 It is abundant in many areas of the world and has unique physical characteristics, such as low cost, porosity (80−90%), small particle size, low thermal conductivity and density26 and it has been approved as a food-grade material by the U.S. Food and Drug Administration.2 Thus, diatomite can be safely used to remove PO4 from eutrophic lake waters. However, diatomite cannot be used directly as an effective adsorbent of PO4 due to its relatively small surface area and the fact that at neutral pH, the surface has a net negative charge. That negative charge would repel the predominant species of PO4 at neutral pH that are HPO42− and H2PO4−. A suitable modification of the surface of diatomite can dramatically improve the surface characteristics and make it more useful for adsorption of PO4 than raw diatomite. Conventional modification methods are purification in HCl25 and calcination,25,27 which have been applied to make treated diatomite more inert for use as a filter support. Treatment of diatomite with NaOH and deposition of manganese oxide on the surface have been used to make it more effective as a scavenger of metal ions. The surface area of modified diatomite can be as great as 80 m2/g.28 Diatomite can be modified with lime and aluminum sulfate (Al2(SO4)3) resulting in aluminum hydroxyl groups on the surface of diatomite, which results in greater chemisorption efficiency for PO4. Efficiency of removal of 80% of total PO4 was obtained by this modified diatomite.29 A ferrihydrite-modified diatomite with a, Brunauer−Emmentt−Teller (BET)-specific surface area of 211.1 m2/g and capacity to adsorb PO4 of 37.3 mg/g at pH 4.02 and a total efficiency for removal of PO4 of 85%.30 In situ formation of magnesium hydroxide (known as MAGIC) is an effective method for preconcentration of nanomolar concentrations of PO4 from fresh and sea waters before quantification.31,32 However, reports on removal of anion by Mg(OH)2 formed in situ are limited. Recently, it was reported that magnesium oxide (MgO) was useful for removal of As(III), MgO nanoflakes that are porous can form Mg(OH)2 in situ by interactions with water, which have high affinity for As(III) anion in aqueous environments.33 The objectives of this study were to (1) use MgO and diatomite, both low cost and environmentally benign materials, to prepare a novel PO4 adsorbent with high surface area and sorption capacity; (2) characterized the mechanism of adsorption of PO4 to modified diatomite; (3) investigate, under laboratory conditions, the potential of modified diatomite to adsorb PO4 for removal from eutrophic lake waters. qe = V (C0 − Ce) m (1) Where qe is the sorption capacity at equilibrium(mg/g), C0 is the initial concentration of PO4 (mg/L), Ce is the equilibrium concentration of PO4 (mg/L), V is the volume of PO4 solutions, and m is the mass of the sorbent (g). Sorption Kinetics. A 500 mg aliquant of MOD was equilibrated with 1000 mL of 50 mg P/L, of which the pH was adjusted to 5.0 in a glass beaker (2000 mL) at 298 K for a fixed period of time (15 min, 30 min, 1 h, 2 h, 3 h, 4 h, 5 h, 6 h, 7 h, 8 h, and 12 h). Approximately 5 mL aliquots were taken from the suspension was filtered using a 0.45 μm filter and concentration of PO4 was measured. The amount of PO4 adsorbed was calculated (eq 1) Data were fitted by using the pseudo first-order equation (eq 2) and the pseudo second-order equation(eq 3).38 ■ MATERIALS AND METHODS Materials. Raw diatomite was obtained through Shanghai Kexi Chemical Co., Ltd., Shanghai, China. The raw diatomite was composed of 86.30% SiO2, 4.91% Al2O3, 2.32% Fe2O3, 2.55% CaO, and 0.58% MgO. All stock solutions were prepared using reagent grade chemicals and deionized water. Stock solutions of PO4 were prepared by dissolving KH2PO4 (Shanghai Chemical Reagent Company, China Pharmacy Group) in deionized water. Soil humic and fulvic acid were extracted from the soil of Jiufeng Mountain (Beijing, China), and have been characterized in detail.34,35 Preparation of MOD. Raw diatomite (15 g) was added to 100 mL of 6 M NaOH at 353K for 2 h to partially dissolve Si. The mixture was immediately added to 100 mL of 2.5 M MgCl2, and stirred at room temperature for 24 h. The mixture was then aged another 24 h and centrifuged to remove the supernatant and ln(qe − qt ) = ln qe‐k1t (2) t 1 1 = + t qt qe k 2qe 2 (3) Where qt (mg/g) is the PO4 sorption capacity at time t, and k1 (1/min) and k2 (g/mg min) are the rate constant of pseudo firstorder adsorption and the equilibrium rate constant of pseudo second-order adsorption, respectively. Sorption Isotherm and Thermodynamics. Solutions of PO4 (100 mL) over a concentration range of 4−50 mg/L were maintained at the pH 5.0 and shaken with MOD (50 mg) at 288, 298, 308, and 318 K, respectively. After 12h of mixing, amounts 583 dx.doi.org/10.1021/es4037379 | Environ. Sci. Technol. 2014, 48, 582−590 Environmental Science & Technology Article Figure 1. Typical scanning electron micrographs for (a) raw diatomite, (b) Mg(OH)2-modified diatomite(MHD), (c) MgO-modified diatomite(MOD). the system, where KF is a measurement of adsorption capacity and 1/n of adsorption intensity. The thermodynamic parameters of the adsorption process were determined from the experimental data obtained at 288, 298, 308, and 318 K after 12 h of mixing (eqs 6, 7, and 840): of PO4 in the supernatant were determined. The sorption isotherm was described using eq 4 and eq 5:39 Ce Ce 1 = + qe Q max b*Q max 1 lgqe = lgCe + lgKF n (4) ΔG 0 = −RT ln K 0 q Kd = e Ce (5) Where: b is a Langmuir constant. Qmax is the theoretical maximum adsorption capacity (mg/g), also known as monolayer coverage of the surface. KF and n are characteristic constants of ln K 0 = 584 ΔSo ΔH o − R RT (6) (7) (8) dx.doi.org/10.1021/es4037379 | Environ. Sci. Technol. 2014, 48, 582−590 Environmental Science & Technology Article Where ΔG0, ΔH0, and ΔS0 are Gibbs free energy change(kJ/ mol), enthalpy change(kJ/mol) and entropy change(J/mol·K), respectively. Kd (L/g) is the distribution coefficient for the adsorption, T(K) is the absolute temperature, R(J/mol·K) is gas constant. Desorption Study. 1000 mL of phosphate solution (50 mg P/L) was maintained at the pH 5.0 and shaken with MOD (500 mg) at 298 K. After 12 h of mixing, the PO4 saturated MOD was filtered and washed gently with deionized water to remove any unadsorbed PO4. Then the PO4 saturated MOD(50 mg) was mixed with 100 mL of 1 mmol and 10 mmol of Ca2+, CO32‑, HCO3−, OH−, SiO32‑ solutions and deionized water at different pH values (5.0−10.0). After 12 h of mixing, the desorbed PO4 was determined. Removal of PO4 from Eutrophic Lake Water. Eutrophic lake water was collected in March 2012 from Chaohu Lake, the fifth largest freshwater lake China, located in the lower reach of the Yangtze floodplain. The sampling sites were on the west side of the lake, close to the city of Hefei. Lake water was filtered using a 0.45 μm filter, and stored in plastic bottles at 277 K. Batch experiments were carried out in 1000 mL glass beakers using 500 mL of eutrophic lake water for measuring PO4 removal efficiency. MOD was added to the lake water at concentrations of 0, 50, 100, 200, 300, 400, or 500 mg/L and then mixed at constant stirring for 12 h at 298 K. Amounts of soluble reactive PO4 in the supernatant were determined after it was filtered through a 0.45 μm membrane filter. Concentrations of total PO4 were measured after acid-persulphate digestion.41 Amounts of Ca and Mg were determined by use of a flame atomic absorption spectrometer (WFX-1E2, Beijing, China). Absorption wavelengths of 422.7 and 285.2 nm were used for Ca and Mg, respectively. The limit of quantification of Ca and Mg were 0.02, 0.006 mg/L and the limit of detection was 5, 0.8 μg/L, respectively. Statistical Analysis. All the experimental data were the averages of triplicate experiments. The relative errors of the data were less than 5%.The curve fitting and statistical analyses were performed using SigmaPlot software (version 10.0, SPSS Inc., Chicago, IL, USA). The correlation coefficient (R2) obtained from regression was used for comparing the model applicability. Figure 2. X-ray diffraction patterns of (a) Raw diatomite, (b) MHD, (c) MOD and (d) MOD after treated with phosphate. * for Mg(OH)2 and # for MgO. ■ to MgO (JCPDS no. 65−0476), and no remaining Mg(OH)2 peaks could be observed (Figure 2c). This observation suggests that Mg(OH)2 was converted to MgO completely after the calcination at 723 K for 2 h. Effect of pH. In the pH range of 5.0−10.0, PO4 exhibit weak adsorption on raw diatomite (Figure 3). The sorption capacity decreased from 4.52 mg/g to 1.09 mg/g as a function of pH. Mg(OH)2 deposited on diatomite resulted in grater sorption capacities of 9.10−4.77 mg/g in the pH range of 5.0−10.0 . After calcining, the surface area and sorption capability of adsorbent became greater. Sorption capacity can reach 45.72 mg/g with an initial pH of 5.0, which is 42-fold greater than that of raw diatomite. This sorption behavior usually can be explained in terms of pHZPC of the adsorbent and PO4 speciation occurring in solution.42 The PO4 in aqueous solution exists mainly as H2PO4− and HPO42‑ in the pH range of 5.0−10.0 (SI Figure S2).At pH< pHzpc, the surface of the adsorbent was positively charged, which is favorable for adsorption of the PO4 anion. Weaker sorption of PO4 by raw diatomite can be attributed to lower surface area and the negative charge on its surface under experimental conditions. Greater soption of PO4 by MOD and MHD can be attributed to the positive charge on its surface in the pH range of 5.0−10.0. The specific surface area of MOD larger than that of MHD is likely more responsible for the enhanced sorption of PO42. Meanwhile, the tendency for sorption of PO4 RESULTS AND DISCUSSION Characterization of Modified Diatomite. Physical parameters of adsorbents (Raw diatomite, MHD and MOD), such as the BET surface areas, total pore volume, average pore diameter and magnesium content, are summarized in Supporting Information (SI) Table S1. Surface morphology of raw diatomite in the present study was disk-like and many pores can be seen on its surface (Figure 1a). The SEM micrograph of MOD reveals that the original geometry of the pores is destroyed by pretreatment with NaOH and deposition of magnesium (Figure 1b and 1c). After being calcined at 723 K for 2 h, MgO nanoflakes were formed on the surface of the diatomite (Figure 1c). The surface area and the total pore volume of raw diatomite were improved significantly. The BET surface area of MOD was 72.53 m2/g, which is 113-fold greater than that of raw diatomite. The pHzpc of raw diatomite, MHD and MOD were experimentally found to be at pH 4.5, 10.9 and 9.6, respectively (SI Figure S1). Two diffraction bands were centered at 22.0° and 36.2° (2θ degree) of the XRD, which is the characteristic peak for amorphous SiO2 (JCPDS 29−0085) (Figure 2a). Four new diffraction peaks were observed at 18.8°, 38.2°, 51.0° and 58.8° in MHD (Figure 2b), which belong to Mg(OH)2 (JCPDS no. 07− 0239). For MOD, the diffraction peaks at 42.9° and 62.3° belong 585 dx.doi.org/10.1021/es4037379 | Environ. Sci. Technol. 2014, 48, 582−590 Environmental Science & Technology Article adsorption on MOD is more negative at higher temperature, which demonstrates that the spontaneity of the adsorption process increases with increasing the temperature (SI Table S4 and Figure S9). The positive value of entropy change (ΔS°) implies an increase in the disorderness of the solid-solution system,46 which is probably because of structural changes take place in adsorbate and adsorbent during the adsorption. A positive value of the standard enthalpy change (ΔH°) indicates that the adsorption process is endothermic.47 Effects of Coexisting Substance. Many coexisting anions, cations and dissolved organic matter such as Na + ,K + , Ca2+,Mg2+,Cl−, NO3−, SiO32−, CO32−‑,SO42‑, fulvic acid, and humic acid in environmental waters can interfere with the adsorption of PO4 on MOD through competitive adsorption. For this purpose, 100 mL of the test solutions containing 5 mg/L of PO4 and various amounts (1.0 and 10.0 mM for ions; 10, 30, and 50 mg C/L for dissolved organic matter) of other coexisting substance were equilibrated with MOD (50 mg) at pH 5.0. The negligible effects of cations on adsorption PO4 were demonstrated by the experimental data in Figure 4. Cl− and NO3− had Figure 3. Effect of pH on the retention of PO4 by MOD, MHD and raw diatomite experimental conditions: Vtotal, 40 mL;adsorbent, 20 mg; T, 298 K. to MOD to be the same as sorption to MHD can be explained by competition adsorption between PO4 anion and OH− with at greater pH. Sorption Kinetics. The time taken for PO4 removal using MOD is of considerable importance for its possible application for remediation of eutrophic lakes. Sorption of PO4 can reach 91% of maximum within 3 h, and then changed slowly until to reach sorption equilibrium (SI Figure S3). The process of sorption consisted of two distinct steps: an initial rapid adsorption which a rapid diffusion of ions from the solution to external adsorbent surfaces, followed by a slower adsorption by diffusion of ions into pores of inner adsorbent surfaces of Donnan spaces.43,44 Under the experimental conditions of continuous stirring, it is unlikely that the external diffusion could control the adsorption process. Chemical reaction might take place on the surfaces of MOD. The obtained data were fitted by using the pseudo first-order equation and the pseudo secondorder equation (SI Figure S4, S5, and Table S2). Correlation coefficients of the pseudo second-order kinetic model (R2 = 0.998) was relatively greater than that of the pseudo first-order kinetic model (R2 = 0.961). The adsorption system obeys pseudo, second-order kinetics for the entire adsorption period and thus supports the assumption that the adsorption is probably via surface reactions until the surface functional sites are fully occupied, thereafter PO4 diffuse into the MOD for further complexation interactions.45 Sorption Isotherms. Sorption capacity of MOD for PO4 increased with increasing of temperature and initial PO4 concentrations and gradually to reach equilibrium (SI Figure S6). Langmuir and Freundlich constants were determined using the adsorption data at different temperature. The adsorption isotherm data fit the Langmuir adsorption model well (Langmuir model: R2 >0.997, Freundlich model: R2 <0.946) (SI Figure S7, S8 and Table S3), indicating the adsorption of PO4 on the surface of MOD was taking place in a monolayer fashion. According to the Langmuir equation, the theoretical maximum sorption capacity of MOD for P was 44.44−52.08 mg/g at experimental conditions. Sorption Thermodynamics. Thermodynamic parameters (ΔGo, ΔSo, and ΔHo) of the adsorption process were calculated using the obtained experiment data at several temperatures. Gibbs free energy changes (ΔG°) during sorption of PO4 Figure 4. Effect of coexisting ions, humic acid and fulvic acid on PO4 removal experimental conditions: Vtotal, 100 mL;adsorbent, 50 mg; initial pH 5.0. little negative effect when the concentration of coexisting ions was increased from 1.0 to 10 mM. Fulvic acid and humic acid also show a slight negative effect on PO4 adsorption. When the concentration of coexisting dissolved organic matter were increased from 10 to 50 mg C/L, the sorption efficiency decreasing from 94.4% and 99.2% to 86.9% and 92.6% for humic acid and fulvic acid, respectively. The presence of CO32‑, SO42‑ and SiO32‑ showed a significantly negative influence on adsorption of PO4 with sorption efficiency decreasing from 90.6%, 91.8%, and 89.6% to 78.5%, 81.4%, and 75.3% for CO32‑, SO42−, and SiO32‑, respectively. The main reason was that the increased concentration of dissolved organic matter and anions could completion or blocks the surface sorption sites and reduced the positive charge density on the surface of adsorbent, which decrease the adsorption of the negative charged PO4 anions through electrostatic force of attraction. Although some of the coexisting anions have negative effects, the experimental results showed that larger than 75% of PO4 ions can be removal even in a solution with a large excess amount of coexisting ions. The results imply that MOD has high sorption selectivity toward PO4 anion and has high potential to removal PO4 from real eutrophic lake water. 586 dx.doi.org/10.1021/es4037379 | Environ. Sci. Technol. 2014, 48, 582−590 Environmental Science & Technology Article Mechanism of Sorption. In order to clarify the mechanism of sorption of PO4 on MOD, the structure changes of MOD before and after the saturation sorption of PO4 was examined with XRD, and the chemical status of phosphate species after its sorption on MOD was examined by high-resolution XPS scans and solid state 31P NMR. The XRD pattern of MOD after sorption in water solution with initial concentration PO4 of 50 mg/L was given in Figure 2(d). After 12h of mixing, peaks attributed to MgO disappeared. Two new diffraction peaks at the 2θ values of 18.8° and 38.2° could be clearly distinguished (Figure 2(d)). According to the intensity and quantity of the diffraction peaks, it can be speculated that MgO on the surface of diatomite was gradually converted in situ into Mg(OH)2.33 The high resolution-spectrum of P 2p was divided into two peaks, 132.9 and 133.8 eV, which corresponded to PO43−and HPO42−, respectively (Figure 5(a)).48 This result demonstrated The chemical shift of physical adsorbed PO4 and the noncrystal structure of Mg3(PO4)2·8H2O and MgHPO4·3H2O appeared at 6.4 ppm,50 0.5 ppm and −2.4 ppm,51,52 respectively. The peak present here attributed to the mixture of physical adsorbed PO4, the noncrystal structure of Mg3(PO4)2·8H2O and MgHPO4· 3H2O. The solid state 31P NMR results showed great accordance with the results of the XPS analysis and imply that new bond formation dominated the sorption process. From the above analysis, the removal of PO4 by MOD is not a simple adsorption process. The whole removal process involves the in situ formation of Mg(OH)2 from MgO on the surface of diatomite; the adsorption of PO4 anion onto in situ formed positive charged Mg(OH)2 on the surface of diatomite through electrostatic attraction to form surface complex; and the reaction between surface Mg(OH)2 and sorbed PO4 to conversion Mg3(PO4)2 and MgHPO4. MOD with high specific surface area were added into the PO4 solution, MgO nanoflakes on the surface of diatomite became hydrated and much more fresh active adsorption site for enhanced PO4 removal was in situ formed (Mg(OH)2) due to their reaction with water. The in situ formed surface hydroxide maybe protonated in acid media and can deprotonated in alkaline solutions. Therefore, the density of positive charged surface groups of MOD change at different pH values. Under the experimental conditions, H2PO4− and HPO42‑ can be formed and the adsorption process is rapid on the surface of MOD. With increasing pH, the number of negatively charged hydroxide groups increases, and the negatively charged adsorbent surface sites do not favor the adsorption of PO4 anion due to the electrostatic repulsion. The pH dependent adsorption indicates that the adsorption is dominated by surface complexation,53 which also showed in the sorption kinetic study. At the same time, surface complexed PO4 species onto MOD could in situ reaction with Mg(OH)2 and to conversion the noncrystal structure compound of Mg3(PO4)2 and MgHPO4, which will be beneficial to the continuous formation of Mg(OH)2. With the increase of contact time, highly dispersed MgO nanoflakes on the surface of diatomite could be in situ converted to Mg(OH)2 completely,33 and the reaction between Mg(OH)2 and PO4 could remove excess PO4 from water continuously. The proposed sorption mechanism of MOD for PO4 from aqueous solution is given (eqs 9-11). DS ≡ Mg = O ⇒ DS ≡ Mg‐O‐OH 2+ (9) DS ≡ Mg‐O‐OH 2+ + HPO4 2 ‐ ⇒ DS ≡ MgHPO4 (10) DS ≡ Mg‐O‐OH 2+ + PO4 3 ‐ ⇒ DS ≡ Mg 2(PO4 )3 (11) (DS = surface of diatomite) Phosphate Desorption. To assess stability of adsorbed PO4 on the surface of MOD, the effect of pH and some chemical water components such as Ca2+, CO32−, HCO3−, OH−, and SiO32‑ on desorption PO4 from MOD saturated with PO4 was studied using batch experimental method. The PO4 desorbability was defined as the ratio of the desorbed PO4 to the total adsorbed PO4 by the adsorbent. The desorbability of PO4 depends on the pH of the extraction solutions(5.0−10.0) and chemical water components were shown in Figure.6 and Table 1. The desorbabilities of PO4 in the different pH solutions were 3.35− 7.67%. Effect of Ca2+, OH− and SiO32‑ on PO4 desorption was negligible (<11%) for all the concentrations of these chemical water components studied. Alkalinity had significant effect on Figure 5. XPS spectra of MOD after treated with PO4. (a) P 2p and (b) Mg 2p. that the surface of MOD was composed of PO43− and HPO42−. To confirm this conclusion deconvolution of the XPS spectra of Mg 2p, (Figure 5 (b)) demonstrated the presence of two compounds. The peak at 52.6 eV probably corresponds to Mg3(PO4)2 and the peak at 51.8 eV to MgHPO4.49 A broad band in the solid state 31P NMR spectrum of MOD after adsorption of PO4 appeared at 1.54 ppm (SI Figure S10). 587 dx.doi.org/10.1021/es4037379 | Environ. Sci. Technol. 2014, 48, 582−590 Environmental Science & Technology Article Comparison with Other Adsorbents for Removal of PO4. Increasing numbers of adsorbents have been used in recent years to remove PO4 from environmental waters. The PO4 sorption capacity is usually chosen as the criterion for adsorbent selection. For there are not standard procedures for batch sorption experiments, direct comparison with other adsorbent materials is difficult due to the different applied experimental conditions.54 To illustrate the potential in the use of MOD for removal of PO4 from waters, a comparative evaluation of the SBET, pHzpc adsorption capacities and experimental conditions of various PO4 adsorbents were provided (Table 2). Under the similar experimental conditions, the PO4 sorption capacity of MOD is slight greater than that of ferrihydrite-modified diatomite (FHMD).2 Some of adsorbent such as biochar,55 Fe loaded skin split waste56 and alunite57 have relative high capacity for PO4, but the initial P concentrations were also higher. Meanwhile, the ratio of material to solution in present study is 0.5g/L, which much less than that of reported adsorbents in Table 2. The results indicate that the removal of PO4 by the MOD was efficient and performance of MOD is better than most of other adsorbents for which data is available. Another advantage of the present adsorbent is its cost-effective and the fact that it leaves no toxic residues or released toxic ions to waters which cause a second pollution like some other adsorbents. Removal of PO4 form Eutrophic Chaohu Lake Water. To test the ability of MOD to remove PO4 from real eutrophic waters, MOD was added to water from Chaohu Lake as a representative eutrophic lake (SI Table S5). The pH value of the water was 8.12, the concentration of total phosphorus was 0.158 mg/L. Lake water parameters such as TP, SRP, pH, Ca, and Mg were determined after using different dose of MOD (Figure 7 and SI Figures S11 and S12). The efficiency of removal of total phosphorus (TP) was proportional to the amount of MOD applied. Efficiencies of removal of TP and SRP were 87.2% and 75% when the concentration of MOD was 250 mg/L. The concentration of TP in the treated lake water samples was 19.9 μg/L, which is comparable with the amounts of alum and ferrihydrite-modified diatomite applied to Lake Sønderby, Denmark 58and Jackfish Lake,Canada.30When the concentration of MOD was 300 mg/L, efficiency of removal of TP was 90% and left a residual concentration of TP of less than 15.0 μg/L, which falls within the oligotrophic range(3.0−17.7 μg/L).59 The pH value of lake water was slight increased from 8.12 to 8.67 due to Figure 6. Effect of pH on PO4 desorption experimental conditions: Vtotal, 100 mL; PO4 saturated adsorbent, 50 mg. Table 1. Effect of Chemical Water Composition on Desorbability of PO4 Saturated MOD desorbability(%) parameters 1 mmol 10 mmol 2+ 0.13 ± 0.05 34.69 ± 1.56 12.75 ± 0.21 3.80 ± 0.15 6.68 ± 0.10 0 38.57 ± 1.94 18.61 ± 0.46 9.29 ± 0.09 11.01 ± 0.34 Ca CO32‑ HCO3− OH− SiO32‑ desorption of PO4 from the surface of MOD. Desorbabilities of PO4 increased from 34.69% and 12.75% to 38.57% and 18.61% with increasing the concentration of CO32− and HCO3− from 1.0 to 10 mM, respectively. Low desorbability at different pH and in present of Ca2+, OH−, SiO32‑ indicates that a small fraction of PO4 is weakly adsorbed or complexed on the surface of MOD. The results from desorption experiments show that PO4 sorbed on MOD is mostly irreversible and that the bond between the MOD and adsorbed PO4 is strong. It is relatively difficult to desorb PO4 from MOD. These results imply that MOD can be a potential adsorbent of PO4 for some eutrophic lake water treatment. Table 2. PO4 Adsorption by Different Adsorbentsa sorbent SBET (m2/ g) pHzpc adsorption capacity (mg/g) reaction condition reference MOD Fe−Mn binary oxide FHMD ACF-LaOH milled furnace slag biochar ZnCl2-activated carbon Fe-EDA-SAMMS La-loaded orange waste Fe loaded skin split waste Al pillared bentonite iron impregnated coir pith alunite 72.53 309 211.1 NA 0.75 336 NA 169 NA 5.23 200 155.2 148 9.6 6.6 10 8.0 NA NA NA NA NA NA NA 5.8 NA 45.7 36 37.3 16.1 43.1 133 4.2 43.3 14 68 5.05 40.55 106.6 pH5.0,T = 298K,adsorbent=0.5g/L,C 0 = 50 mg/L pH5.6, T = 298 ± 1K, adsorbent=0.2g/L, C 0 = 5 mg/L pH4.0, T = room temperature, adsorbent=0.5g/L, C0 = 40 mg/L pH NA,T = room temperature, adsorbent=2.5g/L,C0 = 30 mg/L pH7.0−7.2,T = 293K, adsorbent=10g/L,C0 = 500 mg/L pH7.0,T = 295 ± 0.5K, adsorbent=2g/L,C0 = 640 mg/L pH4.0,T = 308K, adsorbent=6g/L,C0 = 40 mg/L pH5.0,T = room temperature, adsorbent=0.1g/L, C 0 = 18.5 mg/L pH7.5,T = 303K,adsorbent=1.7g/L,C 0 = 40 mg/L pH7.0,T = 303,adsorbent=1g/L,C0 = 93 mg/L pH3.0,T = NA,adsorbent=4g/L,C0 = 20 mg/L pH3.0,T = 303K,adsorbent=2g/L,C0 = 100 mg/L pH5.0,T = 298K,adsorbent=10g/L,C0 = 200 mg/L present study 1 2 60 61 55 62 63 64 56 65 66 57 a C0,initial P concentration NA, Date not available. 588 dx.doi.org/10.1021/es4037379 | Environ. Sci. Technol. 2014, 48, 582−590 Environmental Science & Technology Article (4) Morse, G. K.; Brett, S. W.; Guy, J. A.; Lester, J. N. Review: Phosphorus removal and recovery technologies. Sci. Total Environ. 1998, 212 (1), 69−81. (5) Rittmann, B. E.; Mayer, B.; Westerhoff, P.; Edwards, M. Capturing the lost phosphorus. Chemosphere 2011, 84 (6), 846−853. (6) Hylander, L. D.; Kietlińska, A.; Renman, G.; Simán, G. Phosphorus retention in filter materials for wastewater treatment and its subsequent suitability for plant production. Bioresour. Technol. 2006, 97 (7), 914− 921. (7) Arias, C. A.; Bubba Del, M.; Brix, H. Phosphorus removal by sands for use as media in subsurface flow constructed reed beds. Water Res. 2001, 35, 1159−1168. (8) Del Bubba, M.; Arias, C. A.; Brix, H. Phosphorus adsorption maximum of sands for use as media in subsurface flow constructed reed beds as measured by the Langmuir isotherm. Water Res. 2003, 37 (14), 3390−3400. (9) Zhang, W.; Brown, G. O.; Storm, D. E.; Zhang, H. Fly-ashamended sand as filter media in bioretention cells to improve phosphorus removal. Water Environ. Res. 2008, 80 (6), 507−516. (10) Ugurlu, A.; Salman, B. Phosphorus removal by fly ash. Environ. Int. 1998, 24 (8), 911−918. (11) Bhattacharya, S. S.; Chattopadhyay, G. N. Increasing bioavailability of phosphorus from fly ash through vermicomposting. J. Environ. Qual. 2002, 31 (6), 2116−2119. (12) Xiong, J.; He, Z.; Mahmood, Q.; Liu, D.; Yang, X.; Islam, E. Phosphate removal from solution using steel slag through magnetic separation. J. Hazard. Mater. 2008, 152 (1), 211−215. (13) Huang, W.; Wang, S.; Zhu, Z.; Li, L.; Yao, X.; Rudolph, V.; Haghseresht, F. Phosphate removal from wastewater using red mud. J. Hazard. Mater. 2008, 158 (1), 35−42. (14) Karapinar, N. Application of natural zeolite for phosphorus and ammonium removal from aqueous solutions. J. Hazard. Mater. 2009, 170 (2−3), 1186−1191. (15) Montalvo, S. J.; Guerrero, L. E.; Milan, Z.; Borja, R. Nitrogen and phosphorus removal using a novel integrated system of natural zeolite and lime. J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. 2011, 46 (12), 1385−1391. (16) Wang, S. L.; Cheng, C. Y.; Tzou, Y. M.; Liaw, R. B.; Chang, T. W.; Chen, J. H. Phosphate removal from water using lithium intercalated gibbsite. J. Hazard. Mater. 2007, 147 (1−2), 205−212. (17) Shin, E. W.; Han, J. S.; Jang, M.; Min, S. H.; Park, J. K.; Rowell, R. M. Phosphate adsorption on aluminum-impregnated mesoporous silicates: Surface structure and behavior of adsorbents. Environ. Sci. Technol. 2004, 38 (3), 912−917. (18) Kioussis, D. R.; Wheaton, F. W.; Kofinas, P. Reactive nitrogen and phosphorus removal from aquaculture wastewater effluents using polymer hydrogels. Aquacult. Eng. 2000, 23 (4), 315−332. (19) Ryding, S. O.; Rast, W. The Control of Eutrophication of Lakes and Reservoirs; The Parthenon Publishing Group: Paris, 1989. (20) Higgins, B. P. J.; Mohleji, S. C.; Irvine, R. L. Lake treatment with fly ash, lime, and gypsum. J. Water Pollut. Control 1976, 48, 2153−2164. (21) Drizo, A.; Frost, C. A.; Grace, J.; Smith, J. K. Physico-chemical screening of phosphate-removing substrates for use in constructed wetland systems. Water Res. 1999, 33, 3595−3602. (22) Wadpersdolf, E.; Neumann, T.; Stuben, D. Efficiency of natural calcite precipitation compared to lake marl application used for water quality improvement in an eutrophic lake. Appl. Geochem. 2004, 19, 1687−1698. (23) Reitzel, K.; Andersen, F. Ø.; Egemose, S.; Jensen, H. S. Phosphate adsorption by lanthanum modified bentonite clay in fresh and brackish water. Water Res. 2013, 47 (8), 2787−2796. (24) Hartley, A. M.; House, W. A.; Callow, M. E.; Leadbeater, B. S. C. Coprecipitation of phosphate with calcite in the presence of photosynthesizing green algae. Water Res. 1997, 31 (9), 2261−2268. (25) Goren, R.; Baykara, T.; Marsoglu, M. Effects of purification and heat treatment on pore structure and composition of diatomite. Br. Ceram. Trans. 2002, 101, 177−180. (26) Stoermer, E. F.; Smol, J. P. Applications for the Environmental and Earth Science; Cambridge University Press: Cambridge, 1999. Figure 7. Effects of MOD dose on TP, SRP removal efficiency in the lake water sample. in situ formation of Mg(OH)2. Magnesium ion increased from 14.84 to 35.16 mg/L while calcium ion decreased from 178.15 to 157.88 mg/L in the eutrophic lake water when concentrations of MOD were increases from 0 to 300 mg/L. The reason may be the in situ chemical conversion of magnesium-P compounds to calcium-P compounds and release magnesium ions into the waters. ■ ASSOCIATED CONTENT ■ AUTHOR INFORMATION S Supporting Information * Twelve figures and five tables showing the results of BET data for raw diatomite, MHD and MOD, pHzpc, the data of linear regression, the parameters of sorption kinetic, sorption isotherm and thermodynamics, solid state 31P NMR spectroscopy of MOD after treated with PO4, characteristics of lake water, effects of MOD dose on Ca, Mg concentration and pH in the lake water sample. This material is available free of charge via the Internet at http://pubs.acs.org. Corresponding Author *Phone: +86-10-84915312; fax: +86-10-84931804, e-mail: wufengchang@vip.skleg.cn, lgj@ustc.edu.cn. Notes The authors declare no competing financial interest. ■ ACKNOWLEDGMENTS This work was supported by the National Basic Research Program of China (No.2008CB418200), the National Natural Science Foundation of China (No.21107001) and the China Postdoctoral Science Foundation (2013M541842). ■ REFERENCES (1) Zhang, G.; Liu, H.; Liu, R.; Qu, J. Removal of phosphate from water by a Fe-Mn binary oxide adsorbent. J. Colloid Interface Sci. 2009, 335 (2), 168−174. (2) Xiong, W.; Peng, J. Development and characterization of ferrihydrite-modified diatomite as a phosphorus adsorbent. Water Res. 2008, 42, 4869−4877. (3) Cooke, G. D.; Welch, E. B.; Peterson, S. A.; Nichols, S. A. Restoration and Management of Lakes and Reservoirs, 3rd ed.; Taylor & Francis: New York, 2005. 589 dx.doi.org/10.1021/es4037379 | Environ. Sci. Technol. 2014, 48, 582−590 Environmental Science & Technology Article (48) Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E.-H. A novel phosphate conversion film on Mg−8.8 Li alloy. Surf. Coat. Technol. 2009, 203 (9), 1107−1113. (49) Zhang, W.; Tian, B.; Du, K. Q.; Zhang, H. X.; Wang, F. H. Preparation and corrosion performance of PEO coating with low porosity on magnesium alloy AZ91D in acidic KF system. Int. J. Electrochem. Sci. 2011, 6, 5228−5248. (50) He, Z.; Honeycutt, C. W.; Griffin, T. S.; Cade-Menun, B. J.; Pellechia, P. J.; Dou, Z. Phosphorus forms in conventional and organic dairy manure identified by solution and solid state P-31 NMR spectroscopy. J. Environ. Qual. 2009, 38 (5), 1909−1918. (51) Aramendía, M. A.; Borau, V.; Jiménez, C.; Marinas, J. M.; Romero, F. J.; Ruizl, J. R. Characterization by XRD, DRIFT, and MAS NMR spectroscopies of a Mg2P2O7 Catalyst. J. Colloid Interface Sci. 1998, 202 (2), 456−461. (52) Aramendía, M. A.; Borau, V.; Jiménez, C.; Marinas, J. M.; Romero, F. J.; Ruiz, J. R. XRD and solid-State NMR study of magnesium oxide− magnesium orthophosphate systems. J. Solid State Chem. 1998, 135 (1), 96−102. (53) Yu, S.; Chen, C.; Chang, P.; Wang, T.; Lu, S.; Wang, X. Adsorption of Th (IV) onto Al-pillared rectorite: Effect of pH, ionic strength, temperature, soil humic acid and fulvic acid. Appl. Clay Sci. 2008, 38 (3), 219−226. (54) Cucarella, V.; Renman, G. Phosphorus sorption capacity of filter materials used for on-site wastewater treatment determined in batch experiments-a comparative study. J. Environ. Qual. 2009, 38 (2), 381− 392. (55) Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A. R.; Cao, X.; Pullammanappallil, P.; Yang, L. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011, 190 (1−3), 501−507. (56) Huang, X.; Liao, X.; Shi, B. Adsorption removal of phosphate in industrial wastewater by using metal-loaded skin split waste. J. Hazard. Mater. 2009, 166 (2−3), 1261−1265. (57) Ozacar, M. Adsorption of phosphate from aqueous solution onto alunite. Chemosphere 2003, 51 (4), 321−327. (58) Reitzel, K.; Hansen, J.; Andersen, F. Ø.; Hansen, K. S.; Jensen, H. S. Lake restoration by dosing aluminum relative to mobile phosphorus in the sediment. Environ. Sci. Technol. 2005, 39 (11), 4134−4140. (59) Wetzel, R. G. Limnology Lake and Reservoir Ecosystems, 3rd ed.; Academic: London, 2001. (60) Zhang, L.; Zhou, Q.; Liu, J.; Chang, N.; Wan, L.; Chen, J. Phosphate adsorption on lanthanum hydroxide-doped activated carbon fiber. Chem. Eng. J. 2012, 185−186, 160−167. (61) Xue, Y.; Hou, H.; Zhu, S. Characteristics and mechanisms of phosphate adsorption onto basic oxygen furnace slag. J. Hazard. Mater. 2009, 162 (2−3), 973−980. (62) Namasivayam, C.; Sangeetha, D. Equilibrium and kinetic studies of adsorption of phosphate onto ZnCl2 activated coir pith carbon. J. Colloid Interface Sci. 2004, 280 (2), 359−365. (63) Chouyyok, W.; Wiacek, R. J.; Pattamakomsan, K.; Sangvanich, T.; Grudzien, R. M.; Fryxell, G. E.; Yantasee, W. Phosphate removal by anion binding on functionalized nanoporous sorbents. Environ. Sci. Technol. 2010, 44 (8), 3073−3078. (64) Biswas, B. K.; Inoue, K.; Ghimire, K. N.; Ohta, S.; Harada, H.; Ohto, K.; Kawakita, H. The adsorption of phosphate from an aquatic environment using metal-loaded orange waste. J. Colloid Interface Sci. 2007, 312 (2), 214−223. (65) Yan, L. G.; Xu, Y. Y.; Yu, H. Q.; Xin, X. D.; Wei, Q.; Du, B. Adsorption of phosphate from aqueous solution by hydroxy-aluminum, hydroxy-iron and hydroxy-iron-aluminum pillared bentonites. J. Hazard. Mater. 2010, 179 (1−3), 244−250. (66) Krishnan, K. A.; Haridas, A. Removal of phosphate from aqueous solutions and sewage using natural and surface modified coir pith. J. Hazard. Mater. 2008, 152 (2), 527−535. (27) Khraisheh, M. A. M.; Al-Ghouti, M. A.; Allen, S. J.; Ahmad, M. N. Effect of OH and silanol groups in the removal of dyes from aqueous solution using diatomite. Water Res. 2005, 39 (5), 922−932. (28) Khraisheh, M. A. M.; Al-degs, Y. S.; McMinn, W. A. M. Remediation of wastewater containing heavy metals using raw and modified diatomite. Chem. Eng. J. 2004, 99 (2), 177−184. (29) Wu, J.; Yang, Y. S.; Lin, J. Advanced tertiary treatment of municipal wastewater using raw and modified diatomite. J. Hazard. Mater. 2005, 127 (1−3), 196−203. (30) Xiong, W.; Peng, J. Laboratory-scale investigation of ferrihydritemodified diatomite as a phosphorus co-precipitant. Water, Air, Soil Pollut. 2011, 215 (1), 645−654. (31) Zimmer, L. A.; Cutter, G. A. High resolution determination of nanomolar concentrations of dissolved reactive phosphate in ocean surface waters using long path liquid waveguide capillary cells (LWCC) and spectrometric detection. Limnol. Oceanogr. Methods 2012, 10, 568− 580. (32) Anagnostou, E.; Sherrell, R. M. A MAGIC method for subnanomolar orthophosphate determination in freshwater. Limnol. Oceanogr. Methods 2008, 6, 64−74. (33) Liu, Y.; Li, Q.; Gao, S.; Shang, J. K. Exceptional As (III) sorption capacity by highly porous magnesium oxide nanoflakes made from hydrothermal synthesis. J. Am. Ceram. Soc. 2011, 94 (1), 217−223. (34) Lin, Y.; Wu, F.; Bai, Y.; Xie, F.; Cao, Z.; Su, H. Isolation and characterization of standard fulvic acids from soil and sediments in China. Res. Environ. Sci. 2011, 24, 1142−1148. (35) Lin, Y. Isolation and Characterization of Reference Organic Matter from Soil,Sediment and Blue Algae; Chinese Research Academy of Environmental Seiences: Beijing, 2011. (36) Solhy, A.; Machado, B. F.; Beausoleil, J.; Kihn, Y.; Gonçalves, F.; Pereira, M. F. R.; Ó rfão, J. J. M.; Figueiredo, J. L.; Faria, J. L.; Serp, P. MWCNT activation and its influence on the catalytic performance of Pt/MWCNT catalysts for selective hydrogenation. Carbon 2008, 46, 1194−1207. (37) Murphy, J.; Riley, J. P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31−36. (38) Bulut, E.; Ö zacar, M.; Şengil, I.̇ A. Adsorption of malachite green onto bentonite: Equilibrium and kinetic studies and process design. Microporous Mesoporous Mater. 2008, 115 (3), 234−246. (39) Xie, F.; Lin, X.; Wu, X.; Xie, Z. Solid phase extraction of lead (II), copper (II), cadmium (II) and nickel (II) using gallic acid-modified silica gel prior to determination by flame atomic absorption spectrometry. Talanta 2008, 74 (4), 836−843. (40) Li, W.; Pan, G.; Zhang, M.; Zhao, D.; Yang, Y.; Chen, H.; He, G. EXAFS studies on adsorption irreversibility of Zn(II) on TiO2: Temperature dependence. J. Colloid Interface Sci. 2008, 319 (2), 385− 391. (41) Rowland, A. P.; Haygarth, P. M. Determination of total dissolved phosphorus in soil solutions. J. Environ. Qual. 1997, 26, 410−415. (42) Fiol, N.; Villaescusa, I. Determination of sorbent point zero charge: Usefulness in sorption studies. Environ. Chem. Lett. 2009, 7, 79− 84. (43) Al-Qunaibit, M. H.; Mekhemer, W. K.; Zaghloul, A. A. The adsorption of Cu(II) ions on bentoniteA kinetic study. J. Colloid Interface Sci. 2005, 283 (2), 316−321. (44) Zhao, D.; Chen, C.; Sheng, G.; Wang, X. Effect of environmental conditions on the retention behaviour of Pb(II) by hematite. J. Chem. Technol. Biotechnol. 2011, 86 (8), 1099−1106. (45) Bulut, E.; Ö zacar, M.; Şengil, I.̇ A. Equilibrium and kinetic data and process design for adsorption of Congo Red onto bentonite. J. Hazard. Mater. 2008, 154 (1−3), 613−622. (46) Fan, Q. H.; Tan, X. L.; Li, J. X.; Wang, X. K.; Wu, W. S.; Montavon, G. Sorption of Eu(III) on attapulgite studied by batch, XPS, and EXAFS techniques. Environ. Sci. Technol. 2009, 43 (15), 5776− 5782. (47) Zhao, D.; Yang, X.; Zhang, H.; Chen, C.; Wang, X. Effect of environmental conditions on Pb (II) adsorption on β-MnO2. Chem. Eng. J. 2010, 164 (1), 49−55. 590 dx.doi.org/10.1021/es4037379 | Environ. Sci. Technol. 2014, 48, 582−590 Supporting information for Removal of Phosphate from Eutrophic Lakes through Adsorption by in situ Formation of Magnesium Hydroxide from Diatomite Fazhi Xiea,b,c, Fengchang Wua*, Guijian Liub*, Yunsong Mua, Chenglian Fenga, Huanhua Wanga and John P.Giesyd a State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China b CAS Key Laboratory of Crust-Mantle Materials and Environment, School of Earth and Space Sciences, University of Science and Technology of China, Hefei 230026, China c Key Laboratory of Functional Molecule Design and Interface Process, School of Materials Science and Chemical Engineering, Anhui Jianzhu University, Hefei 230022, China d University of Saskatchewan, Department of Veterinary Biomedical Sciences and Toxicology Centre, 44 Campus Drive, Saskatoon, SK, Canada, S7N 5B3 * Corresponding authors: Tel: +86-10-84915312; Fax: +86-10-84931804, E-mail addresses: wufengchang@vip.skleg.cn, lgj@ustc.edu.cn. Number SI pages: 18 Number the figures: 12 Number the tables: 5 S1 12 MOD MHD Raw Diatomite Final pH 10 8 6 4 2 0 5 10 15 20 25 30 Solid Fraction( %,w/w) Fig. S1 Determination of the point of zero charge of MOD, MHD and Raw Diatomite. Experimental conditions : ionic strength, 0.01mol/L KNO3; T, 298 K. S2 1.2 1.0 Percent( × 100%) 0.8 H3PO4 H2PO4 HPO4 0.6 3- PO4 0.4 0.2 0.0 0 2 4 6 8 10 12 pH Fig. S2 PO4 speciation as a function of pH1 S3 14 - 2- 50 45 40 qt( mg/g) 35 30 25 20 15 10 0 2 4 6 8 10 12 Time(h) Fig.S3 Sorption kinetics of PO4 on MOD Experimental conditions : Vtotal, 1000mL;adsorbent, 500mg; T, 298 K. S4 14 6 y = -0.9748x + 3.9136 2 R = 0.961 4 2 ln(qe-qt) 0 -2 -4 -6 -8 -10 0 2 4 6 8 10 12 14 Time(h) Fig.S4 The linear regression of pseudo first-order kinetic model S5 0.30 y = 0.0206x + 0.0114 R2 = 0.998 0.25 t/qt 0.20 0.15 0.10 0.05 0.00 0 2 4 6 8 10 12 Time(h) Fig.S5 The linear regression of pseudo second-order kinetic model S6 14 60 288 K 298 K 308 K 318 K 50 qe( mg/L) 40 30 20 10 0 0 5 10 15 20 25 30 35 Ce( mg/L) Fig. S6 Equilibrium isotherms of PO4 on MOD, Experimental conditions : Vtotal, 100mL;adsorbent, 50mg; initial pH 5.0. S7 0.8 c e/q e 0.6 288 K 298 K 308 K 318 K 0.4 0.2 0.0 0 5 10 15 20 25 30 35 ce(mg/L) Fig.S7 Experimental data for PO4 absorption fitted to Langmuir isotherm S8 2.0 288 K 298 K 308 K 318 K 1.8 logqe 1.6 1.4 1.2 1.0 0.8 -1.5 -1.0 -0.5 0.0 0.5 1.0 1.5 2.0 logce Fig.S8 Experimental data for PO4 absorption fitted to Freundlich isotherm S9 4.5 4.4 lnK0 4.3 4.2 4.1 4.0 3.10 3.15 3.20 3.25 3.30 3.35 3.40 3.45 3.50 1000/T Fig.S9 Plot of lnK0 versus 1000/T for estimation of thermodynamic parameters S10 250 200 150 100 50 0 ppm -50 -100 -150 -200 -250 Fig.10 Solid state 31P NMR spectroscopy of MOD after treated with PO4 S11 Ca and Mg concentration in lake water(mg/L) 200 Mg Ca 180 160 40 20 0 0 100 200 300 400 500 600 Dose of MOD (mg/L) Fig. S11 Effects of MOD dose on Ca and Mg concentration in the lake water sample S12 9.0 pH 8.5 8.0 7.5 7.0 0 100 200 300 400 500 600 Dose of MOD (mg/L) Fig. S12 Effects of MOD dose on pH in the lake water sample S13 Table S1 Physical parameters for raw diatomite, MHD and MOD Sample SBET (m2/g) Total pore volume (cm3/g) Average pore diameter (nm) Magnesium content (%) Raw Diatomite 0.64 0.00079 49.50 0.35 MHD 49.81 0.19 15.66 24.86 MOD 72.53 0.26 14.41 27.49 SBET,Brunauer–Emmentt–Teller (BET)-specific surface area S14 Table S2 Kinetic parameters for the adsorption of PO4 onto MOD Pseudo first-order kinetic model T 298 K qe,exp(mg/g) 45.72 Pseudo second-order kinetic model k1(h-1) qe,1(mg/g) R2 k2(g/mg·h) qe,2(mg/g) R2 0.97 50.08 0.961 0.037 48.54 0.998 S15 Table S3 The parameters for the Langmuir and Freundlich fitting of PO4 adsorption on MOD Correlation parameters T=288 K T=298 K T=308 K T=318 K qm(mg/g) 44.44 47.39 51.02 52.08 b(L/mg) 0.69 0.65 0.63 0.81 R2 0.9992 0.9982 0.9972 0.9986 1-1/n 1/n L /g) 16.28 K(mg F 17.13 18.16 20.27 n 2.95 2.91 2.82 2.99 R2 0.9374 0.9394 0.9458 0.9427 Langmuir Freundlich S16 Table S4 Thermodynamic parameters for the adsorption of PO4 on MOD T(K) lnKo △Go(kJ/mol) 288 4.08 -9.77 298 4.15 -10.29 308 4.24 -10.86 318 4.42 -11.69 S17 △So(J/mol·K) △Ho (kJ/mol) 62.85 8.40 Table S5 Characteristics of water sampled from Chaohu Lake Parameter Value Parameter Value Na (mg/L) 203 pH 8.12 K (mg/L) 25 Ca (mg/L) 178 SRP(µg/L) 91 Mg (mg/L) 14.84 TP(µg/L) 156 TN (mg/L) 2.58 Reference 1. Karageorgiou, K.; Paschalis, M.; Anastassakis, G. N., Removal of phosphate species from solution by adsorption onto calcite used as natural adsorbent. J. Hazard. Mater. 2006, 139, 447-452. S18





