Enhancement of AhR-mediated activity of selected pollutants and their mixtures
advertisement

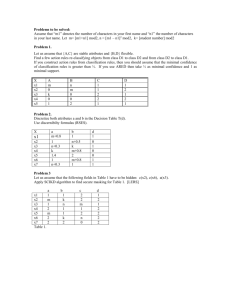
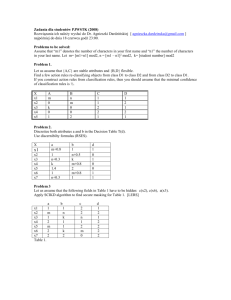
Environment International 37 (2011) 960–964 Contents lists available at ScienceDirect Environment International j o u r n a l h o m e p a g e : w w w. e l s ev i e r. c o m / l o c a t e / e n v i n t Enhancement of AhR-mediated activity of selected pollutants and their mixtures after interaction with dissolved organic matter Michal Bittner a,⁎, Petra Macikova a, John P. Giesy b,c,d,e, Klara Hilscherova a a Research Centre for Toxic Compounds in the Environment, Masaryk University, Kamenice 126/3, CZ62500 Brno, Czech Republic Department of Biomedical Veterinary Science and Toxicology Centre, Univ. of Saskatchewan, Canada Zoology Department and Center for Integrative Toxicology, Michigan State University, East Lansing, USA d Biology and Chemistry Department, City University of Hong Kong, Kowloon, Hong Kong, China e School of Biological Sciences, University of Hong Kong, Hong Kong, China b c a r t i c l e i n f o Article history: Received 13 July 2010 Accepted 17 March 2011 Available online 13 April 2011 Keywords: Humic substances AhR Mixture Interaction H4IIE-luc Organic pollutants a b s t r a c t Dissolved organic matter (DOM) in freshwaters is present at concentrations ranging from 0.5 to 50 mg L−1, and consists of various organic compounds, including humic substances (HS). HS exert a variety of direct and indirect biological effects, including interaction with the aryl hydrocarbon receptor (AhR). AhR is a cytosolic receptor that binds various hydrophobic organic compounds (HOCs) and mediates some of their toxic effects. In vitro effects of binary mixtures of various DOM (mainly HS) with various HOCs on AhR-mediated responses were studied by use of H4IIE-luc cells. Six out of 12 DOM activated the AhR even at environmentally relevant concentrations (17 mg L−1). In simultaneous exposures of H4IIE-luc cells to DOM (17 mg L−1) and each of the model compounds, 2,3,7,8-TCDD, PCB126, PCB169, benzo[a]pyrene, benzo[a]anthracene, dibenz[a,h]anthracene, fluoranthene, a mixture of persistent organic pollutants (POPs), a mixture of polycyclic aromatic hydrocarbons (PAHs), and a mixture of all HOCs, either significant additive or facilitative effects were observed when compared to activities of single HOCs. No significant decrease of effects due to possible sorption of HOCs to DOM was observed, even in subsequent experiments when HOCs + DOM mixtures were preincubated for six days before exposure to H4IIE-luc. Thus, DOM does not seem to protect organisms against AhR-mediated toxic effects of HOCs (as usually predicted due to sorption of HOCs on DOM), but it can actually enhance their potency for AhR-mediated effects in some situations. © 2011 Elsevier Ltd. All rights reserved. 1. Introduction In polluted environments, especially in sediments, soils and water, hydrophobic organic compounds (HOCs) such as polychlorinated dioxins, biphenyls, and polycyclic aromatic hydrocarbons are expected to occur in the presence of dissolved/natural organic matter (D/NOM). An important mechanism of toxicity for some HOCs is binding to the aryl hydrocarbon receptor (AhR). AhR-mediated effects are considered to be markers of exposure to dioxin-like compounds (Whyte et al., 2004). These compounds elicit a variety of adverse effects in organisms, including carcinogenesis, embryotoxicity, teratogenicity, neurotoxicity, endocrine disruption and others (Poland and Knutson, 1982; Janosek et al., 2006). With regard to the adverse biological effects of pollutants such as HOCs, there is a great effort to study their environmental fate which influences their final toxicity. This includes e.g. study on possible interactions and modes of action of HOCs in mixture with naturally ⁎ Corresponding author at: Research Centre for Toxic Compounds in the Environment, RECETOX, Kamenice 126/3, CZ62500 Brno, Czech Republic. Tel.: + 420 549493807; fax: + 420 549492840. E-mail address: bittner@recetox.muni.cz (M. Bittner). 0160-4120/$ – see front matter © 2011 Elsevier Ltd. All rights reserved. doi:10.1016/j.envint.2011.03.016 occurring DOM in waters. To reveal impact of DOM on HOCs' AhRmediated activity, we used in vitro bioassay based on H4IIE-luc cell line. This bioassay is designed for detecting and estimating the relative potency of pure compounds or complex mixtures in various matrices to interact with the AhR. Commercially available clone of this bioassay is registered as DR-CALUX® (Chemical-Activated Luciferase Gene eXpression) assay (Murk et al., 1996), and analogous XDS-CALUX® bioassay has already been approved as U.S. EPA Method 4435 for HOC analysis (US EPA, 2008). In the aquatic environment, approximately 75 to 99% of organic carbon is particulate and dissolved non-living matter (the rest is present in living organisms). Concentrations of DOM in most natural freshwaters are in the range 0.5 to 50 mg L−1. The DOM is predominantly comprised of humic substances (HS) that form about 40–80% of the total DOM (Steinberg et al., 2006). In sediments, organic carbon consisting of HS can represent up to 20% of their total dry weight (Akkanen et al., 2005). HS are natural products formed by diagenesis of organic matter decomposition, which includes degradation and condensation processes. Generally, HS can be divided into humic acids (HA), fulvic acids (FA) and colloidal/insoluble humins, which differ in both their physicochemical and biological properties. Due to the wide range of nominal molecular size and functional M. Bittner et al. / Environment International 37 (2011) 960–964 groups, these compounds are generally functionally defined by their solubilities in alkaline solution or spectral characteristics (Steinberg, 2003). Several processes in aquatic systems can be affected by DOM/ HS. These include acidification and water warming, light penetration and photochemistry, microbial metabolism, ecosystem buffering, indirect and direct biological effects (Tipping, 2002). Indirect effects such as modulation of the availability of toxicants or nutrients via interactions with various HS structures have been known for a long time (Haitzer et al., 1998). Direct physiological effects such as changes of heat shock protein expression and biotransformation enzyme activity, or hormone-like effects in animals have been documented more recently (Steinberg et al., 2008). In a previous in vitro study, DOM-driven changes in AhR-mediated effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin were described (Bittner et al., 2009). In the present paper, in vitro effects of 12 DOM on AhRmediated effects of seven HOCs and their mixtures are investigated. A paired experimental design was used. This consisted of exposure to non-preincubated mixtures of HOCs and DOM, and to mixtures preincubated for six days to simulate two situations that can occur in the environment. At the time of accidental releases of HOCs into the aquatic environment, organisms are exposed to fresh mixtures of DOM and HOCs. This situation was simulated by exposure of H4IIE-luc cells to non-preincubated HOCs + DOM mixtures. Nevertheless, HOCs can interact with DOM during longer co-occurrence in the environment, which can result in modulation of toxicity of these mixtures. The effect of this aging of HOCs + DOM mixtures was examined by exposure of H4IIE-luc to HOCs + DOM mixtures that had been preincubated for six days. 2. Materials and methods DOM isolated from different matrices were purchased from Fluka (now Sigma-Aldrich), Prod. No. 53680 (Prague, Czech Republic) — Fluka HA; Sigma-Aldrich, Prod. No. H16752 (Prague, Czech Republic) — Aldrich HA; Humintech GmbH (Düsseldorf, Germany) — HuminFeed; and the International Humic Substances Society (IHSS, St. Paul, MN, USA) — remaining nine DOM samples. DOMs were dissolved in 0.05 M NaOH to achieve a final concentration of 17 mg L−1. This concentration was chosen based on previous findings where some HA exerted AhR-mediated activity in vitro at this environmentallyrelevant concentration (Bittner et al., 2009). 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) was purchased from Dr. Ehrenstorfer (Augsburg, Germany), 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) and 3,3′,4,4′,5,5′-hexachlorobiphenyl (PCB169) were purchased from Absolute Standards Inc. (Hamden, CT, USA), benzo[a]pyrene (B[a]P), benzo[a]anthracene (B[a]A), dibenz[a,h]anthracene (DB[a,h]A), and fluoranthene (Flu) were purchased from Sigma-Aldrich (Prague, Czech Republic). Experiments were conducted using HOCs dissolved in ethanol. H4IIE-luc (rat hepatocarcinoma) cells stably transfected with the luciferase gene under control of the dioxin response enhancer were used for determination of AhR activation (Murk et al., 1996). In these cells, when the appropriate substrates are added, binding of ligands to the AhR results in emission of light that is proportional to the potency of the mixture. This potency can be reported as 2,3,7,8-TCDD equivalents (TCDD eq.). Cells were grown and maintained in DMEM medium containing 10% fetal calf serum (both PAA laboratories, Pasching, Austria) at 5% CO2 and 37 °C. Once the cells reached about 70% confluence they were passaged and seeded into a sterile 96-well plate at the density of 15,000 cells/well in medium supplemented with 1% gentamicin. After 24 h, H4IIE-luc cells were exposed in triplicate to single HOCs or mixtures of HOCs + DOM for 24 h at 37 °C. Single HOCs were tested at the following concentrations: 1.2 pM 2,3,7,8-TCDD, 10 pM PCB126, 1000 pM PCB169, 40 nM B[a]P, 240 nM B[a]A, 1 nM DB[a,h]A, and 40,000 nM Flu. These concentrations were chosen based on the fact that they are environmentally relevant and 961 they elicited significant in vitro effects of approximately 25% of maximal AhR-mediated light emission caused by AhR saturating concentration of 2,3,7,8-TCDD (500 pM), when compared to blanks. The mixture of polycyclic aromatic hydrocarbons (PAHs Mix) contained equal proportions of each tested PAH (a quarter concentration compared to the concentration of the compound if tested alone): 10 nM B[a]P, 60 nM B[a]A, 0.25 nM DB[a,h]A, and 10,000 nM Flu. The mixture of persistent organic pollutants (POPs Mix) contained equal proportions of 0.4 pM 2,3,7,8-TCDD, 3.3 pM PCB126, and 333.3 pM PCB169 (a third concentration compared to the concentration of the compound if tested alone). The mixture of all studied HOCs (HOCs Mix) contained equal proportions of the PAHs Mix and POPs Mix (a half of each mixture). Each DOM was tested at a concentration of 17 mg L−1 in a mixture with the above mentioned HOCs and their mixtures. Cells exposed to DMEM with 0.5% EtOH and 0.5% 0.05 M NaOH were used as the appropriate vehicle controls. Intensity of luciferase luminescence, which is proportional to activation of AhR-mediated processes, was measured after 24 h of exposure by use of the Promega Steady Glo Kit (Promega, Mannheim, Germany). Three independent assays were conducted for each compound/combination and concentration tested, except for the mixtures with 2,3,7,8-TCDD and PCB169 where six independent assays were conducted. More repetitions of the tests with these exposure variants were conducted because of the greater variance among the measurements. All samples including HOCs alone, and each of the HOCs + DOM mixtures were added to the medium over cells either immediately, or after preincubation. Preincubation was conducted in culture medium supplemented with 1% gentamicin by shaking in glass vials in the dark for the duration of six days. Such paired experimental designs were used to simulate two situations that can occur in real environmental mixtures as described above. The 6-day duration of preincubation was chosen on the basis of previous findings (Bittner et al., 2009), which showed that while the results of tests with shorter incubations were not significantly different from non-preincubated samples, longer incubations usually resulted in bacterial contamination of incubated samples, even when gentamicin was used to prevent such bacterial progress. Bioassay-derived data were examined statistically by use of Statistica for Windows 8.0 (StatSoft, Tulsa, USA). Due to the small sample size (mainly 3), data were not tested for normality or homogeneity of variance. Because a one sample t-test was used to determine if means of HOCs + DOM mixtures were significantly different from a certain fixed value (100% response of pure HOCs) the bias introduced by failure to meet these assumptions would likely be minimal. Experiments were conducted in six independent repetitions (2,3,7,8-TCDD + DOM and PCB169 + DOM) or three independent repetitions (other HOCs + DOM mixtures), each performed in triplicates. An F-test was used only for 2,3,7,8-TCDD + DOM, which was done in 6 repetitions. In that case the data met the assumptions of normality and homogeneity of variance. The level of significance used was p = 0.05. 3. Results Single HOCs at the tested concentrations exerted AhR-mediated effects in the range of 12 to 38% of maximal AhR-mediated response (caused by the AhR saturating concentration of 500 pM 2,3,7,8-TCDD). The observed up-regulations expressed as percent of maxima for each compound were as follows: 1.2 pM TCDD — 17%, 10 pM PCB126 — 38%, 1000 pM PCB169 — 12%, 40 nM B[a]P — 30%, 240 nM B[a]A — 38%, 1 nM DB[a,h]A — 29%, and 40,000 nM Flu — 16%. The AhR-mediated effect of the PAHs Mix was 18%, POPs Mix 59%, and the HOCs Mix 53% of maximal 2,3,7,8-TCDD induction (Table 1, last column). In the case of the 6-day preincubation, there were only insignificant changes in AhR-mediated activities of HOCs compared to activities of nonpreincubated samples. Of the 12 DOMs used in experiments (all at a concentration of 17 mg L−1), six samples (only HA, no FA or NOM samples) elicited significant AhR-mediated activities with and without preincubation. These levels of expression were similar in each experimental design and the values ranged between 7 and 14% of maximal AhRmediated response caused by the AhR saturating concentration of 2,3,7,8-TCDD 962 M. Bittner et al. / Environment International 37 (2011) 960–964 Table 1 Comparison of AhR-mediated activity of DOM relative to AhR-mediated activity of HOCs (%). There are shown AhR-active DOM (all HA) only, activity of other DOM was not significantly different from the activity of vehicle control. Concentration of each DOM sample was 17 mg L−1; concentrations of HOCs are listed in the table. Values were derived from ratio of AhR-mediated activity of tested DOM/AhR-mediated activity of tested HOCs, both expressed as percentage of 500 pM TCDD AhR-mediated activity (results in last column and last row). AhR-mediated activity of DOM × AhR-mediated activity of HOCs (%) Flu 40,000 nM PCB126 0.01 nM PCB169 1 nM B[a]P 40 nM DB[a,h]A 1 nM POPs Mix B[a]A 240 nM TCDD 1.2 pM PAHs Mix HOCs Mix TCDD 500 pM Leonardite HA Fluka HA Elliot Soil HA Aldrich HA HuminFeed Florida Peat HA 81 34 108 43 45 22 34 76 72 25 13 81 34 108 43 45 22 34 76 72 25 13 88 37 117 47 48 24 37 82 78 26 14 56 24 75 30 31 15 24 53 50 17 9 50 21 67 27 28 14 21 47 44 15 8 44 18 58 23 24 12 18 41 39 13 7 % TCDD 500 pM⁎ 16 38 12 30 29 59 38 17 18 53 100 ⁎ Values in this column equal to AhR-mediated activity of HOCs relative to AhR-mediated activity of TCDD 500 pM (%). (500 pM). The proportions of maximal expression caused by DOM themselves were as follows: Leonardite HA — 13%, Fluka HA — 13%, Elliot Soil HA — 14%, Aldrich HA — 9%, HuminFeed — 8%, Florida Peat HA — 7% (Table 1, last row); remaining six DOM samples did not elicit any AhR-mediated response. More detailed information on AhR-mediated effects of DOM has been published previously by Janosek et al. (2007). The proportions of AhR-mediated activity of AhR-active DOM themselves compared to AhR-mediated activity of HOCs are shown in Table 1. Simultaneous exposure of H4IIE-luc cells to both HOCs and DOM resulted in statistically significant modulations of the AhR-mediated activity compared to single HOC exposure in 63 of the 120 combinations without preincubation. All of the observed significant effects were increases of AhR-mediated activity of HOCs in the presence of DOM. Responses of these HOCs + DOM mixtures ranged from 111 to 557% with a median interaction that resulted in 201% compared to each HOCs' alone activity (100%; Table 2). In the case of HOCs + DOM mixtures that were preincubated six days prior to exposure, statistically significant response enhancement was observed for 38 HOCs + DOM combinations. The degree of induction with these mixtures ranged from 127 to 834% with a median of 219% compared to activity of HOCs preincubated alone (100%). Only one DOM type, NOM Nordic Reservoir, caused statistically significant decrease of AhR-mediated expression when co-exposed with PAHs Mix after preincubation. However, the change was relatively small with a decrease from 100% to 93%. Greater decreases in the range of 74 and 76% of the POPs Mix response were observed after preincubation of POPs Mix with several DOM (Table 2), nevertheless, these were not statistically significant due to the assay variability. AhR-mediated responses of single HOCs (or its mixtures) in combinations with various DOM samples differed in dependence on time of preincubation. Nevertheless, there was no uniform trend — both enhancement and decrease of AhR-mediated responses of analogous HOCs + DOM mixtures with/without preincubation were observed. To evaluate the overall influence of the preincubation, the average values of AhR-mediated responses of single HOCs mixed with each DOM sample (i.e. average value from each row in Table 2 was calculated) in the experiment with/without preincubation were compared. If the difference between the average activity of nonpreincubated and preincubated samples was greater than 50% (set arbitrarily), it was deemed to be significant. Preincubation for six days led to significantly different results for four HOCs in mixture with all the types of DOM compared to analogous experiments with non-preincubated samples (depicted in the last column of Table 2). Preincubated mixtures of DOM with PCB126, B[a]P, and POPs Mix resulted in significantly less AhRmediated activity, compared to analogous non-preincubated samples. The average Table 2 Summary of AhR-mediated activities of HOCs + DOM mixtures. Concentration of each DOM sample was 17 mg L−1; concentrations of HOCs are shown in the table. 100% in each row represents AhR-mediated activity of corresponding HOCs at listed concentration. The table presents results for each co-exposure variant dosed to cells either immediately after mixing or after 6-day preincubation. Values represent the mean and SD (values in brackets) of six independent experiments (2,3,7,8-TCDD + DOM and PCB169 + DOM) or three independent experiments (other HOCs+ DOM mixtures), each performed in triplicates. Bold font denotes values significantly different (pb 0.05) from AhR-mediated activity of corresponding HOCs alone (100%). Length of the gray bands graphically represents the values in the table (only the means). Δ indicates changes of AhR-mediated activity of single HOCs in co-exposure with all DOM in experiments with and without preincubation (− denotes no significant change, ↗ denotes increase of activity, ↘ denotes decrease of activity). AhR-mediated activity (% of HOCs induction) Pre- Leonardite Fluka incub. HA HA (days) Flu 40,000 nM PCB126 0.01 nM PCB169 1nM B[a]P 40nM DB[ah]A 1nM POPs Mix B[a]A 240nM TCDD 1.2 pM PAHs Mix HOCs Mix 0 6 0 6 0 6 0 6 0 6 0 6 0 6 0 6 0 6 0 6 557 (91) 548 (228) 442 (79) 396 (14) 477 (255) 671 (179) 305 (121) 273 (78) 319 (90) 299 (69) 339 (69) 169 (14) 232 (51) 228 (60) 176 (39) 168 (68) 149 (17) 143 (60) 147 (32) 150 (40) 383 (130) 462 (191) 499 (143) 403 (6) 469 (256) 834 (421) 256 (90) 253 (75) 231 (30) 296 (41) 256 (31) 158 (20) 203 (15) 184 (10) 174 (33) 158 (29) 114 (6) 145 (3) 121 (9) 158 (38) Elliot Soil Aldrich HA HA 554 (219) 486 (120) 494 (238) 328 (4) 459 (216) 651 (261) 288 (78) 207 (25) 241 (43) 209 (46) 316 (72) 160 (29) 188 (44) 158 (21) 192 (53) 133 (39) 121 (7) 119 (17) 138 (16) 127 (18) 337 (99) 316 (104) 394 (147) 334 (62) 335 (130) 461 (212) 279 (123) 212 (51) 219 (31) 248 (26) 234 (46) 127 (1) 193 (44) 177 (30) 162 (41) 144 (34) 113 (6) 129 (37) 114 (9) 119 (12) HuminFeed Florida Peat HA 394 (115) 377 (136) 392 (77) 306 (44) 320 (150) 454 (104) 262 (41) 189 (10) 214 (15) 218 (28) 200 (47) 117 (29) 186 (41) 162 (15) 157 (30) 128 (32) 105 (6) 95 (9) 110 (6) 107 (3) 309 (101) 275 (102) 338 (128) 239 (13) 330 (155) 367 (128) 231 (69) 144 (26) 201 (66) 174 (29) 285 (54) 130 (30) 168 (44) 149 (25) 145 (22) 113 (25) 145 (12) 144 (33) 148 (10) 122 (22) Waskish Peat HA 250 (39) 312 (162) 212 (55) 230 (79) 148 (33) 307 (168) 214 (62) 224 (51) 198 (31) 220 (39) 126 (11) 97 (15) 182 (30) 179 (29) 131 (15) 124 (33) 111 (5) 108 (10) 114 (14) 107 (7) Suwannee Nordic Suwannee Suwannee Nordic River NOM Reservoir River HA River FA Reservoir FA NOM 256 (41) 237 (90) 190 (109) 156 (61) 139 (35) 183 (73) 253 (75) 160 (48) 211 (75) 170 (42) 143 (24) 74 (27) 186 (60) 157 (35) 138 (16) 118 (26) 111 (3) 123 (57) 113 (9) 108 (12) 234 (31) 260 (99) 220 (46) 198 (16) 123 (27) 168 (77) 236 (40) 188 (71) 202 (41) 191 (22) 119 (11) 76 (35) 181 (65) 158 (45) 129 (20) 110 (29) 103 (15) 111 (44) 104 (3) 96 (11) 233 (56) 200 (15) 200 (34) 218 (59) 125 (21) 227 (131) 223 (45) 172 (41) 170 (20) 154 (42) 139 (15) 99 (25) 157 (20) 192 (46) 124 (14) 112 (20) 106 (5) 118 (29) 116 (5) 108 (13) 196 (44) 223 (119) 164 (72) 148 (49) 124 (46) 260 (113) 223 (49) 173 (46) 194 (32) 174 (33) 102 (20) 81 (23) 195 (59) 144 (19) 131 (10) 111 (29) 97 (1) 112 (23) 106 (11) 101 (1) 197 (46) 136 (19) 165 (94) 92 (8) 190 (68) 139 (89) 161 (23) 112 (16) 154 (66) 99 (9) 176 (29) 90 (14) 134 (22) 120 (9) 125 (6) 93 (17) 138 (8) 93 (2) 126 (7) 109 (11) Δ M. Bittner et al. / Environment International 37 (2011) 960–964 decreases for the various combinations were: 55% for PCB126 + DOM, 52% for B[a]P + DOM, and 88% for the POPs Mix. On the other hand, AhR-mediated response to PCB169 + DOM mixtures that were preincubated prior to exposure was significantly greater compared to analogous non-preincubated samples with average increase of 124%. AhR-mediated activities of other HOCs + DOM mixtures were not significantly influenced by preincubation if compared to non-preincubated samples; the average differences between each appropriate pair ranged from − 23 to 2%. 4. Discussion To visually depict the differences in induction of AhR-mediated response (expression of luciferase) by H4IIE-luc cells among treatments with combinations of DOM and HOCs, combinations of DOM and HOCs that resulted in the greatest AhR-mediated expression are grouped in the upper left corner of Table 2. Alternatively, mixtures resulting in the least AhR-mediated expression are grouped in the lower right corner (Table 2). This arrangement highlights those DOM that most affected AhR-mediated responses of the transactivation assay to HOCs as well as those HOCs that exerted greater AhRmediated response when mixed with DOM. In the case of mixtures of HOCs with AhR-active HA, increases in AhR-mediated expression were probably caused mainly by additive effects. This can be derived from comparison of detected AhRmediated activity of HOCs mixed with AhR-active DOM (first six columns in Table 2) with relative AhR-mediated activity of DOM themselves (Table 1, complementary to Table 2). The mixtures of AhR-active DOM with HOCs were consequently the most AhR-active. Nevertheless, all the observed effects caused by DOM from relatively great up-regulation of AhR-mediated response by HOCs to moderate down-regulation cannot be explained by simple additive effects of HOCs and DOM. There must be mentioned also facilitation, which increases bioavailability of HOCs by both enhancement of its water solubility and changes in biomembrane permeability by DOM (Doring and Marschner, 1998; Glover and Wood, 2005). In addition, uptake of hydrophobic organic compounds into organisms is often limited by the diffusive transport through a thin boundary layer, and it has been established recently that a variety of DOM at environmental levels can enhance diffusive mass transfer in various transport scenarios (Mayer et al., 2007). This mechanism can explain both enhancement of AhRmediated response caused by HOCs in mixture with non-AhR-active DOM (especially FA and NOM), and more than additive effect of AhRactive DOM (HA samples in Table 1 and also in the first six columns of Table 2) on AhR-mediated activity of Fluoranthen, PCB126 and PCB169 (Table 2). Another mechanism, sorption, is a well known ability of HOCs (e.g. POPs and PAHs) to bind onto DOM structures (Haitzer et al., 1998; Perminova et al., 2001), where the decrease of HOCs' toxicity is usually expected. In this paper, the observed decrease of AhRmediated activity of the three groups of HOCs + DOM, i. e. PCB126 + DOM, B[a]P + DOM, and POPs Mix + DOM in preincubated mixtures compared to analogous non-preincubated samples could be explained by sorption. The combination of facilitative and sorptive effects caused by DOM has also been described for copper toxicity to Simocephalus serrulatus (Daphnidae) in DOM-rich pond (Giesy et al., 1983). Specific combination of these involved mechanisms, with facilitation as a most important mechanism that has increased during 6-day preincubation, could also explain the greater AhR-mediated expression after 6-day preincubation (124% greater induction, in average) compared to analogous non-preincubated PCB169 + DOM mixtures (to confirm this quite unexpected observation, six independent experiments with PCB169 + DOM, both with and without preincubation, were performed). Besides the above mentioned mechanisms, cross-talk between AhR and other receptor pathways could be involved in the effects of some compounds or mixtures. For example, recent studies document interactive regulatory cross-talk between glucocorticoid receptor (GR) and AhR (e.g. Dvorak et al. 2008). However, there is no evidence of the potential interaction of DOM with GR. 963 AhR-active DOM including Fluka HA, Aldrich HA, HuminFeed and Leonardite HA are all derived from brown coal and thus are not relevant to effects of DOM in natural environments. However, if coalderived HA are considered to be used as so called “environmentally friendly” sorbents for HOCs decontamination in situ (Perminova et al., 2001), observed AhR-mediated effects including interaction with HOCs should be taken into account as a possible side effect. Other DOM types of environmental origin either had no effect or enhanced the AhR-mediated potency of HOCs. To generalize effects of DOM on HOCs activity, a range of both DOM and HOCs was used. Nevertheless, the significance of our in vitro findings needs to be investigated in vivo before the relevance for ecosystems can be addressed. There is one study with fish describing similar additive effect of coal-derived HA and crude oil (rich for PAHs) on expression of cytochrome P450 1A (Matsuo et al., 2006) that is directly linked to AhR activation. These findings indicate that our in vitro results could be also anticipated if tested in vivo, but more studies with other DOM samples (not only of coal origin) are needed for generalization. Acknowledgments We would like to thank to Dr. Jiri Novak for valuable assistance in in vitro testing, and to Dr. Klara Kubosova for valuable assistance in data analysis. This research has been supported by project GACR 525/ 08/P464, INCHEMBIOL framework project MSM0021622412, and project CETOCOEN (CZ.1.05/2.1.00/01.0001) granted by the European Union and administered by the Ministry of Education, Youth and Sports of the Czech Republic. Prof. Giesy was supported by the Canada Research Chair program and at large Chair Professorship at the Department of Biology and Chemistry and State Key Laboratory in Marine Pollution, City University of Hong Kong. References Akkanen J, Lyytikainen M, Tuikka A, Kukkonen JVK. Dissolved organic matter in pore water of freshwater sediments: effects of separation procedure on quantity, quality and functionality. Chemosphere 2005;60:1608–15. Bittner M, Hilscherova K, Giesy JP. In vitro assessment of AhR-mediated activities of TCDD in mixture with humic substances. Chemosphere 2009;76:1505–8. Doring UM, Marschner B. Water solubility enhancement of benzo(a)pyrene and 2,2′5,5′-terachlorobiphenyl by dissolved organic matter (DOM). Phys Chem Earth 1998;23:193–7. Dvorak Z, Vrzal R, Pavek P, Ulrichova J. An evidence for regulatory cross-talk between aryl hydrocarbon receptor and glucocorticoid receptor in HepG2 cells. Physiol Res 2008;57:427–35. Giesy JP, Newell A, Leversee GJ. copper speciation in soft, acid, humic waters — effects on copper bioaccumulation by and toxicity to Simocephalus serrulatus (Daphnidae). Sci Total Environ 1983;28:23–36. Glover CN, Wood CM. The disruption of Daphnia magna sodium metabolism by humic substances: mechanism of action and effect of humic substance source. Physiol Biochem Zool 2005;78:1005–16. Haitzer M, Hoss S, Traunspurger W, Steinberg CEW. Effects of dissolved organic matter (DOM) on the bioconcentration of organic chemicals in aquatic organisms — a review. Chemosphere 1998;37:1335–62. Janosek J, Hilscherova K, Blaha L, Holoubek I. Environmental xenobiotics and nuclear receptors — interactions, effects and in vitro assessment. Toxicol In Vitro 2006;20: 18–37. Janosek J, Bittner M, Hilscherova K, Blaha L, Giesy JP, Holoubek I. AhR-mediated and antiestrogenic activity of humic substances. Chemosphere 2007;67:1096–101. Matsuo AYO, Woodin BR, Reddy CM, Val AL, Stegeman JJ. Humic substances and crude oil induce cytochrome P450 1A expression in the Amazonian fish species Colossoma macropomum (Tambaqui). Environ Sci Technol 2006;40:2851–8. Mayer P, Fernqvist MM, Christensen PS, Karlson U, Trapp S. Enhanced diffusion of polycyclic aromatic hydrocarhons in artificial and natural aqueous solutions. Environ Sci Technol 2007;41:6148–55. Murk AJ, Legler J, Denison MS, Giesy JP, vandeGuchte C, Brouwer A. Chemical-activated luciferase gene expression (CALUX): a novel in vitro bioassay for Ah receptor active compounds in sediments and pore water. Fundam Appl Toxicol 1996;33:149–60. Perminova IV, Grechishcheva NY, Kovalevskii DV, Kudryavtsev AV, Petrosyan VS, Matorin DN. Quantification and prediction of the detoxifying properties of humic substances related to their chemical binding to polycyclic aromatic hydrocarbons. Environ Sci Technol 2001;35:3841–8. Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-para-dioxin and related halogenated aromatic-hydrocarbons — examination of the mechanism of toxicity. Annu Rev Pharmacol 1982;22:517–54. 964 M. Bittner et al. / Environment International 37 (2011) 960–964 Steinberg CEW. Ecology of humic substances in freshwaters — determinants from geochemistry to ecological niches. Berlin: Springer; 2003. Steinberg CEW, Kamara S, Prokhotskaya VY, Manusadzianas L, Karasyova TA, Timofeyev MA, et al. Dissolved humic substances — ecological driving forces from the individual to the ecosystem level? Freshw Biol 2006;51:1189–210. Steinberg CEW, Meinelt T, Timofeyev M, Bittner M, Menzel R. Humic substances: part 2: interactions with organisms. Environ Sci Pollut R 2008;15:128–35. Tipping E. Cation binding by humic substances. Cambridge: Press Syndicate of the University of Cambridge; 2002. USEPA. Method for toxic equivalents (TEQs) determinations for dioxin-like chemical activity with the CALUX ® bioassay (Method 4435), Revision 0. www.epa.gov/osw/ hazard/testmethods/pdfs/4435.pdf 2008. Whyte JJ, Schmitt CJ, Tillitt DE. The H4IIE cell bioassay as an indicator of dioxin-like chemicals in wildlife and the environment. Crit Rev Toxicol 2004;34:1-83.