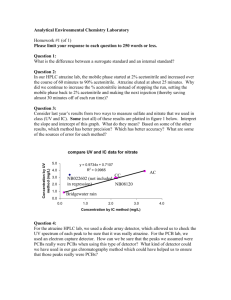
This article was downloaded by: On: 28 October 2008 Access details:
advertisement

This article was downloaded by: On: 28 October 2008 Access details: Access Details: Free Access Publisher Informa Healthcare Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Critical Reviews in Toxicology Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t713401167 Effects of Atrazine on Fish, Amphibians, and Aquatic Reptiles: A Critical Review Keith R. Solomon a; James A. Carr b; Louis H. Du Preez c; John P. Giesy def; Ronald J. Kendall g; Ernest E. Smith g; Glen J. Van Der Kraak h a Department of Environmental Biology and Centre for Toxicology, University of Guelph, Guelph, Ontario, Canada b Department of Biological Sciences, Texas Tech University, Lubbock, Texas c School of Environmental Sciences and Development, North West University, Potchefstroom, South Africa d Toxicology Centre, University of Saskatchewan, Saskatoon, Saskatchewan, Canada e Department of Biology and Chemistry, City University of Hong Kong, Kowloon, Hong Kong, SAR, China f Zoology Department, Michigan State University, East Lansing, Michigan g Institute of Environmental and Human Health and Department of Environmental Toxicology, Texas Tech University, Lubbock, Texas h Department of Integrative Biology, University of Guelph, Guelph, Ontario, Canada Online Publication Date: 01 October 2008 To cite this Article Solomon, Keith R., Carr, James A., Du Preez, Louis H., Giesy, John P., Kendall, Ronald J., Smith, Ernest E. and Van Der Kraak, Glen J.(2008)'Effects of Atrazine on Fish, Amphibians, and Aquatic Reptiles: A Critical Review',Critical Reviews in Toxicology,38:9,721 — 772 To link to this Article: DOI: 10.1080/10408440802116496 URL: http://dx.doi.org/10.1080/10408440802116496 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material. Critical Reviews in Toxicology, 38:721–772, 2008 c 2008 Informa UK Ltd. Copyright ISSN: 1040-8444 print / 1547-6898 online DOI: 10.1080/10408440802116496 Effects of Atrazine on Fish, Amphibians, and Aquatic Reptiles: A Critical Review Keith R. Solomon Department of Environmental Biology and Centre for Toxicology, University of Guelph, Guelph, Ontario, Canada James A. Carr Department of Biological Sciences, Texas Tech University, Lubbock, Texas Louis H. Du Preez School of Environmental Sciences and Development, North West University, Potchefstroom, South Africa John P. Giesy Downloaded At: 15:43 28 October 2008 Toxicology Centre, University of Saskatchewan, Saskatoon, Saskatchewan, Canada, Department of Biology and Chemistry, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong, SAR, China, and Zoology Department, Michigan State University, East Lansing, Michigan Ronald J. Kendall and Ernest E. Smith Institute of Environmental and Human Health and Department of Environmental Toxicology, Texas Tech University, Lubbock, Texas Glen J. Van Der Kraak Department of Integrative Biology, University of Guelph, Guelph, Ontario, Canada The herbicide atrazine is widely used in agriculture for the production of corn and other crops. Because of its physical and chemical properties, atrazine is found in small concentrations in surface waters—habitats for some species. A number of reports on the effects of atrazine on aquatic vertebrates, mostly amphibians, have been published, yet there is inconsistency in the effects reported, and inconsistency between studies in different laboratories. We have brought the results and conclusions of all of the relevant laboratory and field studies together in this critical review and assessed causality using procedures for the identification of causative agents of disease and ecoepidemiology derived from Koch’s postulates and the Bradford–Hill guidelines. Based on a weight of evidence analysis of all of the data, the central theory that environmentally relevant concentrations of atrazine affect reproduction and/or reproductive development in fish, amphibians, and reptiles is not supported by the vast majority of observations. The same conclusions also hold for the supporting theories such as induction of aromatase, the enzyme that converts testosterone to estradiol. For other responses, such as immune function, stress endocrinology, parasitism, or population-level effects, there are no indications of effects or there is such a paucity of good data that definitive conclusions cannot be made. Keywords adverse effects, amphibians, atrazine, fish, endocrine, reptiles Table of Contents I. INTRODUCTION ............................................................................................................................................ 723 II. CHEMICAL, PHYSICAL, AND BIOLOGICAL PROPERTIES ...................................................................... 723 A. Environmental Behavior of Atrazine ............................................................................................................. 723 Address correspondence to Keith Solomon, Centre for Toxicology, University of Guelph, Guelph, ON, N1G 2W1 Canada. E-mail: ksolomon@uoguelph.ca 721 722 K. R. SOLOMON ET AL. Downloaded At: 15:43 28 October 2008 B. Mechanism of Action .................................................................................................................................. 726 C. Bioconcentration/Bioaccumulation ............................................................................................................... 727 III. ACUTE AND CHRONIC TOXICITY .............................................................................................................. 727 A. Lethality and Physiological Effects ............................................................................................................... 727 IV. EXTERNAL DEVELOPMENTAL ABNORMALITIES ................................................................................... 731 A. Trematode Infections ................................................................................................................................... 731 B. Direct Effects of Atrazine on Limb Deformities ............................................................................................. 732 V. SEXUAL DIFFERENTIATION AND DEVELOPMENT .................................................................................. 733 A. The Use of Xenopus laevis as a Model for Endocrine Responses ...................................................................... 733 B. Effects on Sex Ratio .................................................................................................................................... 734 C. Effects on Sexual Development .................................................................................................................... 734 VI. MECHANISMS MEDIATING REPRODUCTIVE EFFECTS .......................................................................... 739 A. Mechanisms Mediated Through the HPG Axis ............................................................................................... 740 B. Mechanisms Mediated Through Aromatase ................................................................................................... 740 C. Effects of Atrazine on Plasma Sex Steroid Hormones in Amphibians and Fish .................................................. 742 VII. EFFECTS ON LARYNGEAL DEVELOPMENT ............................................................................................. 745 A. Effects on the Laryngeal Dilator Muscle ........................................................................................................ 745 VIII. EFFECTS ON THYROID FUNCTION AND DEVELOPMENT ...................................................................... 745 A. Effects of Atrazine on Amphibian Metamorphosis .......................................................................................... 746 B. Effects of Atrazine on Smoltification ............................................................................................................. 746 IX. EFFECTS ON STRESS PHYSIOLOGY .......................................................................................................... 747 A. Effects on Plasma Corticosteroids ................................................................................................................. 747 B. Effects on Adrenal Steroidogenesis and Secretion .......................................................................................... 747 C. Effects of Pesticide Mixtures on Corticosteroid Secretion ............................................................................... 748 X. EFFECTS ON IMMUNE FUNCTION ............................................................................................................. 749 A. Effects of Atrazine on Immune Function in Fish ............................................................................................. 749 B. Effects of Atrazine on Immune Function in Amphibians ................................................................................. 750 XI. EFFECTS OF ATRAZINE ON BEHAVIOR .................................................................................................... 754 A. Effects on Olfactory Neurons and Behavior in Fish ........................................................................................ 754 B. Effects on Behavior in Amphibians ............................................................................................................... 756 XII. EFFECTS OF ATRAZINE AT THE POPULATION LEVEL ........................................................................... 756 A. Atrazine and Reptiles ................................................................................................................................... 756 B. Atrazine and Fish ........................................................................................................................................ 756 C. Atrazine and Amphibians ............................................................................................................................. 757 XIII. OVERALL CONCLUSIONS, AND RESEARCH DIRECTIONS ..................................................................... 762 A. Strengths and Uncertainties .......................................................................................................................... 762 B. Conclusions ................................................................................................................................................ 762 XIV. SUMMARY ..................................................................................................................................................... 764 ACKNOWLEDGMENTS ........................................................................................................................................... 764 REFERENCES .......................................................................................................................................................... 764 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES Downloaded At: 15:43 28 October 2008 I. INTRODUCTION The potential for ecological risks of atrazine (CAS number 1912-24-9) in fresh and estuarine waters has been extensively reviewed with respect to its potential effects on a number of endpoints and processes in ecological systems (Solomon et al., 1996; Giddings et al., 2005). The primary focus was on plants, arthropods, and fish exposed to typical concentrations. These assessments concluded that atrazine did not present significant acute or chronic ecological risks. Although some of the measures of effect used in these assessments, such as those in full-lifecycle studies on several species of fish and those in microcosms, include the aggregate responses of many possible mechanisms of action, these have not specifically characterized reproduction, the endocrine system, or development as possible targets for atrazine. In the 1990s, a number of studies reported that atrazine affected the reproductive system of mammals at large exposure concentrations (≥40 mg/kg). Specifically an increased incidence of mammary tumors was observed in female SpragueDawley (SD) rats. This effect was strain-, sex-, and speciesspecific and judged to not be relevant to humans (U.S. EPA, 2000). Considerable research on the mechanism of this response has been conducted and was recently reviewed (Gammon et al., 2005, Cooper et al., 2007). The mammalian data are discussed briefly in relevant sections of this review, which is focused on the identified lack of data for sublethal endpoints related to development and reproduction in nontarget terrestrial, aquatic, and semi-aquatic species, such as reptiles and amphibians (Solomon et al., 1996, Giddings et al., 2005). Since that time, a number of reports on the effects of atrazine on aquatic wildlife, mostly amphibians, have been published, yet there is inconsistency in the effects reported, and inconsistency between studies in different laboratories. This review was written to provide a critical assessment of current information on atrazine and the effects that it may have on fish, amphibians, and aquatic reptiles. Much of the review is focused on amphibians because more studies have been conducted in these organisms. In conducting this work we followed the general review guidelines as outlined by Weed (1997). We searched the current and historical literature using PubMed, Scopus, Science Direct, and the Agricola databases. In addition, we used books and other reviews to obtain information from the older literature. We also included information presented at meetings where the results were not described by the authors as preliminary and where a physical copy of the presentation was available (such as a poster). In collecting this information, we attempted to be inclusive and did not exclude papers or information based on source. We did, however, critically assess the validity and quality of the study and results based on the published description of the study design and the interpretation of the results by the authors. In assessing all of this evidence, we used guidelines for causality developed from those of Koch (1942) and Bradford-Hill (1965) as modified for assessment of causality associated with adverse effects of substances that are mediated 723 through endocrine and developmental pathways (IPCS, 2002). In organizing this review, we have summarized key information related to the properties of atrazine and how these may affect exposures and then focused the bulk of the review on the effects of atrazine at the level of the individual through the population. Effects on individuals were assessed from the point of view of responses at various levels of biological organization as well as information on mechanisms of action. This review is divided into a number of sections, each with a specific focus. In developing overall conclusions, we assessed the strength of the evidence on the basis of the guidelines for causality and also identified the relevance of these to the conclusions. II. CHEMICAL, PHYSICAL, AND BIOLOGICAL PROPERTIES Detailed descriptions of the chemical and physical characteristics of atrazine have been presented previously (Solomon et al., 1996) and recently updated (Giddings et al., 2005), and only the properties of atrazine most relevant to its environmental fate and effects are presented here. Because of its properties, atrazine is most commonly found in surface waters and, to a small extent, in groundwater. Atrazine is relatively persistent in higher pH surface waters associated with its major uses in agriculture. Thus, much of the focus on atrazine exposures in the literature has been directed to the water route and a similar focus is followed here. In addition, several key points are highlighted. These include the primary mechanism of action of atrazine as a photosynthesis inhibitor—a mode of action that is specific to plants and therefore confers selectivity to other organisms in terms of acute toxicity. The greater persistence of atrazine increases the probability that aquatic organisms will be exposed; however, there is little bioaccumulation in aquatic organisms such as fish and frogs (BCFs range from <1 to about 8), it is rapidly metabolized in fish and frogs, and there is no evidence of concentration in specific tissues such as those implicated in gonadal effects. Lack of bioaccumulation is consistent with water solubility, low K OW , and metabolic degradation and excretion. Thus, although moderately persistent in the environment, atrazine does not bioconcentrate nor does it biomagnify in the food chain. In organisms exposed through the water matrix, concentrations in the organisms will be similar to those in the water and, should these vary, will closely follow them. Transfer of atrazine from the F1 to F2 generation will be negligible. Thus, from the point of view of toxicological and physiological responses, water concentrations during the period of exposure are the critical determinants of potential effects. A. Environmental Behavior of Atrazine Persistence in the Environment The s-triazine ring makes the atrazine molecule somewhat resistant to microbiological degradation in aquatic systems (Howard, 1991). Chemical degradation occurs by hydrolysis and N -dealkylation. Photolysis of atrazine does not occur in water 724 K. R. SOLOMON ET AL. Downloaded At: 15:43 28 October 2008 FIG. 1. Structure of atrazine and its major metabolites. at wavelengths greater than 300 nm. Half-lives in water for six studies ranged from 41 to 237 d with a mean of 159 d (SD = 71 d) (Giddings et al., 2005), and those in anaerobic soil or sediment ranged from 58 to 547 d with a mean of 228 d (SD = 168 d), and field dissipation half lives ranged from 8 to 99 d (Novartis, 2000). The relative persistence of atrazine in surface waters increases the potential for exposure in aquatic organisms, particularly those in static water systems. Transformation Products Known transformation products of atrazine are desethylatrazine (DEA), hydroxyatrazine (HA), desisopropylatrazine (DIA), diaminochlorotriazine (DACT), and two dealkylated hydroxyatrazines, desethylhydroxyatrazine (DEHA), and desisopropylhydroxyatrazine (DIHA) (Fig. 1). Formation of these transformation products has been measured in laboratory studies, and some data are available for transformation products detected in the aquatic environment (summarized in Giddings et al., 2005). Measurements of the persistence of atrazine transformation products are limited. Soil half-lives have been reported as 26 d for DEA, 17 d for DIA, 19 d for DAC, and 121 d for HA (Winkelmann and Klaine, 1991); however, aqueous halflives of atrazine degradates and metabolites are not available. Bioconcentration data for atrazine transformation products are also limited, since studies reporting biological concentration focus on metabolite production in biota upon exposure to atrazine, rather than uptake of these degradates from environmental media. Based on results of studies in mammals and other organisms (Sanderson et al., 2001), these metabolites should be considered in assessing potential risks. Use Pattern and Geographic Distribution Atrazine is usually applied preemergent as a water-dispersed spray, although preplant and postemergent applications are also used. Typically, a single soil/field application of 1 kg/ha (1.1 lbs a.i./acre) is made by ground equipment. The estimated total for all uses of atrazine in 2001 was 35–36 million tonnes active ingredient (a.i.) (U.S. EPA, 2004). Most atrazine use occurs on corn (85% of total), mostly in the Midwestern United States, which is also where the most acres are planted in corn. Applications of atrazine in Illinois, Iowa, Nebraska, Indiana, Ohio, and Missouri alone accounted for approximately 60% of the total atrazine applied to corn in the United States in 1998 (Giddings et al., 2005). Atrazine is used on sorghum throughout the United States, which accounts for approximately 10% of total atrazine use. Atrazine use on sugarcane in parts of Florida and Louisiana represents approximately 2.5% of the total annual use of atrazine. Giddings et al. (2005) combined data on rainfall and atrazine use in a geographic information system (GIS) to define climate-use areas of greatest likelihood of atrazine runoff and showed that measurements from surface water monitoring stations were centered near these greater risk areas. Freshwater ecosystems in these regions are at greatest risk from exposures to atrazine (Fig. 2). Pathways for Exposure in Wildlife Several exposure pathways for aquatic wildlife are possible, but some are more important than others (Fig. 3). Terrestrial wildlife can be exposed to atrazine via consumption of contaminated food or water. Estimates of exposure of terrestrial wildlife range from 60 to 960 mg/kg for a small herbivore (15 g) exposed at the rate of 4 kg/ha used in sugar cane to 16.5–264 mg/kg for a small herbivore exposed to the more typical rate of 1.2 kg/ha used in corn (U.S. EPA, 2003c). Runoff and erosion are the major routes of atrazine entry into surface waters, while leaching and lateral movement through the soil or tile drains are a secondary route of entry (Giddings et al., 2005). Due to typical methods of atrazine application, spray drift is a minor route of exposure (Giddings et al., 2005; Solomon et al., 1996), and volatilization and Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES 725 FIG. 2. Atrazine climate-use regions showing areas with higher use and rainfall and greater potential risk (redrawn from data of Giddings et al., 2005). co-distillation with subsequent rainout are other relatively minor routes of exposure. Habitats where there is possible exposure of aquatic wildlife include natural ponds and ephemeral pools to farm ponds, streams, rivers, reservoirs, lakes, and eventually saltwater. Estuarine and marine environments are not important habitats for amphibians and are only relevant to fish and a few reptiles. Because of dilution in these environments, exposures are likely to be very small and do not present a direct or indirect risk (Solomon et al., 1996; Giddings et al., 2005). Significant differences in exposure regimens exist for the remaining FIG. 3. Possible routes of exposure of wildlife to atrazine. The width of the arrow indicates the relative importance of the exposure route. Downloaded At: 15:43 28 October 2008 726 K. R. SOLOMON ET AL. habitats that are most relevant to fish, amphibians, and possibly reptiles. Analysis of extensive data sets of atrazine concentration measurements in running water showed that exposures are pulses highly correlated with rainfall-driven runoff (Giddings et al., 2005). Thus, fish, amphibians, and reptiles in these environments are likely exposed to atrazine for short durations with intervals of lesser or no exposure between applications. Assessment of these exposures requires consideration of their acute nature and the coincidence of periods of development in the organisms that can confer greater sensitivity. Exposures in lakes, reservoirs, ponds, and pools are likely different from exposures in flowing waters. The relatively long aqueous persistence of atrazine and the lack of flow in lentic systems result in longer exposures more consistent with the type used in chronic laboratory toxicity tests. In these situations, relative inputs will be similar to flowing water but dilutions will be less, and evaporation of water can even increase residue concentrations to values greater than initially present. Thus, exposures in pools and ephemeral ponds near agricultural lands where atrazine is used probably represent greatest exposure scenarios for fish, amphibians, and reptiles. In lakes and large reservoirs, dilution with uncontaminated waters from areas where atrazine is not used will likely result in a much smaller and narrower range of concentrations than that experienced in ponds and pools. However, these exposures will also be chronic in nature. Although ponds and wetland areas are important habitats for amphibians and some reptiles, few studies have reported atrazine concentrations in these types of surface waters. Two ponds receiving runoff from cornfields treated with 2.6 or 4.4 kg atrazine/ha (2–4 times greater than currently recommended label rates) have been studied (Klaasen and Kadoum, 1979). Concentrations from the field receiving the lesser rate ranged from 2 to 282 μg/L, and from 1 to 309 μg/L for the greater rate of application. Only nine sampling times were used in the study, and raw data were not available for distributional analysis. Concentrations of pesticides in a number of rural and farm ponds in southwestern Ontario were investigated between 1975 and 1981 (Frank et al., 1990). In total, 211 water samples from different ponds were analyzed. Some of these samples were from ponds known to be contaminated via spills or other accidents. Triazines were detected in 82 of 124 ponds studied; 73 were atrazine. From ponds where runoff and drift were the major routes of exposure, concentrations ranged from 0.1 to 57 μg/L. Concentrations ranged from 1.1 to 681 μg/L in the eight ponds where spills were known to have occurred. Again, raw data were not available for distributional analysis, and many of the pond water samples were likely submitted for analysis because of suspected contamination (Richard Frank, personal communication). This suggests that some samples may have been biased and not representative of typical exposure. Concentrations of atrazine have been measured in shallow depressions in fields treated with 2.24 kg/ha atrazine (Edwards et al., 1997). Rainfall was simulated with an irrigation device (between 1 and 32 d after application) and water was collected in surface depressions at 5 and 10 min intervals for the first 30 min after irrigation. Initial concentrations (t = 0) of atrazine measured in the no-till field were between 2000 μg/L and 10,000 μg/L. Concentrations decreased with time after irrigation as well as with time after application. The depressions in these fields were ephemeral (the water moved into the soil within hours) and are not representative of suitable habitats for fish or amphibians. These results were similar to those obtained by Baker and Laflen (1979) in studies on dissipation of pesticides from shallow depressions such as tractor ruts. Atrazine concentrations in wheel tracks were 9000 μg/L immediately after application. However, concentrations declined rapidly and were less than 50 μg/L within 100 min of application, presumably as a result of percolation into the soil. Risks to amphibians in these systems should be assessed in the context of their ephemeral nature, likely use by amphibians, and the importance of these habitats in relation to the entire landscape. An extensive characterization of exposure concentrations for atrazine in surface waters of North America has recently been undertaken (Giddings et al., 2005). These authors utilized four tiers of exposure characterization, including several levels of sophistication of models, as well as an extensive analysis of data sets of measured concentrations in surface waters. The models focused on ponds, flowing waters, and reservoirs, while most of the measured data sets were from small and large flowing waters, the Great Lakes, and reservoirs. For example, as reported in Giddings et al. (2005), cumulative probability distributions of annual maximum concentrations based on results of Tier-4 Monte Carlo modeling of 14,000 pond systems × 36 years of meteorology in Ohio (504,000 data points) yielded 90th centile concentrations of less than 10 μg/L. Similarly, the distribution of 30-d maximum concentrations for 14,000 pond systems × 36 years of meteorology × 12 months per year (6,048,000 data points) yielded smaller values (<2 μg/L). There are relatively few data on measured concentrations of atrazine in small water bodies such as farm ponds and almost no data on the temporal trends of these exposures. A study on temporal trends and exposures in an area of intensive atrazine use in South Africa revealed concentrations as great as 9 μg/L and considerable fluctuations in concentration in response to heavier than normal rainfall (Du Preez et al., 2005a). These data emphasize the need to characterize field exposures with due consideration for variation caused by rainfall-driven runoff events and loss through outflow. B. Mechanism of Action Atrazine is an herbicide developed specifically as a phytotoxin through a mechanism of action unique to plants; thus, the toxic potency of atrazine is greater in plants than animals. In target plants, atrazine inhibits photosynthesis via competition with plastoquinone II at its binding site in the process of electron transport in photosystem II (Devine et al., 1993). This inhibition ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES results in the cessation of carbohydrate synthesis, leading to a subsequent reduction in the carbon pool and a buildup of CO2 within the plant cell (Giddings et al., 2005). In plants, the binding of atrazine to the active site is reversible. Removal of the stressor from the site of action results in recovery (Jensen et al., 1977; Hoberg, 2007). When atrazine-exposed plants are removed to uncontaminated media, therefore, levels of photosynthetic activity increase (Brockway et al., 1984; Hamala and Kollig, 1985; Hoagland et al., 1993). If atrazine has effects in nonplant species, these effects must be mediated by other mechanisms; however, if used to control plants, the removal of habitat or food sources can have indirect effects on other organisms. Downloaded At: 15:43 28 October 2008 C. Bioconcentration/Bioaccumulation Bioconcentration and bioaccumulation of atrazine directly affect exposures and can result in exposures of organisms via the food chain. Based on bioconcentration factors (BCFs) and uptake data in the literature, atrazine bioconcentration and food chain biomagnification are negligible (Giddings et al., 2005). Its small octanol–water partition coefficient (log K OW = 2.68 at 25◦ C), relatively large water solubility, and susceptibility to biological metabolism and rapid elimination combine to produce small BCFs (generally less than 10) in most species tested (Giddings et al., 2005; Nikkilä et al., 2001). Consequently, exposure via the food chain is of lesser importance than via the water column. Several studies on toxicokinetics have been conducted in fish. Bioconcentration factors for atrazine in fish were summarized in Giddings et al. (2005) and are generally small, ranging from 12 for bluegill sunfish to <0.27 for brook trout. Some studies have observed uptake, metabolism, and excretion in fish. Rapid absorption of 14 C-atrazine was observed in whitefish (Gunkel and Streit, 1980). Absorption of atrazine into the embryos of zebrafish (Danio rerio) was also rapid and a BCF of 19 was observed (Wiegand et al., 2000). Metabolism in zebrafish was to the glutathione conjugate (Wiegand et al., 2001). The BCFs in various tissues (including gonads) of banded tilapia (Tilapia sparrmanii) were reported to be as great as 8.2 and appeared to be greatest in tissues with greater amounts of lipids. BCFs also increased with increasing exposure concentration (Du Preez and van Vuren, 1992). A terminal excretion half-life of whole-body radioactive residues of atrazine (and metabolites) in juvenile zebrafish of 21 h was reported (Görge and Nagel, 1990). In summary, atrazine is not greatly bioconcentrated in fish, is rapidly excreted and/or metabolized, and does not appear to be significantly accumulated in specific tissues. Three bioconcentration values have been reported for amphibians, 0 for the bull frog (Rana catesbeiana) (Klaasen and Kadoum, 1979), 6 for the leopard frogs (R. pipiens) larvae (Allran and Karasov, 2000), and 1.6 for atrazine and 4.4 for total body residues including atrazine and all its metabolites in metamorphs of the African clawed frog (Xenopus laevis) (Edginton and Rouleau, 2005). These are in the same range as fish. In an ar- 727 ticle, Hayes (2004) stated that the body doses of atrazine in frogs in his studies were 8-fold greater than those in other studies (such as Carr et al., 2003). This argument was based on the volume of the exposure tanks and the total amount of atrazine available for uptake by the frogs. Presumably, the estimate was based on the assumption that atrazine is greatly bioconcentrated in the frogs, a phenomenon that is inconsistent with the known large water solubility of atrazine and its measured small bioconcentration factors (BCF < 12) in several aquatic species, including frogs. In fact, contrary to what has been suggested (Hayes, 2004), the volume of the tanks and the density of larvae would have had little effect on the overall exposure of the individual larvae to atrazine (Edginton and Rouleau, 2005). As observed in fish, atrazine is rapidly metabolized in frogs (Edginton and Rouleau, 2005). In addition, autoradiography studies in X. laevis have not shown tissue-specific accumulation in Nieuwkoop & Faber (NF) stage 66 metamorphs (Nieuwkoop and Faber, 1967) in organs other than those associated with metabolism and excretion—the liver and the gut (Edginton and Rouleau, 2005). This shows that, within the body, large concentrations do not accumulate in gonadal tissues. III. ACUTE AND CHRONIC TOXICITY Based on traditional endpoints such as survival and growth, the toxicity of atrazine to aquatic organisms has been well studied and reported in the literature. These data were reviewed in Giddings et al. (2005), and this section summarizes some representative values and the more recent data for reptiles, fish, and amphibians. Because effect concentrations in the literature are reported in a variety of units, all concentrations in this review have been converted to micrograms per liter for ease of comparison. The potential mechanisms of acute toxic action of atrazine in organisms other than plants are not well understood but are not mediated through the same receptors as in plants. Atrazine is not very acutely toxic to aquatic animals. For amphibians, the smallest LC50 was reported to be 410 μg atrazine/L. Based on other nonlethal endpoints, the most sensitive endpoint reported in the literature was time to development in X. laevis with a lowest-observed-effects concentration (LOEC) of 100 μg/L. The relevance of developmental and other endpoints are discussed in greater detail in other sections of this review since they do not necessarily relate directly to adverse responses at the population level. In the context of risk assessment, the likelihood of acute and chronic toxicity values being exceeded under field conditions is small. A. Lethality and Physiological Effects The mechanism of action of atrazine in fully aquatic organisms is likely related to nonspecific narcosis (Lipnick, 1993), and, consistent with this mechanism, in chronic exposure studies with fish, the responses were reversible when exposure was removed (Whale et al., 1994). The toxicity of atrazine to fish has been presented in detail in two reviews (Solomon et al., Downloaded At: 15:43 28 October 2008 728 K. R. SOLOMON ET AL. 1996; Giddings et al., 2005). Acute toxicity values for freshwater fish ranged from a 96-h LC50 of 4300 μg/L for the guppy (Poecilia reticulata) to a 96-h LC50 of >100,000 μg/L for the carp (Carassius carassius) (Giddings et al., 2005). Chronic noobservable-effect concentration (NOEC) values for fish ranged from 65 μg/L for brook trout (Salvelinus fontinalis) to 4300 μg/L for channel catfish (Ictalurus punctatus) (Giddings et al., 2005). Other than the information in these reviews, no additional data on lethal effects of atrazine on fish have been reported in the literature. A number of studies have been conducted on the effects of atrazine on the survival of frogs. These report effects only at large concentrations (Allran and Karasov, 2000, 2001; Detenbeck et al., 1996; Howe et al., 1998; Hayes et al., 2002; and several others summarized in Giddings et al., 2005). Most of these studies were toxicity tests with lethality or growth as an endpoint and none have specifically addressed reproductive or endocrine endpoints. These results are summarized (Table 1) and the results of several studies pertinent to frogs are discussed in more detail later. The overall conclusion is that atrazine concentrations found to have adverse effects in amphibian embryos and adults were considerably greater than exposure concentrations currently found or predicted in surface waters in North America (Giddings et al., 2005). It is evident that direct toxicity of atrazine is probably not a significant factor in recent amphibian declines (also see Allran and Karasov, 2001). Studies using the Frog Embryo Teratogenic Assay Xenopus (FETAX) assay suggest that atrazine has effects on embryonic and early postembryonic development only at large concentrations. Embryotoxicity and teratogenicity to X. laevis embryos occur only at concentrations approaching maximum solubility in water (30,000 μg/L, Morgan et al., 1996). In studies on wildcollected R. pipiens and the American toad (B. americanus) larvae raised in the laboratory to Gosner stage 29 and 40, the 96-h LC50s for atrazine in the two stages of tadpoles of R. pipiens were found to be 47,500 and 14,500 μg/L, respectively, while those for B. americanus were 26,500 and 10,700 μg/L, respectively (Howe et al., 1998). The authors used a commercial formulation of atrazine (4L) but confirmed exposures through immunoassay to be within 10% of nominal concentrations. The authors also reported that alachlor (CAS number 15972-60-8) and atrazine appeared to act synergistically (more than additive toxicity) when present in a 50:50 mixture; however, they used formulated product in their assays and the concentrations where effects were observed were greater than those that would be expected in surface waters. The authors also estimated chronic no-observable-effect concentrations (NOECs) from their acute data. They suggested that these concentrations (690 to 5100 μg/L) can occur in ponds and pools exposed to runoff from atrazine-treated fields; however, from the modeling conducted by Giddings et al. (2005) these concentrations would be extremely rare in farm ponds and have not been observed in field studies (Du Preez et al., 2005a; McDaniel et al., 2008; Smith, 2007 personal communication). It has been reported that atrazine exposures of 20 and 200 μg/L had no effect on development rate, percent metamorphosis, time to metamorphosis, percent survival, mass at metamorphosis, or hematocrit in R. pipiens larvae when exposed from first feeding (Gosner stage 25) through metamorphosis in a laboratory study (Allran and Karasov, 2000). Measured concentrations in the atrazine exposure solutions were 19.2 ± 0.3 and 192 ± 4.2 μg/L. In the same study, the authors assessed the effects of nitrate at 5, and 30 mg NO3 (as nitrogen)/L and found a statistically significant decrease in growth rate of larvae; however, they suggested that this effect of nitrate was probably not biologically important when compared with natural variation in the environment. Their conclusion was that concentrations of atrazine and nitrate commonly found in the environment do not appear to pose a significant threat to R. pipiens larvae through direct toxicity. In a subsequent study, the same authors reported that concentrations of atrazine as large as 20,000 μg/L did not affect hatchability of embryos or 96-h posthatch mortality of larvae of R. pipiens, the wood frog (R. sylvatica), or B. americanus (Allran and Karasov, 2001). Atrazine also had no effect on swimming speed in R. pipiens. However, the authors reported that there was a concentration-dependent increase in deformed larvae of all three species with increasing atrazine concentration (NOEC = 2590 μg/L). In adult R. pipiens exposed to atrazine, buccal and thoracic ventilation rates were greater than in untreated frogs, which indicated respiratory distress. The NOECs for these responses were 4320 and 12,000 μg/L, respectively. Frogs exposed to the greatest atrazine concentrations (>12,000 μg/L) stopped eating immediately after treatment began and did not eat during the 14-d experiment. Disruption of organ development was reported in larvae of X. laevis (NF stage 47) exposed to concentrations ≥10,000 μg/L (Lenkowski et al., 2008). A study of the effects of atrazine on the development of the streamside salamander (Ambystoma barbouri) found effects on survival, size at metamorphosis, and behavior (Rohr et al., 2004). Animals from field-collected eggs were exposed to atrazine in two studies conducted over 2 years in the laboratory. In the first year, treatments of atrazine were applied by dissolving 80% pure technical material in dimethyl sulfoxide (DMSO), while in the second year, solutions of 99% pure technical material from a different source were applied in acetone. Exposure solutions were changed every week; however, from the methods (“The aquaria . . . . contained 9.5 L of constantly aerated, charcoalfiltered, dechlorinated municipal water (pH 8, 158C),” it appeared that charcoal filters were used in the tanks. Although the authors described analyses of exposure solutions only in the first year of the study, these data were not reported in the paper. Since charcoal will absorb atrazine (U.S. EPA, 1989), this may have affected exposure concentrations; however, the extent of this is not known and may seriously have compromised the study. In analyzing the data, the authors combined the data from the 2 years and presented the results as year-standardized means using Z -scores (Rohr et al., 2004). Although this may have been a statistically valid approach, the facts that different sources of 729 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES TABLE 1 Acute and chronic toxicity data for atrazine in amphibians Effect measure (μg/L) Species NOEC LOEC LC50 Downloaded At: 15:43 28 October 2008 Bufo americanus B. americanus (early life stage) B. americanus (later life stage) Comment 86 d >400 A. barbouri larvae A. barbouri Pleurodeles walti Duration 250 μg/L reduced growth (1) 37 d No effects on survival of embryos at 4, 40, and 400 μg/L but a greater response to noise/vibration in animals exposed to 400 μg/L atrazine (2). 47 and 56 d Decreased survival in one year but not another. Reduced size at metamorphosis. Ambystoma tigrinum A. barbouri (embryos) EC50 400 >300 ≥4 12 d 300 μg/L did not alter erythrocytes (1, 2) (1) (3) 1,900 >48,000 26,500 8d 96 h 690 10,700 30 d 96 h Reference (Larson et al.; 1998) (Rohr et al.; 2003) (Rohr et al.; 2004) (Fernandez et al.; 1993, L’Haridon et al.; 1993) (Birge et al.; 1983) (Howe et al.; 1998) (Howe et al.; 1998) 30 d B. americanus Hyla versicolor 20,000 Hatching 2,590 20 to 200 200 to 2000 H. versicolor Rana catesbeiana R. catesbeiana 2000 410 28 d 8d 24 h Deformities Based on mass and length at metamorphosis Mortality 4,810 μg/L damaged DNA (1, 2) (3) R. pipiens (early life stage) 5,100 47,000 96 h R. pipiens (later life stage) 650 14,500 30 d 96 h R. pipiens 200 30 d 138 d Growth 20,000 10 d Hatching R. pipiens, R. sylvatica 2,590 (Allran and Karasov 2001) (Diana et al.; 2000) (Mazanti et al.; 2003) (Birge et al.; 1983) (Clements et al.; 1997) (Howe et al.; 1998) (Howe et al.; 1998) (Allran and Karasov 2000) (Allran and Karasov 2001) Deformities (Continued on next page) 730 K. R. SOLOMON ET AL. TABLE 1 Acute and chronic toxicity data for atrazine in amphibians (Continued) Effect measure (μg/L) Species NOEC LOEC LC50 Duration Comment R. pipiens adults 4,320 14 d Buccal ventilation Xenopus laevis 12,000 12,000 800 14 d 14 d 35 d Thoracic ventilation Feeding Lethality 100 21 d Uptake of propidium iodide in nuclei from exposed larvae. Time to development Malformations reported at low water hardness (4) Malformations reported at high water hardness Buffer solution (5) Natural water Buffer solution Natural water Buffer solution Natural water 800 X. laevis Downloaded At: 15:43 28 October 2008 EC50 X. laevis 30 28 d 96 h 100 96 h 33,000 <8,000 96 h 100,000 126,000 3,030 1,100 Reference (Allran and Karasov 2001) (Freeman and Rayburn 2005) (Napier et al.; 1998) (Morgan et al.; 1996) Note. (1) Standard toxicity value not reported (i.e., LC50 and EC50 values for acute studies generally 96 h or less in duration and NOEC, LOEC, MATC/chronic values for chronic studies exceeding 10 d in duration). (2) Nonstandard measurement endpoint not clearly related to survival, growth, and reproduction was used (i.e., enzyme activity, blood parameters). (3) Toxicity value from a more sensitive life stage was used. (4) May have been affected by lack of calcium in the medium. (5) A smaller toxicity value from a low water hardness condition was used. atrazine were used in the two studies and that concentrations were not reported raise serious questions about the analysis of the results and whether the reported effects were related to exposure or not. Absolute differences in the percent hatch, survival until d 16, day of hatching, percent larvae in refuge, mean day of metamorphosis, and snout–vent length at metamorphosis (author Table 1) were not large and are of questionable biological significance in view of the potential problems in the study design. In a follow-up study, newly metamorphosed A. barbouri, exposed to atrazine during larval development as described in the second year above, were followed for a further 410–433 d after metamorphosis (Rohr et al., 2006). Animals were transferred to terreria at metamorphosis and received no further exposures to atrazine. Results, presented as standardized means and Z scores, indicated statistically significant reductions in survival (increased mortality) at all exposure concentrations and with an apparent relationship to exposure concentrations (author Figure 2D). Actual mortality values were not presented so are difficult to interpret in terms of population-level effects. The interpreta- tion of the responses by regression of transformed data (log x+ 2) was also in error since the control, with zero nominal concentration, cannot be made proportional to the other nominal concentrations and should only have been used as a reference point. In addition, this study is based on a previous study (Rohr et al., 2004) where no measurements of exposure were made and where the presence of activated charcoal in the system may have affected exposures. Because of errors in design, these results cannot be interpreted and the conclusions are speculative at best. EC50 values based on malformations in the FETAX assay were reported to occur 30 μg/L (meeting poster Napier et al., 1998) in low-hardness water and may have been confounded by lack of calcium in the medium, a necessary element for normal development in X. laevis (ASTM, 1992). Since detailed descriptions of these data have not been published, these effects cannot be interpreted. An assay was developed with X. laevis in which flow cytometric histograms of developing larvae were observed to be representative of developmental stage when native (unfixed) nuclei Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES were analyzed (Freeman and Rayburn, 2004). Using these techniques, studies of the effects of atrazine (technical) on nuclei of X. laevis exposed for 35 d showed no statistical differences at measured concentrations ≤600 μg/L but the authors did observe effects at 800 μg/L (Freeman and Rayburn, 2005). The biological significance of these observations is difficult to determine in terms of survival or development but the authors did observe longer development times in larvae exposed for 28 d to 100 μg atrazine/L. A study of the effects of exposures to a commercial formulation of atrazine reported on the development of tadpoles of spring peepers (Pseudacris crucifer), American toad (B. americanus), green frogs (Rana clamitans), and wood frogs (R. sylvatica) (Storrs and Kiesecker, 2004). Tadpoles were exposed to concentrations of atrazine prepared from AAtrex Nine-O (85.5% atrazine) at nominal concentrations of 3, 30, and 100 μg/L for 30 d. Based on concentrations measured in two samples, exposures were 2.84 ± 0.05 μg/L, 25.20 ± 1.82 μg/L, and 64.80 ± 2.88 μg/L (mean ± SD). The authors reported “counterintuitive” responses in that survivorship (across species and stage) in frogs was less (20%) at the nominal concentration of 3 μg/L, compared to 50% at 30 and 100 μg/L and 15% in the control. Although endocrine-mediated responses were not specifically measured in this study, the authors invoked the concept of the nonmonotonic concentration-response curve reported by others for some endocrine-mediated effects to explain their results. Given the lack of concordance with observations in other studies, other factors such as the presence of unknown stressors and/or confounders are more likely explanations. Preliminary results of a laboratory study on the effects of exposures of R. pipiens larvae (Gosner stage 25 to 42) to concentrations of 0.1 and 1.8 μg atrazine/L (as AAtrex Liquid 480) reported an exposuredependent reduction in rate of development and size of the ovary (poster by Fridgen et al., 2005); however, this study may have been confounded by the use of formulated product containing surfactants and the results are not yet published. A study on R. pipiens reported a significant decrease in weight and snout-vent length in animals exposed to 0.1 μg atrazine/L from Gosner stage 21 to metamorphosis (Hayes et al., 2006a). However, only one concentration was tested, and the only control used was for solvent. In addition, the differences in size and weight were small (<10%). Other laboratory studies investigating endocrine responses in frogs at concentrations ranging from 0.1 to 100 μg atrazine/L have not reported adverse effects on larval growth, developmental rate, mortality, time to metamorphosis, or size at metamorphosis in female or male frogs (Hayes et al., 2002; Carr et al., 2003; Coady et al., 2004, 2005; Kloas et al., 2008). All of these data suggest that, at environmentally realistic exposures, atrazine does not cause lethality during larval development and/or metamorphosis and that it does not affect size at metamorphosis. Specific studies on the toxicity and non-reproductive developmental effects of atrazine in reptiles were not found in the literature. The effects reported in alligators in Lake Apopka, 731 Florida (Guillette et al., 1994, 1996) have not been linked to atrazine exposures (Crain et al., 1997, 1999). IV. EXTERNAL DEVELOPMENTAL ABNORMALITIES A number of studies have investigated the possible causes of deformities in frogs and there is no evidence that synthetic chemicals were directly responsible. While a number of other possible causes have been suggested, parasitism has been identified as a major factor (Blaustein and Johnson, 2003; Sessions et al., 1999; Johnson et al., 1999). Some studies on the effects of pesticides on limb deformities in frogs have been reported in the literature but there is a need to clarify the contribution of changes in habitat structure and function, parasite density, and the frequency of dysmorphogenesis in amphibians under field conditions. A. Trematode Infections Cercariae of trematode parasites induced severe limb abnormalities in the Pacific tree frog (Hyla regilla) in laboratory studies (Johnson et al., 1999). These abnormalities were reported to closely match those observed in the field. In addition, increased parasite density caused an increase in the frequency of abnormalities with an associated decline in tadpole survivorship (Johnson et al., 1999). More recently, a study on trematode infections in R. sylvatica was conducted to assess the potential effects of pesticides, including atrazine on the induction of limb deformities caused by trematode infection (Kiesecker, 2002). The author concluded that some pesticides, including atrazine, could increase the susceptibility of R. sylvatica to parasite infection, which could lead to a greater incidence of limb deformities. However, several key deficiencies in the experimental design of this study make its interpretation difficult. In the field component of the study, tadpoles of R. sylvatica were exposed to the cercaria (the mobile infective stage) of the trematode parasite Ribeiroia sp. in mesh enclosures placed in ponds close to agriculture and ponds remote from agriculture. More frogs from the agricultural ponds developed limb deformities than did frogs from nonagricultural sites. Snail and parasite densities in the ponds were reported in the paper but no measures of cercarial density inside the enclosures were reported. Thus, it is not certain what the exposures to cercaria inside the enclosures were and if they were the same between sites. Unfortunately, the infection rates in the field-exposed frogs were not reported. Since not all meta-cercarial cysts result in limb abnormalities, the number of cysts may have been a better index of interactions between exposure to agricultural runoff and infectivity. Although more frogs exposed to cercaria at the agricultural sites developed limb deformities, the lack of information on number of meta-cercarial cysts makes it difficult to know whether, in general, this was the result of greater infection rates or more infections in the limb buds of tadpoles at these sites. Water samples from the ponds were analyzed for pesticide residues; however, the exact identity of the pesticides was not stated except that “both organochlorine pesticides and Downloaded At: 15:43 28 October 2008 732 K. R. SOLOMON ET AL. organophosphorus compounds (e.g., atrazine and malathion) were detected” (Kiesecker, 2002). The detection limits of the method, and measured concentrations were not reported. It also is uncertain what changes in concentration occurred over the course of the exposure period. Thus, it is impossible to use the results of these studies to make any conclusions about the potential effects of atrazine on immune function or the effect on the rate of infection with trematodes. Analyses for other potential confounding factors such as other agricultural chemicals, including other classes of insecticides, herbicides, and fungicides, heavy metals, nutrients such as nitrate/nitrite and phosphate, or ultraviolet (UV) light that are known to affect frog development (Ankley et al., 2002; Peterson et al., 2002; Diamond et al., 2002) were not reported. The omission of these particular measurements is unfortunate since the author cites each of these factors as possible co-factors that are related to population declines of amphibians (Kiesecker, 2002). The temperatures of the ponds were not reported. Since temperature has an effect on amphibian development, this also may have confounded the comparisons between ponds. The ability to make conclusions about the possible interactions between atrazine and trematode infections based on the laboratory component of this study (Kiesecker, 2002) was also limited. The laboratory study used three model pesticides, atrazine, malathion, and es-fenvalerate. The nominal exposure concentrations for atrazine were 3 and 30 μg/L. Tadpoles (presumably Gosner stage 25) were exposed to the pesticides for 4 weeks. Exposure solutions were replaced every 2 d and tadpoles were fed ad libitum. Measurements of atrazine concentrations in the exposure solutions were not made. After 4 weeks, the tadpoles were individually exposed to 50 cercaria in a test chamber for 4 h. Tadpoles were then placed in uncontaminated water for 1 week, after which a blood sample was taken to enumerate eosinophils (the type of white blood cell responsible for cellmediated immune response) and the frogs were then prepared for determination of the number of cercaria that had successfully encysted to form meta-cercaria. The author reported that meta-cercarial infection rates for two trematodes (Telorchis sp. and Riberiroia sp.) in the atrazineexposed frogs were greater than in the solvent controls and that number of eosinophils were less in the exposed frogs. The implications of these observations on the possible immunotoxic effects of atrazine are discussed later, in the section on immunotoxicology. The number of eosinophils appeared to be inversely correlated with the proportion of cercaria that formed metacercarial cysts and also with pesticide exposure. But, since only two concentrations were tested, a concentration-response relationship could not be developed, thus limiting the interpretation of the results. More recent reports on the effects of atrazine (AAtrex, 40.8% active atrazine) on the infectivity of cercaria in frogs have reported negative or no effects. In a study of the effects of atrazine on the infectivity of cercaria of the trematode parasite Echinostoma trivolvis in R. clamitans, it was reported that 200 μg atrazine/L caused increased mortality of cercaria and that concentrations of 20 or 200 μg/L decreased infectivity in tadpoles of R. clamitans (Koprivnikar et al., 2006). The exposures were not reported to have adverse effects on the tadpoles (Gosner stage 25/26; 20 tadpoles per treatment). Toxicity values for cercaria of several other species of trematode were also reported and 12-h LC50s ranged from <20 μg/L for Haematoloechus sp., 92 μg/L for Alaria sp., 110 μg/L for E. trivolvis, to >850 μg/L for Megalodiscus sp. (Koprivnikar et al., 2006). Unfortunately, concentrations in the exposure solutions were not verified by analysis. In a subsequent paper by the same authors on the infectivity of cercaria of E. trivolvis in tadpoles of R. sylvatica, different responses from those in R. clamitans were reported (Koprivnikar et al., 2007). Using the same formulated product also without verification of exposure concentration, there was no effect on intensity of parasitism when both cercaria and Gosner stage 28/29 tadpoles were exposed to 3 or 30 μg/L atrazine. However, if only the tadpoles were exposed to 30 μg/L (but not 3 μg/L), intensity of infection increased by a factor of approximately 2. Given the differences in response reported in these studies and the relatively small number of tadpoles per replicate (21 per treatment), these results are difficult to interpret but neither study showed increased parasitism when both tadpoles and cercaria were exposed to atrazine, which is what would occur under field conditions. The responses observed may have been due to formulants in the commercial product used (AAtrex, 40.8% active ingredient) and thus may only be representative of direct contamination of frog habitats, such as from spray drift. Under most conditions of exposure in the field, atrazine and its formulants would be expected to not co-occur. Regardless of the reasons, the results of the study do not appear to be internally consistent or reproducible and are not ecologically relevant. Several authors have pointed out that anthropogenic activities can result in changes in the physical and biological properties of water bodies. Some of these changes, such as eutrophication, can increase the prevalence of both the snail hosts and trematode parasites that they carry (Johnson et al., 2002; Kiesecker, 2002; Blaustein and Johnson, 2003). Eutrophication itself has not been shown to affect survival of tadpoles of R. sylvatica infected with cercaria of E. trivolvis (Belden, 2006), but infectivity of cercaria of the trematode parasite Ribeiroia ondatrae in tadpoles of the green frog (R. clamitans) was increased by eutrophication. The mechanism by which eutrophication promoted parasitism was via increasing the density of infected snail hosts and enhancing per-snail production of cercaria (Johnson et al., 2007). This potentially confounding variable was not addressed in the field study by Kiesecker (2002). B. Direct Effects of Atrazine on Limb Deformities In another study on limb deformities, exposures to a mixture of atrazine and carbaryl were included as treatments in a study of R. pipiens; however, no responses were observed (Bridges et al., 2004). A mixture of 5 μg/L atrazine and 5 μg/L carbaryl did not affect the incidence of embryonic deformities, hatching Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES success, survival to metamorphosis, tadpole mass, bony triangles, skin webbing, or multiple deformities. Although only one concentration of atrazine (in a mixture) was tested with this species, the results are consistent with the lack of effect seen in the FETAX embryonic frog development assay at greater concentrations (1100 to 26,000 μg/L) (Morgan et al., 1996). In contrast, extracts of water from a reference pond in Minnesota were associated with a small incidence (5%) of deformities, indicating that other environmental contaminants or factors are potentially responsible for the observed abnormalities noted under field conditions (Bridges et al., 2004). Overall, there is no credible evidence to suggest that atrazine directly causes or contributes to limb deformities caused by parasites in frogs. However, it has been shown that activities that increase populations of the snail vectors of trematode parasites increase parasite pressure and increase the frequency of deformities in frogs by increasing populations of snail intermediate hosts and increasing production of cercaria. Concentrations of atrazine in ponds from runoff in agricultural areas are unlikely to exceed thresholds for effects in macrophytes (Giddings et al., 2005) and thus cause eutrophication. However, deliberate (offlabel) use to control weeds in ponds could affect eutrophication. This would also occur if other herbicides were used and is not a specific response to atrazine. Changes in habitat have the potential to induce dysmorphogenesis in amphibians. Loss of habitat has impacted amphibians for decades. In addition, UV-B irradiation, emerging diseases, the introduction of alien species, direct exploitation, changes in land use, and climate change (Beebee and Griffiths, 2005) can potentially interact and contribute to abnormalities that are observed under field conditions. Agricultural activity, including the use of pesticides, can contribute to eutrophication of surface waters; however, this is not specific to particular pesticides and would only occur at concentrations that directly affect aquatic plants. The likelihood of this occurring is judged to be small unless the products are specifically used for weed control in ponds. SEXUAL DIFFERENTIATION AND DEVELOPMENT Much of the current research investigating the risks posed to wildlife by exposure to atrazine has focused on the potential for adverse effects on reproduction and sexual development. This concern is justified given the paramount role of reproduction in population sustainability and reports that atrazine, at relatively high concentrations, negatively affects reproduction in the laboratory rodents and may disrupt reproductive endocrine function in mammals (Cooper et al., 2000, 2007; IPCS, 2002). Investigation of the reproductive responses is a broad topic encompassing sexual differentiation, gonadal development, and studies of mechanisms of reproductive dysfunction, through to actions on sex-dependent processes. Investigations have at times been hampered by a limited knowledge of reproductive and developmental processes in wildlife. While this is most evident in the case of species in the wild, it has become apparent that there are significant gaps in our knowledge of these processes in species 733 such as X. laevis despite it having being used a laboratory model species for over 70 years (OECD, 2004). A. The Use of Xenopus laevis as a Model for Endocrine Responses While X. laevis is a popular and well-studied model for examining the effects of chemicals on sexual differentiation, recent tests have shown that husbandry conditions may well have a marked effect on the sensitivity of amphibian species to chemicals (Kloas et al., 2008). It is also used to study several endocrine responses, such as the control of metamorphosis by thyroxine. In this regard, it has been suggested as the test organism for assessing sensitivity to chemicals that interact with the thyroid axis (OECD, 2004). While some of its popularity stems from its ease of breeding and maintenance in the laboratory, that fact that it is exclusively aquatic makes exposures more easily controlled than other amphibians that are terrestrial in the adult stage. Ease of use does not mean that the species is very sensitive—a necessary attribute for a sentinel species—and this question has been raised with respect to responses to steroid hormones. Compared to other amphibians, X. laevis is relatively sensitive to estradiol. Using the least concentration of water-borne exposures to estradiol that caused 100% feminization of developing frog larvae (Hayes, 1998) and urodeles (Wallace et al., 1999) that had been reported in the literature, a species sensitivity distribution was constructed using published methods (Solomon and Takacs, 2002). From these data (Fig. 4) it is apparent that, although not the most sensitive species to estradiol, X. laevis is close to the 20th centile for sensitivity in static exposures and V. FIG. 4. Distributional analysis of sensitivity of sexual differentiation in larval frogs (•) and urodeles (◦) exposed to estradiol in static water-borne test systems. Data for urodeles were excluded from the regression. Data from Hayes (1998), Wallace et al. (1999), Kloas et al. (2008), and Lutz et al. (2008). 734 K. R. SOLOMON ET AL. is only two-fold less sensitive than the most sensitive species, R. sylvatica. Sensitivity of X. laevis in flow-through exposures was greater (Kloas et al., 2008), probably because of the greater body dose resulting from this exposure method, but this is the only species to be tested in this way. This observation is important since it points out that flow-through protocols should be used when assessing possible effects of hydrophobic endocrineactive substances, such as estradiol. Thus, we conclude that X. laevis is a useful and sensitive model for affects of estradiol and that ease of exposure via the matrix makes it a useful sentinel animal for assessment of steroid and thyroid-mediated effects, provided that this is done under flow-through conditions. Downloaded At: 15:43 28 October 2008 B. Effects on Sex Ratio Effects of atrazine exposures on determination of gender have not been studied in fish or terrestrial wildlife species; however, atrazine does not appear to affect sex ratios in mammals, reptiles, or frogs. No effects on sex ratios in mammals were reported in multigeneration studies (U.S. EPA, 2000, Gammon et al., 2005). It has been reported that atrazine exposures did not result in any adverse effects on developing alligators (Alligator mississippiensis) either in the laboratory or under field conditions (Crain et al., 1997, 1999). In fact, the authors of those studies concluded that atrazine did not affect sexual development in A. mississippiensis hatchlings and was not responsible for effects on sexual development observed in wild A. mississippiensis. Treatment of A. mississippiensis eggs with atrazine at concentrations as great as 14,000 μg/kg applied to the surface of the egg in ethanol did not alter the sex ratio of hatchlings, nor did it alter the height of epithelial cells in the Müllerian duct, affect degeneration of the ovarian medulla, or the diameter of the sex-cord (Crain et al., 1999). All of these parameters responded to estradiol exposure as a positive control. A study on the effect of several pesticides on the eggs of the broad-snouted caiman (Caiman latirostris) reported no effects on sex ratio in eggs treated topically with 15 μg/egg dissolved in 50 μl ethanol (Beldomenico et al., 2007). This is equivalent to ∼178 μg/kg, given an egg weight of ∼84 g as has been reported in the literature (Groombridge, 1982). Treatment with estradiol (105 μg/egg; ∼1250 μg/kg) resulted in 100% females. Treatment of eggs with atrazine or several other pesticides resulted in greater loss of weight during incubation when compared to the vehicle control (11.3% for atrazine vs. 8.6%) and decreased weight of hatchling (as percent of initial weight 65.1% vs. 67%). Given the fact that weights were determined to the nearest gram, the small differences were close to the limits of detection and raise questions about possible measurement errors. In addition, the physiological significance of these differences in weight was not tested in the study (Beldomenico et al., 2007). Other studies on eggs of red-eared slider turtle (Pseudemys elegans) and A. mississippiensis showed no responses in terms of sex ratio to nominal aqueous exposures of as great as 500 μg atrazine/L used to drench the eggs (Gross, 1999a, 1999b). Although topical treatment of eggs has been shown to give inconsistent penetration into the egg (Muller et al., 2007a, 2007b), this is a more realistic route of environmental exposure for a water soluble substance, such as atrazine. The lack of response confirms the lack of effect of atrazine in the development and sexual differentiation of reptiles such as turtles and alligators under realistic exposure conditions. A study by Suzawa and Ingraham (2008) reported that zebrafish (Danio rerio) exposed to atrazine for 60 d at concentrations of 0.1, 1, or 10 μM (21.7, 217, 2167 μg/L) beginning on posthatch d 17 displayed a dose-dependent increase in the percentage of females and a decrease in the percentage of males. The data for sex ratios are unclear in author Figure 2A (Suzawa and Ingraham, 2008). As the fish were not sexually differentiated at the beginning of the study, the authors could not know how many males and females there were in each treatment. However, given a sex ratio of 50:50, 7 or 8 of the 15 fish used per concentration would be expected to be females. Author Figure 2A shows that females increased in proportion by 400%, a 4-fold increase relative to the control. If there were 7 or 8 females at the start of the exposure, a 4-fold increase would mean there were 28–32 females in each beaker by the end of the exposure. This is impossible and is inconsistent with the decrease in number of males (50% ≈ 4 fish) reported at 2167 μg/L, which could only have produced 4 sex-changed fish. These data are uninterpretable and, in addition, the experimental design was flawed as the treatment unit was the tank and there was only one replicate. Few effects on sex ratio have been reported in frogs. Laboratory studies on R. clamitans exposed to atrazine at concentrations as great as 25 μg/L (Coady et al., 2004) and X. laevis at concentrations as great as 100 μg/L did not reveal any effects on sex ratio (Coady et al., 2005; Kloas et al., 2008). Studies in wild populations of X. laevis in areas of corn production and atrazine use and in reference areas did not reveal effects on sex ratios of adults or metamorphs (Du Preez et al., 2005b); sex ratios were near 50:50. Similar results were observed in a field study on R. catesbeiana in Iowa (Smith, 2007 personal communication). No effects on sex ratio were observed in NF stage 66 metamorphs in studies on X. laevis larvae exposed to atrazine at concentrations as great as 30 μg/L in outdoor microcosms, (Jooste et al., 2005). No transgenerational effects on sex ratio were observed in F2 larvae of F1 X. laevis exposed to concentrations of atrazine as great as 25 μg/L from 96 h of age to breeding (Du Preez et al., 2008b). One study reported an effect on sex ratio in X. laevis at concentrations with significantly more females at 10 and 100 μg/L but not at 0.1 and 1 μg/L (Oka et al., 2008). Because of high mortality at100 μg/L, the analysis was based on only one replicate of 30 animals. The reason for this is not clear. Aromatase mRNA was not induced at any exposure concentration, nor was there any evidence of estrogenicity based a hepatic vitellogenin assay. Sex ratio in all ZZ males was unaffected at exposures of 0.1 and 1 μg atrazine/L; however, greater concentrations were not tested. C. Effects on Sexual Development If atrazine were to affect maturation of the gonads, this would be manifested in terms of a quantitative difference in the ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES FIG. 5. Proportion of different sex cell types in the testis of C. auratus exposed to 100 and 1000 μg/L atrazine at 0, 7, 14 and 21 d. Redrawn from data of Spanó et al. (2004). Downloaded At: 15:43 28 October 2008 distribution of cell types within the testis and ovary, through effects on sperm production, or fecundity. There is no evidence to support the contention that atrazine affects sexual development in fish or amphibians. Testicular Cell Types and Gonadal Development In goldfish exposed to relatively large concentrations of atrazine (100 and 1000 μg/L) for 21 d, there were no differences in the relative size, number of sperm, or the relative proportions of each cell type cell type (Fig. 5) (Spanó et al., 2004). A number of measures of testicular development were made in a study of fathead minnows (Pimephales promelas) exposed to measured concentrations of 224 and 25 μg atrazine/L for 21 d (U.S. EPA, 2005). Males in the control group and the two atrazine-treated groups had well-developed testes and there was no difference in the mean stage of development between treatments. There were slight differences in the proportion of testis cells in stage 2A (primary spermatogonia) between treatments, varying from 0% in controls to 0.3 and 0.5% of the testis cells in the 25 and 224 μg atrazine/L groups, respectively. Mean seminiferous tubule diameter in males from the greatest atrazine exposure group was smaller than those of males in the control group (123.8 versus 153.8 μm). However, the biological relevance of these histological changes has been questioned considering that no other abnormalities were reported (U.S. EPA, 2005). The lack of effect on vitellogenin, which is a sensitive indicator of exposure to estrogen, suggests that atrazine was neither directly nor indirectly estrogenic. There were no differences in vitellogenin concentrations between fish exposed to either atrazine concentration or that of control fish for either males or females (U.S. EPA, 2005). Overall, this study suggests that atrazine at exposures as great as 224 μg/L had no significant effects on important reproductive endpoints in P. promelas. It is important to place these results in the context that this was a robust and well-characterized bioassay procedure that has been used to identify the adverse effects of a variety of endocrine active compounds. 735 No differences in the absolute or relative numbers of testicular cell types were observed in X. laevis from corn and non-corngrowing areas in South Africa where atrazine concentrations ranged from 0 to 9 μg/L (Smith et al., 2005). This included an assessment of the fractional volume of the testis occupied by spermatagonia, spermatocytes, sperm, connective tissues, and blood cells. All frogs appeared normal when evaluated histologically and fully developed sperm were observed in all frogs (Smith et al., 2005). Similar results were obtained in a field study on bull frog (Rana catesbeiana) in agricultural and nonagricultural areas in Iowa (Smith, 2007 personal communication). Atrazine concentrations ranged from less than the limit of detection (LOD) to 40 μg/L in the agricultural area but were consistently less than the LOD in the nonagricultural areas. In a study on R. pipiens from areas associated with row crop agriculture (and where atrazine and other pesticides were found), no differences were detected in the gonadosomatic indices or stage of spermatogenesis between frogs from agricultural and nonagricultural regions ( p > .05) (McDaniel et al., 2008). In a study of R. pipiens metamorphs exposed to 15 μg atrazine/L throughout metamorphosis, Orton et al. (2006) reported no difference in the total number of spermatogenic cells. However, they did report an increase in the percentage of testicular cells in the latter stages of spermatogenesis relative to controls (38% vs. 20% in the controls). The biological significance of this effect is unclear since there were no other effects of atrazine on testicular development, and only one atrazine concentration was tested. A series of laboratory studies on the effects of atrazine on gonadal and kidney development in male and female X. laevis tadpoles were reported in a thesis and two published papers (Tavera-Mendoza 2001; Tavera-Mendoza et al., 2002a, 2002b). After quantitatively assessing testicular volume, primary spermatogonial cell nests, and nursing cells from histological sections, they reported a 57% reduction in testicular volume in tadpoles exposed to atrazine at 18 μg/L relative to controls (TaveraMendoza et al., 2002a). Similarly, primary spermatogonial cell nests were reported to be 70% fewer than in unexposed individuals, while the nursing cells that provide the nutritive support for development of germ cells were reported to be 74% fewer (Tavera-Mendoza et al., 2002a). Unfortunately, the data in the published papers and in the thesis are inconsistent. For example, the numbers of animals used in the study were not clearly reported and are different in the description of the methods and the figure captions. In the Tavera-Mendoza thesis (2001), it is stated that six tanks were used with 15 tadpoles each; however, in the published paper (Tavera-Mendoza et al., 2002a), it is stated that two control tanks and two exposed tanks were used, each with 24 tadpoles. In another experiment, more tadpoles were sampled than were initially stated to be present in the tanks. Responses at greater concentrations may have been confounded by general necrosis observed in several tissues of exposed tadpoles (Tavera-Mendoza, 2001). The cause of the necrosis was not clear. The authors reported that testicular volume was reduced in atrazine-exposed tadpoles but they did not make measurements 736 K. R. SOLOMON ET AL. Downloaded At: 15:43 28 October 2008 of testicular volume in a subsample of animals before exposure to atrazine. Furthermore, the responses reported in the testes were inconsistent with those reported elsewhere (Hayes et al., 2001; Coady et al., 2005; Carr et al., 2003). Because of incomplete descriptions in the methods and inconsistencies in the data, the results in these papers (Tavera-Mendoza et al., 2002a, 2002b) and the thesis (Tavera-Mendoza, 2001) are essentially uninterpretable and cannot be cited as supporting any adverse effects of atrazine on gonadal development in frogs. Intersex Testis and Testicular Ovarian Follicles It has been known for many yrs that exposure of developing X. laevis to estradiol causes sex reversal in genetic males, with males developing ovaries instead of testes. In some cases, estradiol-exposed animals can exhibit gonads that are intermediate in appearance between testes and ovaries (Chang and Witschi, 1956), a condition that has been variously referred to as hermaphroditic or intersex gonads (see Hecker et al., 2006, for a review of the terminology). Given that gonadal differentiation in X. laevis has been reported to be sensitive to several types of aquatic contaminants ranging from PCBs (Qin et al., 2003) to bisphenol A (Levy et al., 2004), and alkylphenols (Bögi et al., 2003), it is logical that several studies have focused on this model to examine the potential effects of atrazine on gonadal development. Laboratory Studies. The first study to report on this response stated that atrazine concentrations ranging from 0.1 to 200 μg atrazine/L produced gonadal abnormalities in developing X. laevis exposed from hatching until completion of metamorphosis (Hayes et al., 2002). These authors reported that 16–20% of the exposed animals had multiple gonads or were “hermaphrodites” with multiple testes and ovaries. The incidence of gonadal abnormalities at each test concentration was not reported, making it impossible to determine if these effects were concentration-related, although the authors claim that similar gonadal abnormalities were never observed in over 10,000 control animals examined over a 6-year period in their laboratory (Hayes, 2004). This claim is also not clear since it was later stated that, “By definition, ‘gonadal malformations” were defined initially as morphologies observed in atrazine-exposed larvae but not in controls” (Hayes et al., 2006b, p. 135). This begs the question as to how gonadal malformations that did occur in control animals were dealt with in terms of data reporting and statistical analysis. In another paper, Hayes (2004) plotted the data from the 2002 study as a function of atrazine test concentration. Based on this report, Hayes observed hermaphroditism and/or “single sex polygonadism” in as many as 15% of the animals exposed to atrazine. There was no apparent association between atrazine concentration and the incidence of hermaphroditism or single-sex polygonadism. A concentration-related increase in the incidence of segmented and anomalous gonads was observed in X. laevis exposed to atrazine from <24 h after fertilization for 70 d (Carr et al., 2003). The incidence of abnormal gonads based on gross morphology and categorized as intersex individuals differed significantly from controls only in animals exposed to 25 μg atrazine/L (4.7% relative to 0.6% in controls) (Figure 3 in Carr et al., 2003). In their study, Carr et al. (2003) defined intersex gonads as those gonads that could not be unambiguously identified as testis or ovary because of shared or undifferentiated traits in size, shape, and physical appearance. Phenotype-specific (male versus female) characteristics of differentiated gonads in developing X. laevis include size, shape, and pigmentation differences (Carr et al., 2003), as well as more obvious differences in germ-cell type (oogonia, spermatogonia) and presence/absence of cortex and medulla in differentiated gonads. While gonad size, shape, and pigmentation can be qualitatively assessed based on gross appearance, germ cells, and the organization of cortex, medulla and ovarian cavity can only be observed at the histological level. Subsequent histological evaluation of the gonads from the 25μg/L group, as described in Carr et al. (2003) and more recently by independent analysis of the same slides by pathologists from EPL Laboratories (Wolf, 2007), confirmed (as originally reported by Carr et al., 2003) that gonads identified as intersex based upon their outward appearance showed no evidence of mixed ovarian and testicular tissue. It is important to note that the definition of intersex as it appeared in Carr et al. (2003) was based on the ambiguous physical appearance of gonads at the gross morphological level and differs from more recent definitions of the term, which are based solely on the simultaneous presence of male and female germ cells within the same gonadal tissue. While many papers have cited the Carr et al. (2003) paper as providing evidence that atrazine causes a mixture of male and female germ cells within differentiating gonadal tissue in X. laevis, this was never reported by Carr et al. (2003) nor in the more recent analysis by Wolf (2007). In fact, to our knowledge, the only circumstances in which mixed ovarian and testicular tissue have been consistently observed in developing X. laevis is when the animals are exposed to estradiol (Carr et al., 2003; Kloas et al., 2008; Hu et al., 2008) or other estrogenic pollutants such as nonyl-phenol (Mosconi et al., 2002) or polychlorinated biphenyls (PCBs) (Qin et al., 2003). The biological significance of the effects reported by Carr et al. (2003) remains unclear since mixed ovarian/testicular tissue was not observed at the histological level. In another study, Coady et al. (2005) expanded on these observations by examining gonadal development in X. laevis exposed to atrazine or sex steroids (dihydrotestosterone or estradiol) throughout larval development and continuing for 2–3 months postmetamorphosis. These authors reported a small incidence of rudimentary hermaphroditism (van Tienhoven, 1983) based on gross gonadal morphology in animals exposed to sex steroid from completion of metamorphosis and 2–3 months postmetamorphosis (Coady et al., 2005). Detailed histological analysis revealed that 8% of the control animals possessed testicular ovarian follicles (TOFs), defined as oocytes with an intact nucleus, nucleoli, and a surrounding squamous epithelial layer. Testicular Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES ovarian follicles have been described as testicular oocytes (TOs) in a number of papers, but the more correct term—testicular ovarian follicles—is used in this review. There was no apparent relationship between atrazine concentration and the incidence of TOFs, although they were more common in animals treated with estradiol (32%) or ethanol (20%) relative to the FETAX controls or atrazine treatment groups (Coady et al., 2005). The TOFs reported by Coady et al. (2005) were generally small, nonvitellogenic, and represented a very small percentage of the total tissue. The observation of TOFs by Coady et al. (2005) is interesting, given previous reports of this phenomenon by Gallien (1974) and recent data from microcosm studies in which male X. laevis exposed to atrazine or control medium during larval development exhibited TOFs regardless of whether they were exposed to atrazine or not (Jooste et al., 2005). In this latter study, the incidence of TOFs declined in 10-month-old animals from all treatment groups and were virtually absent in 2-yearold animals (See Fig. 6). The maximum number of TOFs per testis furthermore reduced from 58 at NF stage 66 to 5 after a 10-month period to 1 follicle in 1 of 4 frogs at 2 years of age (Du Preez et al., 2008b). In addition, there was no indication of any transgenerational response to atrazine exposures in adult F1 frogs of a number of parameters including of frequency of F2 metamorphs with TOFs and number of oocytes per frog (Du Preez et al., 2008b). In recently reported studies conducted under the requirements and guidance of the U.S. EPA and recommendations of its 2003 Scientific Advisory Panel (U.S. EPA, 2003a), X. laevis were exposed to concentrations of atrazine ranging 4 orders of magnitude from 0.01 μg/L to 100 μg/L from NF stage 47–48 through to metamorphosis (NF stage 66). Two similar studies were conducted under Good Laboratory Practices (GLP) with full quality FIG. 6. Mean number of TOFs found per specimen in the reference and atrazine-exposed NF stage 66, 10-mo grow-out juvenile male frogs and 2-year-old adult frogs. ∗ A single TOF was observed in 1 of 4 adult frogs in the 25 μg atrazine/L treated group. Redrawn from data of Jooste et al. (2005) and Du Preez et al. (2008b). 737 assurance/quality control (QA/QC). No effects of atrazine on metamorphosis, sexual differentiation, frequency of males, or mixed sex were observed. Testicular ovarian follicles were not observed in negative controls or in larvae exposed to atrazine but one TOF was observed in the positive control larvae exposed to 17β estradiol at 0.2 μg/L (Kloas et al., 2008). No TOFs or other indications of intersex were observed in a study of X. laevis exposed to concentrations ranging from 0.1 to 100 μg atrazine/L (Oka et al., 2008). Exposures were static with renewal every 3 d. Analysis by GC-MS showed exposure concentrations from 100 to 200% of nominal, a relatively large range. Only a few studies have examined effects of atrazine on gonadal development in frogs native to the United States, where most atrazine is used. In a laboratory study, Hayes et al. (2003) exposed R. pipiens to 0.1 μg atrazine/L or 25 μg atrazine/L throughout larval development. They reported the presence of TOFs in males from the 0.1- and 25-μg atrazine/L treatment groups but not in the controls. This observation is confusing because it is also reported that two control animals with TOFs were observed (Hayes et al., 2003, p. 570). Two other studies examining the effects of atrazine on ranid frogs have reported the presence of TOFs in males irrespective of treatment and no effect of atrazine on the incidence of hermaphroditism. Based on laboratory exposures of R. clamitans, Coady et al. (2004) reported TOFs in 12% of the control frogs and 0% of the frogs exposed to 10 or 25 μg atrazine/L. There were no atrazine-related effects on incidence of intersex or phenotypic sex ratio (based upon gonadal appearance), although exposure to dihydrotestosterone resulted in a shift toward more phenotypic males (Coady et al., 2004). In a similar study, Orton et al. (2006) tested only one concentration of atrazine (10 μg atrazine/L nominal) and found no significant difference in the incidence of males with TOFs between controls (4 of 40) and atrazine treated (2 of 68) R. pipiens exposed from shortly after hatching through metamorphosis. Field Studies. In general, field studies have not observed a link between exposure to atrazine and the presence of TOFs in frogs. In a field study conducted on R. pipiens in 2002, Hayes et al. (2003) reported that only frogs collected from sites with measurable atrazine concentrations exhibited TOFs that were similar to those observed in the laboratory after exposure to atrazine. This was interpreted as suggesting that hermaphroditism or “sex reversal” never occurs in the absence of atrazine and that the phenomenon is solely the result of exposure to atrazine. As has been pointed out (Gammon et al., 2005), there was no consistent response of gonadal abnormalities to concentration of atrazine. This does not support a causal relationship. In addition, the study design (Hayes et al., 2003) was flawed because concentrations of atrazine were only measured at the time of collection of sexually differentiated frogs and there is no knowledge of their exposure when they were undergoing sexual differentiation prior to the time of collection. Since concentrations were not known during critical developmental stages, any conclusions reported by Hayes et al. (2003) are speculative. In addition, for some sites, Downloaded At: 15:43 28 October 2008 738 K. R. SOLOMON ET AL. there was no likely source of atrazine identified. In one site in Wyoming, atrazine was detected only in 2001, at which time 92% of the male R. pipiens had one or more TOFs. These results are inconsistent and cannot be interpreted as atrazine having a causative relationship with TOFs. As far as we are aware, this phenomenon has not been observed again. It is also instructive to note that there seems to have been a robust population of frogs in the subsequent year, which indicates that the occurrence of TOFs the previous year did not have a measurable impact on the population in the following year. A field study on frogs in Ontario, Canada reported the incidence of TOFs in R. pipiens from areas associated with row-crop agriculture as well as in reference areas (McDaniel et al., 2008). Mean proportion of R. pipiens with one or more TOFs were 25 and 42% in the two agricultural sites where atrazine was detected (0.068 to 3.13 μg atrazine/L), 25% in an agricultural reference site (0.044 to 0.39 μg atrazine/L), to 7% in the nonagricultural site (atrazine 0.015 to 0.090 μg atrazine/L). Residues of pesticides were measured only during the growing season from August 2003 to July 2005. There was no correlation between the proportion of R. pipiens with TOFs and measured atrazine concentrations and the authors speculated that there may be a natural background incidence of this phenomenon. Atrazine was present in greater concentrations in the agricultural sites than in the reference sites; however, several other pesticides such as the phenoxy herbicides were also detected in agricultural locations. Thus, causality could not be assigned. A review of the available literature demonstrates that the appearance of rudimentary hermaphroditism and small incidence of TOFs is a widespread phenomenon particularly in Ranids (see Hecker et al., 2006, for a review of the terminology), with many cases having been reported decades prior to the introduction of atrazine (in 1959). As early as 1929, TOFs were reported in R. clamitans (Witschi, 1929). Also, historical studies conducted on museum specimens of cricket frog (Acris crepitans) collected before and after the introduction of atrazine have found TOFs (Reeder et al., 2005). These studies reported a historical increase and subsequent decrease in the incidences of TOFs, which does not match the temporal trends in the use of atrazine. In fact, these authors speculate on the potential effects of organochlorine compounds such as PCBs as being a possible cause and conclude that it is unlikely that the incidence was related to exposure to atrazine. In addition, studies of frog populations conducted after the introduction of atrazine have not found a relationship between the exposures of frogs to atrazine and the incidence of TOFs (Smith et al., 2005; Coady et al., 2005; Jooste et al., 2005; McDaniel et al., 2008; Smith, 2007 personal communication) or a statistically significant association to the presence of atrazine (Reeder et al., 1998). Testicular Ovarian Follicles in Frogs as a Natural Phenomenon. Whether TOFs are a natural or induced phenomenon has been extensively debated. A recent study of X. laevis from a number of locations in South Africa reported that TOFs were observed in frogs from a number of locations NE of the Cape- fold Mountains (Du Preez et al., 2008a). Some of these locations were remote from atrazine use and had no detectable concentrations of atrazine in the water (MDL 0.025 μg atrazine/L). No TOFs were observed in frogs southwest of the Cape-fold Mountains where no atrazine is used (see later discussion). Phylogenetic analysis of mtDNA and two nuclear DNA sequences in these frogs showed that they belonged to two distinct haplotypes and that the presence of TOFs was likely haplotype-specific. This has serious implications for the use of TOFs as a marker of endocrine-modulated responses in this species. Unless the genetic background of the frogs being used and the homogeneity of the culture are known, results may be confounded. This is particularly true for X. laevis where mating in cultured animals is artificially stimulated and interhaplotype (or interspecies) mating can occur (Blackler et al., 1965; Blackler and Fischberg, 1968). This haplotype-specificity must be considered in the interpretation of laboratory studies with X. laevis. In frogs from northeast of the Cape-fold Mountains, TOFs have been consistently observed in controls as well as atrazine exposed animals but there was no concentration-response to atrazine (Smith et al., 2005; Jooste et al., 2005; Du Preez et al., 2008b). In frogs from southwest of the Cape-fold Mountains, the source of most of the frogs exported to other locations, no TOFs were observed in animals captured in the wild (Du Preez et al., 2008a) or in laboratory studies where frogs, identified by haplotype to be from this area, were exposed to concentrations from 0.1 to 100 μg atrazine/L (Kloas et al., 2008). TOFs were not observed in control or treated animals in a study on X. laevis tadpoles exposed from NF stage 49 to 66 to concentrations of atrazine between 0.1 to 100 μg/L (Oka et al., 2008). Although not specifically identified to genotype, these frogs were from a commercial source and were most probably collected from southwest of the Cape-fold mountains. In other studies of X. laevis, such as those conducted by Hayes et al., the provenance of the culture and the genetic homogeneity is unknown or unclear, which may explain some of the anomalous results. Similar phenomena may apply to other species of frogs and there may be a need to consider genotype in these frogs as well. Testicular Ovarian Follicles in Fish and Reptiles Although there have been no reported studies on the effects of atrazine on the incidence of TOFs in fish, this endpoint has been used in assays for endocrine-disrupting substances (reviewed in Grim et al., 2007). As in the case of frogs, it is important to consider background incidence of this response in controls. In a review of unexposed control Japanese medaka (Oryzias latipes) used in 41 studies on a number of chemicals, TOFs (TOs) were observed in 30% of all studies but with large variation between the four laboratories in the study (0 to 100%) (Grim et al., 2007). These observations suggest that, as in frogs, the presence of TOFs in O. latipes is a natural phenomenon and that there may be variations between strains of fish. ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES Downloaded At: 15:43 28 October 2008 A study where eggs of snapping turtles (Chelydra serpentina) were exposed to atrazine via soil revealed no effects on thyroid gland morphology or on the number of TOFs (TOs in De Solla et al., 2006). Eggs from a nonagricultural site were incubated in soil treated with 1× and 10× the concentration of atrazine (as Atrazine 480 formulated product) applied in the field (3.1 and 31 L/ha) at a temperature of 25◦ C. Although the testes of some hatchlings from the atrazine soil had 1 or more TOFs, there were no statistically significant differences between these and the unexposed control. It is also possible that TOFs, which had a diameter of about 50 μm, were missed because sections were taken at 175-μm intervals. These authors suggest that small numbers of males with TOFs may be a natural phenomenon, as has been reported by other authors, and does not appear to affect fertility (Pieau et al., 1999). Effects on the Ovary There is little evidence to suggest that atrazine affects ovarian development in Xenopus. Tavera-Mendoza et al. (2002b) evaluated the frequency of occurrence of primary and secondary oogonia, which are the only stages of oogenesis present in the ovary during sexual differentiation. They also evaluated the frequency of occurrence of atresia among primary and secondary oogonia. Following exposure of stage-56 tadpoles to 21 μg/L atrazine for 48 h, the frequency of occurrence of primary oogonia was reduced but the frequency of secondary oogonia and atresia was significantly greater (Tavera-Mendoza et al., 2002b). These observations were inconsistent with the lack of effects reported in the ovary of amphibians by others (Hayes et al., 2001; Carr et al., 2003; Orton et al., 2006) and may have been compromised by the experimental design and other factors (see earlier discussion). A study with P. promelas showed that exposure of adults to measured concentrations 25 and 224 μg atrazine/L for 21 d had no effect on a range of parameters in females, including body weight, gonadal somatic index (ratio of gonad mass to body mass; GSI), stage of ovarian development, proportion of atretic follicles or postovulatory follicles, number of eggs produced, or the number of eggs hatched (U.S. EPA, 2005). In other studies, Hayes reported that nonpigmented ovaries, which occurred at relatively high frequencies in atrazine-treated X. laevis larvae, were found in 4 individuals out of more than 400 control examined (1%) (Hayes et al., 2006b). Exposure of X. laevis to the androgen receptor antagonist (cyproterone acetate, CPA) from NF stages 50 to 66 resulted in a high proportion of animals (36%) with nonpigmented ovaries (Hayes et al., 2006b). By comparison, exposure to estradiol had no effect on ovary pigmentation. Hayes (2006b) speculated that the induction of unpigmented ovaries by CPA suggests that this malformation is the result of androgen depletion in atrazine-treated larvae, potentially as a result of the induction of aromatase. This interpretation should be considered with some caution due to the lack of evidence that atrazine induces aromatase in vivo and the high mortality (42%) in the CPA-treated groups in the study. Differ- 739 ences in numbers of ovarian melanophores (pigmented cells) between control and treated X. laevis were observed in only one of two laboratories in the recent study reported by Kloas et al. (2008). At the one laboratory, by gross observation and by histological examination, the proportion of NF stage 66 females with fewer melanophores in the ovaries was statistically significant by trend test only and then only when all treatments were included. The actual differences were not great (10% incidence of frogs with fewer melanophores in the control and 15% in the 100 μg/L atrazine treatment) and a similar trend was not observed in the study at the other laboratory (Kloas et al., 2008). Observations such as these have not been reported in other studies and the biological significance of changes in numbers of ovarian melanophores is unknown. Based on the available literature, there is little evidence to suggest an effect of atrazine on sex differentiation or gonadal development in reptiles, fish, or amphibians. Studies in the laboratory and field have failed to demonstrate an effect of atrazine on sex ratio of reptiles or amphibians. Hayes has reported that small concentrations of atrazine (≤0.1 μg/L) affect sexual development through effects on gross anatomy (multiple gonads) and functional morphology (hermaphroditism) in X. laevis. However, these responses were not confirmed in a series of investigations working with X. laevis in the laboratory and in its native habitat in South Africa. Hayes also reported that atrazine, at concentrations as little as 0.1 μg/L, contributed to the development of TOFs in R. pipiens. However, subsequent studies showed that the presence of TOFs is widespread among many species of frogs and that these have been reported in museum specimens collected before the introduction of atrazine. It is likely that the presence of TOFs is a result of the general plasticity of gonadal development in some amphibian species. While effects on reproduction would represent a significant concern, the weight of the available evidence does not substantiate claims that atrazine is a reproductive toxicant that feminizes and demasculinizes male frogs. VI. MECHANISMS MEDIATING REPRODUCTIVE EFFECTS Atrazine has been proposed to exert adverse effects on the reproductive fitness of animals including mammals, fish, and amphibians. Some of these effects are well substantiated while others are not. Furthermore, mechanisms observed in one species are often uncritically cited as support of proposed mechanisms in other species and responses to relatively large exposures are cited as support of theories to explain purported effects caused by smaller exposures. Here we present each of the proposed mechanisms of toxic action and discuss the species-specific responses and dose/concentration-response relationships and put them into perspective relative to potential effects in frogs and other aquatic vertebrates. Based on the available information, the only evidence for effects of atrazine on concentrations of steroid hormones in blood plasma are those reported by Hayes et al. (2002). While Hayes and coworkers have hypothesized 740 K. R. SOLOMON ET AL. that upregulation of CYP19 is a cause for adverse effects in frogs, they have never reported any measurements of CYP19 mRNA or aromatase enzyme activity. In the one study where they reported a decrease in concentration of testosterone, they failed to measure estrogen and a mass balance, which would have been supportive of this hypothesis, could not be calculated. When other workers have measured both CYP19 mRNA expression and aromatase activity, no effects of atrazine have ever been observed. There is no evidence to support the hypothesis that atrazine modulates CYP19 expression or the associated aromatase activity in vivo. Atrazine has been found to upregulate CYP19 expression in some transformed cell systems, but not others. The concentrations of atrazine required to affect CYP19 expression in vitro are much greater than those observed in the environment or tissues of animals exposed to environmentally relevant concentrations. Thus, there is essentially no support for the aromatase hypothesis. Downloaded At: 15:43 28 October 2008 A. Mechanisms Mediated Through the HPG Axis It has been hypothesized that atrazine or its degradation products can modulate the endocrine system through the central nervous system (CNS). Atrazine has been found to suppress the amplitude of the luteinizing hormone (LH) surge and prolactin concentrations in Sprague-Dawley and Long-Evans female rats by altering the hypothalamic control of these hormones (Cooper et al., 2000). Similar effects were observed recently when atrazine degradation products were found to affect the onset of puberty and thyroid function in male Wistar rats via actions on the CNS and its subsequent control of the pituitarygonadal axis (Stoker et al., 2002). These effects have only been reported for rodents, and it is unknown whether amphibians would respond in a similar manner. Regardless, because of the small bioconcentration factor of atrazine for aquatic organisms (see earlier description), the doses at which atrazine caused the observed effects in rodents (100–200 mg/kg bw, via oral administration) are much greater than those to which frogs or other aquatic organisms are likely to be exposed. For example, based on a BCF of 1.5, these are equivalent to water exposures of 66,000–133,000 μg/L, well above the maximum water solubility of atrazine (33,000 μg/L) and several orders of magnitude greater than typical environmental concentrations. Therefore, it can be concluded that these types of effects are not ecologically relevant in aquatic organisms. This is a good example of the need to consider the fundamental difference between body dose and matrix exposure concentration when citing papers as supporting a particular mechanism of action. B. Mechanisms Mediated Through Aromatase It has been proposed that atrazine can increase estradiol availability by upregulating expression of CYP19 (aromatase gene) mRNA and thereby increasing aromatase activity (Fig. 7). This could result in an increase in local availability of estradiol, depending upon where CYP19 is expressed. Since testosterone is FIG. 7. Illustration of the function of aromatase in the synthesis of E2. Depending on the action of aromatase, the ratios of testosterone and estradiol will change with resulting changes in the expression of sexual characteristics that are determined by these hormones. the endogenous substrate for aromatase, this could also result in increased plasma estradiol concentrations with a concomitant decrease in plasma concentrations of testosterone. It has been further proposed that this change in the ratio of testosterone to estradiol can result in changes in the expression of those sexual characteristics that are under the control of these hormones. While atrazine and several other triazine herbicides have been shown to upregulate CYP19 expression and aromatase activity in certain cell lines in vitro (Sanderson et al., 2000, 2001), other cell lines have been found to be unresponsive (Heneweer et al., 2004). These studies were conducted, in part, to explain anomalous results of in vitro studies that indicated that atrazine was estrogenic (Davis et al., 1993). Atrazine has never been shown to affect aromatase activity in vivo, although one study reported increased Cyp19A1 gene expression in ovaries of zebrafish (Danio rerio) exposed to atrazine for 3 d at concentrations as small as 2.17 μg/L (Suzawa and Ingraham, 2008). Later in this paper we review and critique the results of the studies that have examined the potential for effects of atrazine on aromatase in aquatic organisms. Aromatase activity in juvenile zebrafish was not affected by exposure to atrazine (Kazeto et al., 2004). Transcription of aromatase (CYP19 A1 and A2) in juvenile zebrafish was not significantly affected by exposure to concentrations as great as 1000 μg atrazine/L (Fig. 8). The report of increased expression of Cyp19A1 in ovaries of D. rerio (Suzawa and Ingraham, 2008) is in contrast to the findings of Kazeto et al. (2004) but may have been the result experimental design. There was no replication of treatments (one tank of 15 fish only per concentration), concentrations were not measured, and pooling of samples (5 fish each) for analysis may have obscured interindividual variability. In the study by Kazeto et al. (2004), exposure to ethinyl estradiol as a positive control caused reduced transcription. Fish were not exposed to estradiol as a negative control in the concentrationresponse study by Suzawa and Ingraham (2008). The results of Kazeto et al. (2004) are similar to those in frogs (discussed below) and are not consistent with the theory the atrazine induces aromatase activity during in vivo exposures. Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES FIG. 8. Effects of ethinyl estradiol (positive control) and atrazine on CYP19A1 transcript abundance. Zebrafish juveniles at 17 d post fertilization were exposed for 3 d. The results represent the mean ± S.E.M. of six samples: ∗ statistically different from the control ( p < .05), ∗∗ data not actually given in original figure but were described in text as not being different from 1000 μg/L. Redrawn from data of Kazeto et al. (2004). A study on frogs exposed to atrazine under field conditions in South Africa, where X. laevis is native, found no effects on aromatase activity (Hecker et al., 2004) as did a similar field study on R. clamitans in Michigan (Murphy et al., 2006a). Aromatase was not induced in X. laevis tadpoles (NF stage 49 to 66) exposed to concentrations of 0.1 to 100 μg atrazine/L (Oka et al., 2008). In the same study, atrazine did not induce the production of vitellogenin in tadpoles at concentrations up to 100 μg/L (Oka et al., 2008). These studies included concentrations in excess of the 0.1 μg/L that has been suggested by Hayes et al. (2002) as the threshold above which this effect causes adverse effects in frogs. It has been suggested that induction of aromatase activity results in the estrogen-like effects that are responsible for the hypothesized feminization and demasculinization of male frogs (Hayes et al., 2002). This theory was based on experiments with adult maleX. laevis that reportedly exhibited lesser plasma testosterone concentrations when treated with atrazine (Hayes et al., 2002). Unfortunately, neither CYP19 mRNA expression nor aromatase activity was measured in this study. Furthermore, concentrations of estradiol, the product of aromatase action, which could have confirmed the hypothesis, were not measured by Hayes et al. (2002). However, a different study with adult male X. laevis exposed to atrazine concentrations ranging from 1 to 250 μg/L showed no significant effects on aromatase activity or CYP19 mRNA expression (Hecker et al., 2005a). Because aromatase activity can be small in testes, it is often difficult to detect. However, the amplification methods developed by Park et al. (2006) allow detection of as little as a single copy of CYP19 mRNA. Furthermore, it needs to be recognized that there are several forms of CYP19, with the “A” type occurring in gonad and the “B” form occurring in brain. 741 The mechanism of induction of aromatase activity in cancer cell lines (KGN—human ovarian granulosa-like tumor cell line, H295R—adrenal carcinoma cells, and NIH/3T3—mouse fibroblasts) has been investigated (Fan et al., 2007). Aromatase activity had previously been shown to be induced by atrazine and simazine in H295R and JEG-3 cells in vitro (Sanderson et al., 2000, 2001), but only at relatively large concentrations and not in the rat R2C cell line (Heneweer et al., 2004). In this study, Fan et al. (2007) provided evidence that mammalian cell lines cells expressing the transcription factor steroidogenic factor-1 (SF-1) and the aromatase promoter (ArPII), which is activated by SF-1, were responsive to atrazine and simazine in terms of upregulated mRNA expression and aromatase activity. However, this was a highly artificial situation whereby cell lines were transfected with multiple copies of the ArPII promoter, high copy levels of SF-1, and exposed to very large concentrations of atrazine or simazine (often >2000 μg/L). Even in these cases, CYP19 mRNA expression was often only about 1.5-fold greater than the control levels. Given the complexity of this artificial cell system and the very large concentrations of the triazines required to mediate these effects, the significance of these observations in whole organisms and tissues other than these cell lines is questionable. As discussed elsewhere in this section, environmentally relevant concentrations of atrazine do not induce aromatase in vivo in frogs or fish, which suggests that the responses observed in mutated cells in vitro should not be extrapolated to whole organisms in the field. This also suggests that the extrapolations to human cancers discussed in the paper (Fan et al., 2007) are highly speculative at best and are not supported by the greater weight of evidence in the literature. Because they were unable to make measurements of E2 or CYP19 mRNA or aromatase activity and were thus unable to test the aromatase hypothesis directly, Hayes et al. attempted to investigate the mechanism of action of atrazine in frogs using two model chemicals with different endocrine modes of action, the anti-androgen cyproterone acetate (CPA) and the estrogen 17β-estradiol (Hayes et al., 2006b). They compared the effects of a range concentrations of atrazine (0.1, 0.4, 0.8, 1.0, and 25 μg/L) on gonad morphology of early X. laevis life stages from a previous study (Hayes et al., 2002) with those caused by exposure to CPA or estradiol. Larvae of X. laevis were exposed to atrazine and CPA from NF stage 50 through 66. In the estradiol experiments, larvae were treated for three different time periods: 7 d (NF stages 50–53), 14 d (NF stages 50–55), or 49 d (NF stages 50–66). Each of the positive control chemicals was tested at a single concentration (estradiol = 100 μg/L; CPA = 5 g/L). Based on gross morphological and histological analyses, several malformations were reported to occur in juvenile X. laevis exposed to atrazine (Hayes et al., 2006b). These included lobed testes, unpigmented ovaries, the occurrence of TOFs, and, in some rare occasions, multiple combinations of testes and ovaries in the same individual. To support their findings, Hayes et al. (2006b) presented a photograph of gross morphology and micrographs, which was already published in an earlier manuscript. Author Figure 5 in Hayes et al. (2006b) states that the exposure Downloaded At: 15:43 28 October 2008 742 K. R. SOLOMON ET AL. concentration was 0.1 μg/L (0.1 ppb), but the identical photographs also appeared in author Figure 2 in Hayes et al. (2002) where the text and caption state that the exposure was 1 μg/L (1 ppb). The reason for the use of this figure and difference in treatment concentration is unclear. No concentration-response relationships were observed for any of the malformations described. The descriptions of the procedures used in the study were unclear and difficult to follow and there was inconsistency between the methods and the results in terms of the number of animals reportedly used in the study. Not surprisingly, given the large concentration (5 g/L = 5,000,000 μg/L), mortality in the only concentration of CPA tested was 35%, which suggests that any responses observed may have been artifacts of general toxicity. The authors stated that the results presented in this study “suggest that atrazine-induced gonadal malformations result from the depletion of androgens and production of estrogens, perhaps subsequent to the induction of aromatase by atrazine a mechanism established in fish, amphibians, reptiles, and mammals (rodents and humans)” (2006b). However, neither the study design nor the results they reported allow such conclusions to be drawn. In fact, the authors (Hayes et al., 2006b) did not analyze concentrations of testosterone or dihydrotestosterone in plasma or tissue or the activity of steroidogenic enzymes such as17βHSD or CYP19. The authors were thus unable to test whether a depletion of androgens or an increased production of estrogens had occurred. The analysis of gross morphological and histological endpoints, as presented in this study, does not provide sufficient information to extrapolate to a specific mode of action. As for the statement that the aromatase mechanism has been established in fish, amphibians, reptiles, and mammals, there is no information given in the cited papers to support this statement. As discussed earlier, the phenomenon of upregulation of CYP19 activity has been observed only in transformed cell lines but not in other cell lines. The current information suggests that while atrazine at relatively great concentrations can upregulate in vitro expression of CYP19 in some cell lines, the phenomenon has never been demonstrated in amphibians in vivo, in the laboratory, or in the field. C. Effects of Atrazine on Plasma Sex Steroid Hormones in Amphibians and Fish Plasma Sex Steroids in Frogs Interpreting the effects of chemicals, such as atrazine, on plasma hormones in frogs is difficult due to the relatively great degree of variation among individuals. Changes in plasma hormone concentrations may reflect alterations in synthesis, secretion, binding to plasma binding proteins, or changes in metabolism of the hormone. This variation is exacerbated by seasonal effects and the duration of the breeding season (Licht et al., 1983; Fasano et al., 1989; Mosconi et al., 1994). This is an important consideration since some frogs can breed once whereas others can breed repeatedly or skip a season. In addition to the factors just listed, the role of testosterone, 11-ketotestosterone (KT), and dihydrotestosterone (DHT) in sexual development and reproduction of amphibians is not clear. Effects of Atrazine on Plasma Hormones in Frogs If the putative aromatase-mediated mechanism of feminization and/or demasculinization of male frogs as proposed by Hayes et al. (2002) were correct, one would expect to observe a decrease in plasma testosterone concentrations and an increase in plasma estradiol concentrations. Few studies have reported effects of atrazine on plasma testosterone or estradiol concentrations in frogs. Only one study has reported a change in testosterone in response to exposure to atrazine at environmentally realistic concentrations (Hayes et al., 2002). In that study, it was reported that exposure of adult, male X. laevis to 25 μg atrazine/L for 46 d resulted in a statistically significant decrease in plasma testosterone concentrations to values that were the same as those observed in unexposed, adult females. Concentrations of estradiol were not measured in the study by Hayes et al. (2002) and only four animals were used. As discussed earlier, it was postulated that upregulation of the CYP19 gene was the cause of this effect; however neither aromatase activity nor CYP19 gene expression were measured in the study. If this effect was indeed a result of induction of aromatase activity, then a decrease in testosterone should be accompanied with an increase in estradiol. A study in which juvenile X. laevis were exposed to a range of atrazine concentrations (0.1–25 μg/L) from 72 h posthatch until 2 to 3 months postmetamorphosis found no statistically significant differences in plasma testosterone concentrations among atrazine treatments and between treatments and the controls (Coady et al., 2005). There were also no statistically significant effects of exposure to waterborne atrazine on plasma concentrations of estradiol in females. However, in males exposed to 1.0 μg/L atrazine, plasma concentrations of estradiol were significantly less than those of controls, but not at greater or lesser exposures, such that there was no consistent concentration-response relationship (Coady et al., 2005). Furthermore, the decrease in concentrations of estradiol was opposite to the effects that would have been caused by induction of aromatase. No studies, conducted under controlled conditions, have been able to repeat the observation of a decrease in plasma concentration of testosterone in male X. laevis at environmentally realistic exposure concentrations, in either the laboratory (Coady et al., 2005; Hecker et al., 2005a) or the field (Hecker et al., 2004). It was reported that atrazine exposure reduced the concentration of testosterone in plasma of adult male X. laevis (Hecker et al., 2005a), but the lowest-observed-effect concentration for this response was 250 μg atrazine/L. The concentrations of testosterone in plasma shown in (Fig. 9) illustrate an error made in the article by Hayes (2004) when he compared concentrations from his study to those of Hecker et al. (2003). Because the frogs used in the Hecker et al. study were juveniles, the results should be compared to other studies on juveniles such as the Kang et al. (1995, data shown for Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES 743 FIG. 9. Testosterone concentrations in plasma of Xenopus laevis in a number of studies. Mean and SE data except for field study in South Africa (Field SA) where the median of medians is shown. PM4 and PM5 are post-metamorphic juveniles not exposed to atrazine. Estradiol is a positive control. Redrawn from data from Kang et al. (1995), Hecker et al. (2003), Hecker et al. (2005b), Hecker et al. (2005a), Hecker et al. (2004), and Hayes et al. (2002), as referenced in the figure. postmetamorphic stages 4 and 5), not to the adults tested by Hayes et al. (2002) or the laboratory and field studies on adults by Hecker et al. (2005a). As is clear from the observations in juveniles and adults (Fig. 9), the only study that claims an effect of atrazine at environmentally realistic concentrations is that of Hayes. Given the lack of effect seen on aromatase (discussed earlier) and the fact that concentrations of estradiol were not reported by Hayes et al. (2002), the significance and physiological mechanisms underlying this response remain unclear. Since the results reported by Hecker et al. (2005a) found that atrazine reduced plasma testosterone only at the greatest concentration tested (250 μg/L) and did not result in an concomitant increase in plasma estradiol concentrations, CYP19 gene expression, or aromatase activity, to date there is no published in vivo information supporting the proposed aromatase mode of atrazine action in frogs. Several studies have examined the relationship between concentrations of atrazine in the field and plasma hormone con- centrations in frogs. A negative correlation was found between concentrations of atrazine in water and concentrations of testosterone in plasma of adult female X. laevis inhabiting atrazineexposed ponds in South Africa; however, due to the presence of other confounding factors, such as other agricultural chemicals, it was impossible to establish a direct cause–effect relationship between atrazine and plasma testosterone concentrations. Concentrations of testosterone and 11-ketotestosterone in plasma of R. clamitans and R. pipiens from wetland areas in Ontario showed no correlation with concentrations of atrazine (McDaniel et al., 2008). Concentrations of the hormones, testosterone, estradiol, and 11-ketotestosterone in plasma were measured in R. clamitans inhabiting Michigan (Murphy et al., 2006b). Estradiol, testosterone, and 11-ketotestosterone concentrations, as well as the ratios of estradiol/testosterone and 11ketotestosterone/testosterone, of adult male frogs were significantly different among all of the locations. Atrazine 744 K. R. SOLOMON ET AL. Downloaded At: 15:43 28 October 2008 concentrations were significantly and positively correlated with 11-ketotestosterone in adult females in 2002 and with testosterone in juvenile males in 2003, but were not significantly correlated with any other parameter. Estradiol/testosterone, 11-ketotestosterone, and 11-ketotestosterone/testosterone ratios were significantly greater at agricultural sites than nonagricultural sites (Murphy et al., 2006b), with a power (1 – β) to detect differences in GSI, testosterone, and estradiol in 2002 that was less than 0.20. The conclusion of Murphy et al. (2006b) was that there were no consistent effects on plasma hormone concentrations along an atrazine exposure concentration gradient. Overall, there is no evidence from either laboratory or field studies that exposure to atrazine at realistic environmental concentrations leads to changes in the concentrations of estradiol or testosterone in the blood plasma of male frogs or that this subsequently results in feminization. Effects of Atrazine on Plasma Hormones in Fish Several studies have evaluated the effects of atrazine on sex steroids in fish. Most of these studies have been on adults and have determined levels of sex steroids in the plasma, while some studies on small-bodied fish have included measurement of levels of steroids within the whole body or the gonads. Overall, changes in sex steroid levels have been minimal, and when the effects have been seen this has occurred at atrazine concentrations much greater than those typically observed in the environment. No effects on the concentration of testosterone, estradiol, or 11-ketotestosterone in the testes were observed when sexually mature goldfish (Carassius auratus) were exposed to atrazine (nominal concentration of100 or 1000 μg/L) for 21 d (Spanó et al., 2004). Exposure to atrazine at 1000 μg/L, but not at 100 μg/L, caused a decrease in testosterone and 11-keto-testosterone concentrations in plasma and increased plasma estradiol concentrations in males. There were no changes in the concentration of vitellogenin in plasma, which is an estradiol-dependent response (Spanó et al., 2004). This result indicates that the changes observed in plasma concentrations of the steroid hormones were either transitory, in error, or not sufficiently great to cause adverse effects on reproductive function of C. auratus. Largemouth bass (Micropterus salmoides), approximately 2 y of age, were exposed to technical atrazine at nominal concentrations of 0, 25, 35, 50, 75, or 100 μg/L in the water column for 20 d during the nonreproductive season. An additional treatment of 100 μg/L commercial formulation of atrazine was also utilized, which contained surfactants and other inert ingredients (Gross et al., 1997). Both studies showed small effects on concentrations of some steroid hormones and vitellogenin (Fig. 10), but there was no concentration-dependent relationship and the statistical differences were not consistent so no overall conclusions could be made. Results for female bass indicated an inconsistent, non-concentration-dependent re- FIG. 10. Plasma concentration of steroid hormones associated with reproduction and vitellogenin in male and female bass exposed to technical and formulated atrazine (100 μg/L). The star symbol indicates statistically significant differences from the control. Redrawn from data of Gross et al. (1997). sponse for testosterone in plasma with no statistically significant differences between control and treatments. Concentrations of 11-ketotestosterone in plasma of female M. salmoides were the same, regardless of atrazine exposure concentration. Results for female M. salmoides did, however, indicate significantly greater estradiol concentrations in plasma of fish exposed to 100 μg/L commercial formulated atrazine and almost significantly greater concentrations in plasma of fish exposed to 100 μg/L technical atrazine. No statistically significant responses in plasma vitellogenin concentrations, which are a sensitive estradiol-dependent response in fish, were observed, which suggests that there were no functional changes due to the small, inconsistent, and transient changes in plasma estradiol concentrations. Results for male M. salmoides did not indicate any significant effects of atrazine on plasma concentration of estradiol, testosterone, or plasma vitellogenin, regardless of atrazine exposure concentration or whether formulated or technical atrazine was used. Concentrations of 11-ketotestosterone in plasma were, however, significantly less at exposure concentrations greater than or equal to 50 μg atrazine/L, regardless of formulation. The 11-ketotestosterone response in male fish appeared to be a Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES threshold response. Previous, preliminary studies with greater atrazine exposures did not report significant changes in plasma 11-ketotestosterone concentrations (Gross et al., 1997; Grady et al., 1998). A study of Atlantic salmon (Salmo salar) reported that exposure to atrazine at nominal concentrations of 2 or 20 μg/L resulted in changes in response to the priming effect of ovulated female salmon urine, changes in androgen secretion, and changes in steroid concentrations in the bile (Moore and Waring, 1998). The authors hypothesized that atrazine can affect testosterone concentrations by increasing enzymatic transformation of the hormone. However, no estradiol measurements were made in that study. Increased metabolic activity is a common response to exposure to environmental pollutants as well as being a natural phenomenon in the recrudescence cycle. Thus, it cannot be excluded as a possible cause for the observed response. Results of a 21-d reproduction bioassay where P. promelas were exposed to atrazine at measured concentrations of 25 and 224 μg/L showed no treatment-related effects on estradiol or testosterone in females or testosterone and 11-ketotestosterone in males (U.S. EPA, 2005). Based on the available information, the only evidence for effects of atrazine on concentrations of steroid hormones in blood plasma are those reported by Hayes et al. (2002). No other researchers have been able to reproduce these results, and the study by Hayes et al. (2002) does not report concentrations of estradiol so it is impossible to make inferences about the potential mechanisms of action. VII. EFFECTS ON LARYNGEAL DEVELOPMENT Laryngeal development in frogs is a sexually dimorphic process, and the formation of a larynx capable of male-calling behavior is androgen dependent. It has been hypothesized that atrazine could act as an endocrine disruptor by decreasing plasma concentrations of testosterone in X. laevis, which results in the development of a laryngeal dilator muscle with smaller volume (Hayes et al., 2002). Theoretically, this endpoint could serve as an integrating measure of androgen-dependent processes that would respond to subtle changes in androgen status during critical periods of development. A. Effects on the Laryngeal Dilator Muscle Under normal conditions, the laryngeal dilator muscle of male X. laevis is larger than that of females (Sassoon and Kelley 1986; Tobias et al., 1993). It has been reported that exposure of X. laevis to concentrations of atrazine as little as 0.1 μg/L resulted in smaller laryngeal adductor muscles at NF stage 66 than in unexposed males (Hayes et al., 2002). In contrast, Carr et al. (2003) and Coady et al. (2005) found no evidence based on laboratory studies to suggest that atrazine at concentrations as great as 25 μg/L reduced laryngeal muscle size in male X. laevis. In the field, there were no differences in larynx weight in either male or female X. laevis from the corn-growing regions and the non-corn-growing regions in South Africa (Smith et al., 2005). 745 The differences between the findings of Carr et al. (2003) and Coady et al. (2005) and those of Hayes et al. (2002) may be explained by differences in sampling, both at the level at which animals were selected for analysis as well as how laryngeal size was determined. Xenopus laevis that completed metamorphosis earlier were found to always be larger than their siblings that completed metamorphosis a few weeks later (Carr et al., 2003). For this reason, Carr et al. (2003) randomly selected animals that represented the entire range of body sizes from each tank. In contrast, Hayes et al. (2002) systematically selected animals that completed metamorphosis early. Whether differences in body size confounded their analysis is unknown. An additional difference is the method used to determine dilator muscle size. The dilator muscles are not uniform in shape, and Carr et al. (2003) employed a method for determining dilator muscle volume that previously has been used by other investigators to determine laryngeal muscle volume in frogs (McClelland et al., 1996, 1998). This analysis utilized measurements from evenly spaced sections through the rostral–caudal extent of the muscle, rather than sampling from one point in the muscle. In contrast, Hayes et al. (2002) determined dilator muscle size by subjectively selecting the “largest section” through the muscle and determining the cross-sectional area of this section. Even though the Carr et al. (2003) subsequent analyses have determined that largest crosssectional area through the muscle is the best descriptor of sex differences in muscle size, their method of choosing the largest section was quite different from that of Hayes et al. (2002). The studies on the laryngeal muscle provide important insights into the endocrine physiology of frogs and their susceptibility to atrazine. Measurable differences in muscle size at NF stage 66 between sexes suggests that androgen secretion begins prior to completion of metamorphosis in X. laevis. It is evident that the laryngeal muscle is responsive to androgens since exposure to dihydrotestosterone caused the laryngeal dilator muscle cross-sectional area to be greater in exposed than unexposed X. laevis, an expected response for the positive control. Based on differences observed in laryngeal dilator muscle size between sexes, the data suggest that larval androgen secretion proceeds normally in frogs exposed to atrazine (Carr et al., 2003). Overall, there is little evidence to support the theory that exposure to atrazine affects laryngeal development, either directly or indirectly. VIII. EFFECTS ON THYROID FUNCTION AND DEVELOPMENT The thyroid hormones (TH: triiodothyronine, T3; tetraiodothyronine, T4) have a constellation of effects on wildlife, ranging from the control of postembryonic growth and tissue differentiation to effects on reproduction (Carr and Norris, 2006). Given the particular importance of TH in developing organisms, it is not surprising that several studies have considered the thyroid as a possible target for the effects of atrazine. In theory, chemicals can affect TH levels in the blood 746 K. R. SOLOMON ET AL. via a number of pathways including (1) disruption of thyroidal iodide uptake and TH synthesis, (2) alteration in plasma TH binding protein levels, (3) effects on deiodination of T4 to T3, (4) effects on TH metabolism, or (5) direct action on TH receptors. Two model systems in particular have been studied with respect to atrazine effects on the thyroid axis: amphibian metamorphosis and smoltification in juvenile salmonids. Most studies have reported no consistent effects of atrazine on amphibian metamorphosis. Some effects of atrazine have been reported in smolting salmon, although there appear to be no consistent patterns of effect, with some studies demonstrating elevated plasma TH while others report decreased levels of TH after atrazine exposure. Downloaded At: 15:43 28 October 2008 A. Effects of Atrazine on Amphibian Metamorphosis Since most amphibian species require normal TH synthesis to complete metamorphosis, metamorphosis is a particularly sensitive endpoint for assessing effects on thyroid function. Disruption of TH synthesis during metamorphosis can lead to reduced hind limb growth, a delay in metamorphosis, and feminization of the gonads (in anuran amphibians, which require TH for androgen receptor expression, Robertson and Kelley, 1996). In general, studies examining effects of atrazine on metamorphosis suggest no consistent concentration-related effect of atrazine on thyroid function (Allran and Karasov, 2001; Hayes et al., 2002, 2003; Sullivan and Spence, 2003; Jooste et al., 2005; Carr et al., 2003; Orton et al., 2006; Kloas et al., 2008). Several studies have reported no effect of atrazine on the time to metamorphosis (Hayes et al., 2002, 2003; Carr et al., 2003; Kloas et al., 2008) in X. laevis or R. pipiens (Allran and Karasov, 2000; Orton et al., 2006), while Coady et al (2004) reported that 10 μg atrazine/L, but not 25 μg atrazine/L, inhibited metamorphosis relative to controls in larval R. clamitans. When X. laevis tadpoles were exposed to concentrations from 20 to 320 μg atrazine/L, Sullivan and Spence (2003) reported a significant positive relationship between atrazine concentrations and time to metamorphosis. These effects are difficult to interpret considering that individual concentrations of atrazine both accelerated (20 μg atrazine/L) and slowed (320 μg atrazine/L) time to metamorphosis to a small degree, whereas concentrations between 40 μg atrazine/L and 160 μg atrazine/L had no effect on metamorphosis (Sullivan and Spence, 2003). Metamorphosis was slightly delayed by approximately 5 d relative to controls in larval tiger salamanders (Ambystoma tigrinum) exposed to 75 μg atrazine/L (Larson et al., 1998). The same authors found that salamanders exposed to 250 μg atrazine/L actually reached later stages of metamorphosis more quickly (by 1–2 d) than controls (Larson et al., 1998). They also reported that exposures to 75 and 250 μg atrazine/L resulted in elevation of thyroxine in plasma of stage-IV larvae (from 0.9 μg/ml in control to 1.5 μg/ml) but with no concentration response, possibly explaining the shorter time to metamorphosis. This effect was not observed in stage-II larvae (Larson et al., 1998). The mechanism by which elevation of thyroxin occurred was not investigated but could have been related to toxic stress. B. Effects of Atrazine on Smoltification Thyroid hormones, prolactin, corticosteroids, and growth hormones (GHs) are all required for normal smoltification, a developmental process that accompanies migration from fresh water (FW) to salt water (SW) in anadromous salmon (Barron, 1986). Waring and Moore (2004) reported that exposure to atrazine concentrations between 1 and 10 μg/L resulted in less Na+ /K+ -ATPase activity in gills in Atlantic salmon (Salmo salar) smolts exposed for ≤7 d in fresh water. There were no effects of atrazine on plasma T3 or T4 in smolts exposed in fresh water (Waring and Moore, 2004). The authors reported a concentration-related increase in plasma T4 but not T3 in smolts exposed to atrazine ranging from 1.1 μg atrazine/L to 22.7 μg atrazine/L in fresh water and then transferred to seawater (Waring and Moore, 2004). The mechanism underlying the effect of atrazine on plasma T3 in smolts after transfer to seawater is unknown. In another study (Nieves-Puigdoller et al., 2007), S. salar smolts were exposed to atrazine at measured concentrations of 8.5 ± 1.1 (SEM) and 84.3 ± 1.3 μg/L in fresh water (FW) for 21 d, then exposed to a saltwater challenge for 24 h, and then returned to FW for a further 3-month period of observation. At 8.5 μg/L, atrazine had no effect on plasma levels of cortisol, growth hormone (GH), insulin growth factor I (IGF-I), T4 and T3, Cl− , Mg2+ , Na+ , or Ca2+ in FW or after SW challenge. No effect on plasma levels of GH, IGF-I, T4, or T3 was found in FW smolts exposed to atrazine at 84 μg/L, but, following SW challenge, fish had significant increases in hematocrit, plasma cortisol, Cl− , Mg2+ , Na+ , and Ca2+ and a decrease in plasma levels of T4 and T3. These data are not consistent with an effect on deiodinase activity, since plasma T4 and T3 were both decreased in fish exposed to 84 μg atrazine/L. Whether these effects are related to changes in plasma TH binding proteins is unclear since differences in free versus bound T4 and T3 were not reported. The reported lessening of plasma T3 is different from the effects of atrazine on plasma T3 reported by Waring and Moore (2004) and was only observed at the greater test concentration. The authors did report mortality (9%) in the smolts exposed to atrazine at 84 μg/L but not at 8.5 μg/L. In addition, feeding was reduced by close to 75% after 10 d of exposure and by 100% after 15 d of exposure to 84 μg atrazine/L but not in fish exposed to 8.5 μg/L (Nieves-Puigdoller et al., 2007). Reduced food consumption was accompanied by a significant loss of weight at the end of the exposure period when compared to all other exposures. Fish exposed to 84 μg atrazine/L were stressed based on the elevated blood glucose and cortisol levels and anorexia observed in these animals. Dietary restriction has been reported to affect concentrations of testosterone in rats (Trentacoste et al., 2001), but reports of similar effects in fish were not found in the literature. The reason why animals exposed to 84 μg atrazine/L exhibited a stress response is unclear, since Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES atrazine would not be expected to be acutely toxic to S. salar, although it has not yet been tested in this species. The 10th centile for the 96-h LC50 species sensitivity data in SW and FW fish was 3840 μg/L and the 10th centile for chronic exposures in all aquatic animal studies was 40 μg/L (Giddings et al., 2005). The most sensitive fish to chronic exposures was S. fontinalis with a NOEC of 65 μg/L for a 308-d exposure. Thus, the effects reported in this study may be caused by general toxicity and are not a specific hormone-mediated response. The authors of the study suggest that atrazine can have adverse effects on salinity tolerance in anadromous fish, but they also point out (correctly) that the concentration at which responses were observed would be rarely observed in the environment, especially in flowing water. Overall, there are no consistent reported effects of atrazine on metamorphosis in fish or amphibians. Given the fact that amphibian metamorphosis is a very sensitive indicator of thyroid function in frogs, and has been recommended as a Tier I test for EDCs by the U.S. EPA Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC, U.S. EPA, 1998), these results suggest that atrazine does not consistently affect thyroid function in developing amphibians. Atrazine has inconsistent effects on plasma TH levels in salmon smolts, with no clear mechanism of action. IX. EFFECTS ON STRESS PHYSIOLOGY Exposure to a wide range of environmental or physiological stressors can increase the activity of the hypothalamus-pituitaryadrenal axis, leading to greater concentrations of corticotrophin (ACTH) and adrenal corticosteroids in plasma. There are reports of atrazine causing greater plasma corticosteroid concentrations in salmon. Some effects of atrazine on in vitro adrenal steroidogenesis have been reported at high atrazine concentrations, although the effects appear to be species specific. Overall these studies suggest that atrazine at environmentally relevant concentrations has limited effects on the adrenal axis. A. Effects on Plasma Corticosteroids The major corticosteroids secreted by adrenal steroidogenic tissue can differ depending upon the species; in most fish species cortisol is produced, whereas corticosterone is the primary corticosteroid in amphibians and reptiles. There are only a few studies examining the effects of atrazine on cortisol in plasma of aquatic vertebrates. Exposure of carp (Cyprinus carpio) to 100 μg atrazine/L for 72 h or less resulted in greater plasma cortisol concentrations (Gluth and Hanke, 1985). This effect was dependent upon exposure temperature, since carp exposed to 100 μg atrazine/L exhibited a 5-fold increase in plasma cortisol relative to controls at 17o C but only a 3-fold increase when exposed at 22o C (Gluth and Hanke, 1985) for 72 h. Exposure to atrazine resulted in greater plasma glucose concentrations as well, possibly an effect that was secondary to elevated cortisol concentrations. Interestingly, similar cortisol responses were observed after 72 h of exposure to a wide variety of contaminants (100 747 μg/L aldrin, 50 μg/L DDT, 20 μg/L dieldrin, 2 μg/L endrin, l00 μg/L lindane) and industrial chemicals (100 μg/L toluene; 1 mL/L methanol), which suggests that this was a nonspecific stress response. Exposure to atrazine, at concentrations between 6.5 and 22.7 μg/L, was reported to cause a concentration-related increase in plasma cortisol concentrations in S. salar smolts exposed in fresh water (Waring and Moore, 2004). Similarly, concentrations of cortisol in plasma of S. salar smolts were greater when they were exposed to 84 μg atrazine/L (Nieves-Puigdoller et al., 2007), although these authors did not find an effect of lesser (8.4 μg/L) atrazine concentrations. Salmo salar exposed to 84 μg atrazine/L also exhibited hyperglycemia and anorexia (Nieves-Puigdoller et al., 2007), suggestive of a stress response. The mechanisms underlying these effects on plasma cortisol is unclear since atrazine is not overtly toxic at these concentrations and plasma ACTH and corticosteroid binding globulin were not measured in any of these studies. The effects of atrazine on plasma corticosterone concentrations were studied in larval A. tigrinum reared in the laboratory (Larson et al., 1998). Animals were exposed to atrazine at concentrations as great as 250 μg/L in a static renewal protocol for 86 d. The authors reported differences in plasma concentrations of corticosterone between stage II and stage IV but no concentration response to atrazine; however, only two concentrations were tested. They concluded that atrazine does not directly affect corticosterone at the concentrations tested. B. Effects on Adrenal Steroidogenesis and Secretion Adrenocortical tissue in fish and amphibians is intermingled with kidney tissue, making it impossible to directly isolate adrenal tissues for in vitro studies in these species. Thus, the few studies that have examined a direct effect of atrazine on adrenal steroidogenesis have used mixed kidney and adrenocortical tissue. The direct effects of atrazine on the responsiveness of adrenal cortical tissue to an ACTH challenge have been examined in trout head-kidney cells in vitro (Bisson and Hontela, 2002). Atrazine had no effect on viability of cells, even at the greatest concentration tested (500 μM ≈ 100,000 μg/L, Fig. 11). A significant increase in cortisol secretion was observed at 500 μM atrazine (≈ 100,000 μg/L, Fig. 11). When cells were stimulated with ACTH but not with a membrane-permeable form of cAMP, dibutyryl adenosine-cyclic monophosphate (dbcAMP). This concentration of atrazine is very unrealistic, even if one assumes some bioconcentration of atrazine in fish—the median value for the bioconcentration factor in fish reported in the literature is 2 and the maximum, 12 (Giddings et al., 2005). A reduction in ACTH-stimulated cortisol secretion was observed at concentrations of atrazine between 0.005 and 5 μM (≈ 1 to 1000 μg/L, Fig. 11). Stimulation of cortisol secretion with dbcAMP resulted in an increase in cortisol secretion but no concentration response to atrazine exposures was observed. Using two different types of suspended cell preparations from X. laevis and R. catesbeiana, Goulet and Hontela (2003) examined the effects of atrazine on adrenal corticosterone Downloaded At: 15:43 28 October 2008 748 K. R. SOLOMON ET AL. FIG. 11. ACTH- and dbcAMP-stimulated cortisol secretion and viability (± SEM) of trout head-kidney cells following in vitro exposure to atrazine. Statistical significance was evaluated by Dunnett’s test ( p < .05) and Student’s t-test ( p < .05). The number of replicates was five to eight for ACTH and dbcAMP and three to six for viability. Redrawn from data of Bisson and Hontela (2002) to show concentrations in μg/L. secretion. Kidney cells from X. laevis and “adrenal” cells from R. catesbeiana were used, although the degree to which the adrenal cell preparation was contaminated with kidney cells was not reported. Atrazine had no effect on cell viability or corticosterone secretion induced by ACTH or dbcAMP at concentrations ranging from 10–8 M to 10–4 M atrazine (2.16 to 21,600 μg atrazine/L for X. laevis kidney cells). Atrazine significantly decreased ACTH- and dbcAMP-evoked corticosterone secretion from R. catesbeiana adrenal cells at concentrations of 10 μM and greater (2160 μg atrazine/L). The ecological significance of these results is unclear given that atrazine was only effective at concentrations (>1000 μg/L) unlikely to be found under natural conditions. The authors claim that R. catesbeiana is a more sensitive model for studying contaminant effects on adrenal function. However, the basis for such a direct comparison of sensitivities to atrazine is weak since it is based upon different cell preparations (kidney cells for X. laevis vs. adrenal cells for R. catesbeiana). C. Effects of Pesticide Mixtures on Corticosteroid Secretion A study was conducted to evaluate the effects of nine different pesticides, alone and in combination, on early development in R. pipiens and corticosteroid homeostasis in adult X. laevis (Hayes et al., 2006a). Pesticides used in the exposure experiments were four herbicides (atrazine, metolachlor, alachlor, and nicosulfuron), three insecticides (cyfluthrin, cyhalothrin, and tebupirimphos), and two fungicides (metalaxyl and propiconazole). Effects were assessed either for each pesticide alone or for the mixture of all nine compounds. In addition, a binary mixture of atrazine and S-metolachlor and the commercial formulation Bicep II Magnum, which contains both of these herbicides, was investigated. Responses to individual pesticides were assessed at a concentration of 0.1 μg/L only. The binary mixtures of atrazine and metolachlor were tested at 0.1 and 10 μg/L. In addition to the individual studies, a nine-chemical mixture was also assessed with each pesticide at a concentration of 0.1 μg/L. Not surprisingly, the authors reported that the pesticide mixture had a much greater effect on larval growth and development than did the individual chemicals when tested alone. Furthermore, the authors reported that exposure to the nine-pesticide mixture resulted in damage to the thymus and theorized that this resulted in subsequent immuno-suppression and greater incidence of infection with Flavobacterium menigosepticum in tadpoles and young frogs (Hayes et al., 2006a). They also reported increased plasma corticosterone levels in adult X. laevis exposed to the nine-pesticide mixture. Gonadal development was not assessed in this study. The experimental design in this study was seriously flawed. It is possible to test interactions between two or three substances using the isobologram approach but more complex mixtures require a ray or multifactorial design (McConkey et al., 2000). Furthermore, tests for additivity or interactions must be based on potency, rather than amount of the chemical. This requires that the concentration response be separately characterized for all components of the mixture before any combinations are assessed. That the nine-component mixture caused a greater response is because the mixture was nine times more concentrated than the individual components. Because of problems in ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES the experimental design, it is not possible to determine whether the observed effects were the result of potency addition, response addition, synergism, antagonism, or a combination of all of these (LeBlanc and Wang, 2006). Thus, this study was neither informative with respect to the potential effects of mixtures, nor did it offer any elucidation on the potential effects of atrazine. Thus, although atrazine exposure has been reported to elevate plasma cortisol in salmon smolts, the mechanism underlying these effects is not clear. Large concentrations of atrazine have been reported to alter adrenal steroid secretion in vitro in frogs (>1000 μg/L) and fishes (84 μg/L), but the responses are inconsistent between species and may be a general stress response to large exposures that would be rarely found in the environment. Downloaded At: 15:43 28 October 2008 X. EFFECTS ON IMMUNE FUNCTION Few studies of the immunotoxic potential of atrazine are available. The majority of studies on the immunotoxic properties of atrazine that have been published so far were conducted using mammalian in vitro or in vivo test systems. The effects observed for the exposure of mammalian systems to a range of concentrations of atrazine included alterations of both the humoral and/or cellular immune response. Responses to subacutely toxic exposures in mammals were decreased numbers of natural killer cells (Whalen et al., 2003), decreased numbers of T and B lymphocytes (Filipov et al., 2005; Fournier et al., 1992; Karrow et al., 2005), increased macrophage phagocytotic activity (Fournier et al., 1992), decreased cytokine production (Hooghe et al., 2000), decreased lymphocyte activation capacities by PHA (Pistl et al., 2002), and decreased B16F10 tumor challenge capacity in mice (Karrow et al., 2005). In a review of the regulatory and published studies conducted to evaluate the potential effect of atrazine and/or its chlorometabolites on immune system parameters in mammals, Pastoor et al., (2008) reported that there were few studies that reported an observed association between exposure to atrazine and effects on the immune system at realistic exposures. Two sensitization studies conducted on technical atrazine in guinea pig were positive, while a third was negative and a sensitization study conducted in humans was negative. There was no evidence of effects of atrazine or its chlorometabolites on the immune system of rats, rabbits, or dogs at chronic doses that resulted in low to moderate reductions in body weight gain (5% to 15%—below the maximum tolerated dose—MTD). Doses in excess of the MTD (i.e., body weight gain reductions greater that 15%) were associated with reduced spleen and/or thymus weight along with reductions in other organ weights. In addition, such high doses appeared to delay the development of hematopoiesis in bone marrow. This was primarily reflected as reduced erythroid parameters in such animals and, in extreme cases, by the activation of extramedullary hematopoiesis in the liver. Multigeneration reproduction studies conducted in rats did not indicate any increased sensitivity of the immune system resulting from exposure to atrazine during development. There was no evidence 749 of increased incidence or onset of cancers originating in the immune system and no evidence of increased disease susceptibility resulting from lifetime treatment of rats or mice with MTDs of atrazine. Some effects in immune parameters were reported in short-term, high-dose studies conducted either in vivo or in vitro with cell models. Many of these were conducted at concentrations that were near the solubility limit of atrazine (30,000 μg/L) or at acute and repeat doses that greatly exceeded the MTD (Pastoor et al., 2008). These effects were judged to be secondary to a generalize stress response as been observed by others for atrazine and other substances (Pruett et al., 2003). Effects observed for the exposure to atrazine using artificial or in vivo exposure systems raised the question whether atrazine may cause similar responses in aquatic species such as fish and amphibians. Compared to the literature on the effects of atrazine on the mammalian immune system, however, little information is available on similar effects in fish or amphibians. A. Effects of Atrazine on Immune Function in Fish Effects of atrazine exposure on the immune system of fish included several aspects of the cellular immune response such as degeneration of macrophages, an increase in the number and size of hepatic melano-macrophage centers in the euryhaline muglid fish species Liza aurata and L. ramada (BiagiantiRisbourg, 1990), and leucopenia and atrophy of lymphoid organs in salmonids (Walsh and Ribelin, 1975). Other effects on salmonid species (Oncorhynchus kisutch, Salvelinus namaycush) included reduction of spleen weight and decreased number of lymphocytes (reviewed in Zeeman and Brindley, 1981). A series of studies on the effects of atrazine on immune function of C. carpio did not find any significant alterations at concentrations as great as 28,000 μg atrazine/L (Cossarinidunier and Hattenberger, 1988; Cossarinidunier, 1987) or with a dose of 10,000 μg atrazine/kg body weight (b.w.) (Cossarinidunier et al., 1988) in vitro or in vivo, respectively. A summary of the effects of a range of atrazine concentrations on the immune system of teleost fish is given in Table 2. It appears that there are distinct differences in the sensitivity of fish species in terms of their immune response to exposure with atrazine. While L. aurata, L. ramada, and some salmonid species exhibited the first signs of an alteration in their cellular immune response at concentrations as small as 25 and 100 μg atrazine/L, respectively (Walsh and Ribelin, 1975; BiagiantiRisbourg, 1990), no effects on either the humoral or the cellular level, were observed in C. carpio at concentrations as great as 28,000 μg atrazine/L (Cossarinidunier and Hattenberger, 1988; Cossarinidunier, 1987). Concentrations of atrazine have been reported to exceed 20 μg atrazine/L only in rare occasions, even directly after application (Battaglin et al., 2000; Solomon et al., 1996; Giddings et al., 2005). These concentrations typically occur only for a short time in environments that are contiguous to agricultural lands, and seldom exceed the threshold concentration of 25 μg atrazine/L for which immunological effects have been observed in a single study (Biagianti-Risbourg, 750 K. R. SOLOMON ET AL. TABLE 2 Effects of atrazine on the immune system of teleost fish Species Cyprinus carpio Downloaded At: 15:43 28 October 2008 Liza auratus, Liza ramada. Salmonidae (species not specified) Oncorhynchus kisutch, Salvelinus namaycush Endpoint Humoral immune response Phagocytosis Head kidney macrophages chemiluminesense response Replication of spring viraemia of carp virus Macrophages (liver) Effect System Exposure No In vivo 100–10,000 μg/kg BW No No In vivo In vitro No In vitro Degeneration In vitro/ex vivo ≥ 25μg/L Atrophy No effect/decrease In vivo In vivo Number of lymphocytes No effec/decrease In vivo (Cossarinidunier et al.; 1988) 7000–28,000 μg/L 28,000 μg/L Number of endocytes Increase In vitro/ex vivo ≥ 25μg/L (liver) Increase in Increase In vitro/ex vivo ≥ 25μg/L melano-macrophage centres (liver) White blood cells Decrease (leucopenia) In vivo 100–1000 μg/L Lymphoid organs Spleen weight Reference 1,500–13,500 μg/L (BiagiantiRisbourg; 1990) (Walsh and Ribelin; 1975) (Zeeman and Brindley; 1981) BW = bodyweight, (Data from Dunier and Siwicki 1993). 1990). Furthermore, maximum exposures to atrazine occur in environments such as small ponds or lowland streams that are typically not inhabited by the more sensitive species such as some salmonids or the euryhaline muglid species L. aurata and L. ramada. Considering factors such as exposure likelihood and maximum environmental concentrations, the risk of exposure to atrazine compromising the immune system of fish in the wild appears to be small. To our knowledge, no ecotoxicological studies are available that tried to link exposure to atrazine in the wild with effects on the immune system, and therefore, the environmental relevance of possible immunological consequences of the exposure of fish to atrazine remains unclear. In general, the overall body of information regarding the effects of atrazine on the immune system of fish is very scarce, and to be able to conduct an appropriate risk assessment of the immunotoxic properties of atrazine, additional studies are needed. These studies should specifically address the complexity and multiplicity of immune responses as well as differences in species sensitivity, as currently only patches of information are available for individual species, making it difficult or impossible to compare results among studies. B. Effects of Atrazine on Immune Function in Amphibians There have been few original research papers on amphibians that report on the potential effects of atrazine on the immune system of anurans (Table 3). Three of these studies tested effects of mixtures of pesticides, making it difficult to characterize the contribution of atrazine to the observed results. In one study, exposure of R. pipiens to mixture of pesticides (metribuzin, aldicarb, dieldrin, endosulfan, lindane, and atrazine, Table 4) resulted in a decrease in magnitude of a number of responses (Christin et al., 2003). Cellularity of frog splenocytes was determined before and after infection. A significant limitation with this data set is that it was normalized and reported as percent of control where 100% is arbitrarily assigned to both of the water control groups (i.e., infected and uninfected). Although the authors state that the infected water control frogs exhibited a mean value of 58.15 × 104 cells/ml, they failed to provide the mean value of cells/ml for the uninfected water-control group. Therefore, it is not possible to determine the change in spleen cellularity due to parasitic infection. In addition, by reporting the results as cells/ml, it is difficult, if not impossible, to determine the 751 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES TABLE 3 Effects of atrazine on the immune system of anurans Species Endpoint Downloaded At: 15:43 28 October 2008 Rana pipiens Cellularity of splenocytes(1) Viability of splenocytes (1) Number of phagocytic splenocytes(1) T-cell proliferation (1) Phagocytic activity of splenocytes(1) R. pipiens Thymic plaques Thymic lymphocytes R. pipiens Formation of thymic plaques(1) R. pipiens Thioglycollate-stimulated recruitment of white blood cells to the peritoneal cavity R. sylvatica Number of white blood cells(3) Xenopus laevis Cellularity of splenocytes(1) Viability of splenocytes(1) Number of phagocytic splenocytes(1) T-cell proliferation(1) Phagocytic activity of splenocytes (1) Effect System Exposure No In vivo (2–2,100 μg/L) (4) No Decrease In vivo (≥2 μg/L)(4) Decrease No In vivo (≥2 μg/L) (4) In vivo Decrease No effect Increase Decrease In vivo In vivo In vivo In vivo Decrease (2) Decrease Decrease Decrease In vivo 3 and 30 μg/L In vivo (≥210 μg/L) (4) In vivo In vivo No Increase In vivo (2–2,100 μg/L) (4) In vivo (≥21 μg/L) (4) ≥ 1μg/L (30 d) ≤ 10μg/L (60 d) (0.1 μg/L) (5) 10 to 0.01 μg/L Reference (Christin et al.; 2003, Christin et al.; 2004) (Houck and Sessions; 2006) (Hayes et al.; 2006a) (Brodkin et al.; 2007) (Kiesecker; 2002) (Christin et al.; 2004) Note. (1) Mixture study. Effect cannot be assigned to atrazine because other pesticides were tested in the mixture. (2) No information on significance of effect given. (3) Not clear if effect is due to exposure to atrazine or increased infection with trematode cercaria. (4) Number in brackets = concentrations of atrazine in mixture with 5 other pesticides. (5) Number in brackets = concentration of atrazine alone or in mixture with nine other pesticides. total number of spleen cells per frog. No significant pesticideassociated effects on spleen cellularity were observed in any of the treatment groups. The effect of the pesticide mixtures on T cell proliferative responses induced by two separate mitogens, concanavalin A (ConA) and phytohemaglutinin (PHA) were measured pre- and postinfection. A significant confounding factor to this set of experiments is that both Con A or PHA produced a very weak induction of T cell proliferation, which, at best, was 2-fold in spleen cells from uninfected frogs. The modest proliferative responses suggest that both ConA and PHA have weak mitogenic activity in frog T cells or that the conditions for this assay have not been optimized. Regardless of the reasons, due to the extremely poor level of stimulation with each of the mitogens, it is difficult to evaluate the biological relevance of the decreased proliferation in the presence of pesticide exposure. The authors also observed that the overall magnitude of proliferation was greater in mitogen-activated spleen cells from infected frogs (author Figure 2B). However, even under these conditions, only a twofold increase in proliferation (mitogen stimulated versus no mitogen stimulation) was observed. In addition, that the spleen cells from infected frogs were modestly TABLE 4 Nominal concentrations of pesticides used in studies on frogs Mixture 0.1× 1× 10× 100× Atrazine μg/L Metribuzin (μg/L) Aldicarb (μg/L) Dieldrin (ng/L) Endosulfan (ng/L) 2.1 21 210 2, 100 0.056 0.56 5.6 56 1.7 17 170 1, 700 0.015 0.15 1.5 15 0.002 0.02 0.2 2 Note. Data from Christin et al. (2004) and Gendron et al. (2003). Lindane (ng/L) 0.033 0.33 3.3 33 Downloaded At: 15:43 28 October 2008 752 K. R. SOLOMON ET AL. more responsive to mitogen-induced proliferation versus spleen cells from uninfected frogs is not too surprising. Within the environment of an active immune response, the lymphocytes from the infected frogs were likely primed as well as exposed to a milieu of growth-promoting cytokines. No statistically significant effect on either PHA- or Con A-induced proliferation by spleen cells isolated from infected frogs was observed due to pesticide treatment at any of the dose groups. In addition, no statistically significant pesticide-associated treatment effects were observed on phagocytosis by spleen-derived phagocytic cells and no statistically significant pesticide-associated treatment effects were observed on the prevalence of lung infection by R. ranae. However, since no tests were conducted during this study with individual compounds, it is impossible to separate possible effects of atrazine from those that might have been caused by the other chemicals. In a second study of the same mixture on X. laevis and R. pipiens, a significant decrease in total spleen cell number was observed in X. laevis in the 10× and/or 100× treatment groups (Christin et al., 2004). However, there was mortality (15 of 30 of frogs in the 100× and 2 of 30 in the 10× group). It is quite common to observe a decrease in lymphoid organ cellularity when overt toxicity is chemically induced. This can be attributed to a variety of nonspecific effects, including stress. Therefore, a major shortcoming of this study was that body and spleen weights were not measured and reported in light of the indications that overt toxicity was being produced in some of the treatment groups. There was also a decrease in phagocytic activity in spleen cells from R. pipiens, which occurred in the absence of a decrease in spleen cellularity. This is contradictory to the results from an identical study in 2003 (Christin et al., 2003) where no effect on phagocytic activity was observed at any of the concentrations of the pesticide mixture. As with the previous study, no tests were conducted with individual compounds. Therefore, it is impossible to separate possible effects of atrazine from those that might have been caused by the other chemicals. A study on R. pipiens reported on the effects of exposure to atrazine on the formation of hemolytic plaques and lymphocytes in the spleens of frogs challenged with sheep red blood cells (Houck and Sessions, 2006). Frogs of unreported age and size and from an unknown source were exposed to nominal concentrations of 0, 1, and 10 μg atrazine/L in glass containers of unreported volume and kept at room temperature (unreported) for 30 and 60 d. Four frogs were exposed to each concentration, except for the control in the 60-d study where only three frogs were used. Exposure solutions were replaced every 3 d but concentrations were not measured. At the end of the exposures, the frogs were injected intraperitoneally (ip) with the equivalent of 0.5 ml of washed sheep red blood cells for 5 consecutive days. Frogs were then sacrificed, the spleen removed, and flushed to collect cells for counting and for the hemolytic plaque assay, performed with sheep red blood cells and guinea pig complement (GPC). The results of the 30-d exposure (only three of the four exposed frogs were used) showed a statistically significant decrease in the formation of the hemolytic plaques at 1 and 10 μg atrazine/L. In the 60-d exposure, treatments did not produce scorable plaques (reasons not reported), counts of lymphocytes and lymphocytes and red blood cells were not statistically different between control and treatments, and there was no concentration response. The methods used in this study were poorly reported, small numbers of animals were used, and the same assay endpoints were not reported for the two exposure periods. Thus, the results are essentially uninterpretable and do not justify the very speculative and over-extrapolated discussion in the article. At best, the results reported in this article are preliminary and a better designed study should be conducted to test this hypothesis. Another study reported effects of atrazine in combination with exposure to trematode cercariae (Ribeiroia sp. and Telorchis sp.) on eosinophil counts and susceptibility to successful encystation of cercariae in R. sylvatica (Kiesecker, 2002). The only immunological parameter investigated was the number of eosinophils in circulating blood. In light of the fact that the author’s goal was to draw a linkage between chemical exposure and decreased immune competence, it is puzzling that information concerning immune status of the frogs was not included in the paper. It appeared that the number of eosinophils inversely correlated with the formation of meta-cercarial cysts, and to increasing concentrations of atrazine. However, no statistical comparisons were reported between the controls and atrazineexposed frogs, leaving the question open whether there was a significant decrease in eosinophil counts due to atrazine exposure. Furthermore, blood samples for immunological analyses were taken after infestation of frogs with cercariae, making it impossible to determine whether the observed effect on the immune response was due to the infection or herbicide exposure. The author’s conclusion that the pesticide exposure resulted in a “dramatic effect” on the immune system would perhaps be justifiable if eosinophils had been observed to be reduced in pesticide-exposed frogs before exposure to cercaria, and this reduction showed a consistent concentration-response. In the absence of these data, it could be argued that the reduction in eosinophils in the blood was a result of the cercarial infection. Eosinophils are known to migrate from the blood vessels to sites of parasite infection (Guyton and Hall 1996; Dhabhar et al., 1993, 1994), and this may have been the cause of the apparent reduction in numbers circulating in the blood. The study on the effects of pesticide mixtures on frogs discussed earlier (Hayes et al., 2006a) also reported effects on the thymus gland in R. pipiens. This effect was “discovered” because of unexpected infections in the frogs with F. menigosepticum and was not related to a specific hypothesis test, nor was the study designed to test this hypothesis. The authors reported an increased incidence of “thymic plaques” in animals exposed to atrazine, metolachlor (0.1 μg/L), the formulated mixture of atrazine and metolachlor (0.1 μg/L atrazine), and the nine-pesticide mixture (total concentration of 0.9 μg/L). Incidence was greatest (26%) in the latter exposure. There is no reference to the literature that Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES would causally link the presence of “thymic plaques” to reduced immunocompetence and it appeared to be a nonspecific response related only to exposure to pesticides in general. There is no indication of whether this was a response to infection, the cause of the infection, or whether the incidence of thymic plaques increased at greater exposure concentrations, nor was the experiment designed to elucidate this. The extrapolation of this effect to suggest that declines of amphibians caused by an “inability to mount proper immune responses as a result of pesticide exposure” is overinterpretation of chance observations and needs to be properly tested in a well-designed study. A recent study reported the effects of atrazine on the innate immune system in adult R. pipiens at exposure concentrations as small as 0.1 μg/L (Brodkin et al., 2007). The frogs employed in this study were caught in the wild and there was no knowledge of prior exposure to environmental contaminants, a fact acknowledged by the authors. No analysis of tissues was performed at the end of the study to assess the potential impact of exposure to other agents may have had on the study results prior to captivity. The experimental model consisted of maintaining 6 individual frogs for 8 d at each exposure concentration in separate containers with 500 ml of aged tap water or atrazine-supplemented water, about 1 cm deep. Because the frogs were not fully immersed, there may have been unquantified differences in exposures between replicates as well as between treatments. The sex of the frogs was not reported. In measurements of innate immune responses, thioglycollate-induced peritoneal cells were employed. Historically, thioglycollate has been used in many studies to increase the total number of phagocytes that can be isolated from the peritoneal cavity. However, this approach is now not commonly employed, especially for assessment of immunotoxicity. This is due to the well-known fact that, in addition to elicitation of cells into the peritoneal cavity, thioglycollate elicitation also activates phagocytic cells. The concern is that the thioglycollate activation may confound the assessment of functional responses by macrophages and other phagocytic cells. This is especially a concern in the present study since phagocytosis is used to both identify phagocytic cells as well as serve as a functional measure of immune status. The authors reported an almost complete inhibition of thioglycollate elicitation of leukocytes into the peritoneum by exposure of frogs to 21 μg/L atrazine. The same effect was observed in a concentration response study (0.01–10 μg/L atrazine in thioglycollate-stimulated frogs). Although 8 d of atrazine exposure markedly suppressed leukocyte recruitment into the peritoneal cavity of thioglycollate-stimulated frogs, there was no assessment of the response of frogs to a range of concentrations of atrazine alone, a logical question to ask since it had been shown to be stimulatory at 21 μg/L. In addition, no attempt was made to phenotype the composition of the thioglycollate elicited cell exudates. Both of these omissions are major flaws of the study. The authors also characterized the percentage of phagocytes present in the cells retrieved from the peritoneal cavity. 753 These studies showed that after thioglycollate elicitation, approximately 23% of the cells in the exudate were phagocytes, as assessed by their ability to take up fluorescent spheres, which decreased to approximately 3% in frogs exposed to 21 μg/L atrazine, approximately an 80% difference between the two treatment groups. In contrast, frogs injected ip with Ringers (control) possessed approximately 2% phagocytic cells in their peritoneum, which increased to about 10% when treated with atrazine. These results, at least without further investigation, are somewhat paradoxical since atrazine in the presence of thioglycollate-elicitation suppressed the percentage of phagocytic cells yet in the absence of thioglycollate (i.e., no elicitation) increased the percentage of phagocytic cells. In fact, these results serve as a good illustration of why thioglycollate-elicitation is no longer commonly employed when assessing the effects of an agent on innate immune function. The authors noted these paradoxical effects of atrazine in the discussion section and reiterate that atrazine appears to stimulate the phagocytic activity of resident peritoneal cells while inhibiting phagocytic activity in the thioglycollate-induced peritoneal cells. This may or not be the case. Since it is unclear whether atrazine actually alters phagocytosis, whether atrazine alters the profile of cell types recruited to the peritoneum in the presence of thioglycollate, or whether thioglycollate-activated peritoneal cells are more susceptible to modulation of phagocytic activity by atrazine than are resident peritoneal cells, drawing conclusions from these results is highly speculative at best. The authors also presented data indicating that atrazine treatment increased the number of nonphagocytic cells after an ip injection of thioglycollate. As already discussed, it is difficult to discern the meaning of these results since “nonphagocytic” cells are defined functionally (i.e., do not possess fluorescent spheres). Therefore it is unclear whether the increase in nonphagocyic cells following atrazine treatment is due to a change in the profile of cell types recruited to the peritoneum or whether there is no change in the profile of cell types recruited but merely a decrease in the phagocytic activity of the phagocytic peritoneal cells. The authors’ primary conclusion from this study was that atrazine treatment acts as an “innate immune response disruptor” (Brodkin et al., 2007). The conclusion is based on the observation that atrazine treatment suppressed thioglycollate-induced recruitment of leukocytes into the peritoneum as assessed by cell counts. In the context of the experimental conditions and the concentrations of atrazine employed, the effects appear to be real. However, it is unclear whether the effects are directly mediated by atrazine, which cell types are being affected, and/or whether the effects are unique to thioglycollate elicitation. Moreover, although the authors state in the discussion that thioglycollateinduced peritonitis is a common model to study inflammation, it is not a common approach to assess the immunotoxicity of an agent on innate immune responses, which was the stated objective for this study. Many of the published studies on the effects of atrazine on immune function in amphibians have suffered from flaws in the 754 K. R. SOLOMON ET AL. Downloaded At: 15:43 28 October 2008 design, poor descriptions of methods, and the use of inappropriate techniques. Given the very limited information on the immunological effects of atrazine in aquatic organisms as a whole, it is impossible at the current state of research to evaluate the possible status of atrazine as an immunotoxic or suppressive compound for amphibians. As for fish, there have been no efforts so far to determine possible links between the exposure to atrazine and effects on the immune system in frogs from the wild. Further studies using better designed protocols will be necessary to address the potential of atrazine to interfere with the immune system of amphibians before any definitive conclusions can be drawn. XI. EFFECTS OF ATRAZINE ON BEHAVIOR The ability of organisms to survive to reproductive age requires that they make appropriate behavioral responses to changes in their environment. Behavior is an important determinant of reproductive success and, as such, is an important target for evolutionary selective forces, which can act to mold and shape species-specific behaviors. As a result, it can be difficult to generalize about a contaminant affecting “behavior,” as an organism’s response to changes in the environment may be species-specific. Ultimately, any behavior requires that an organism detect changes in its environment, integrate this information within the central nervous system, and then elicit appropriate motor commands to carry out the behavior. Major challenges remain in extrapolating from experimental data to populationlevel effects (Beebee and Griffiths, 2005), particularly utilizing wildlife populations (Kendall and Lacher, 1994). In response to potential behavioral effects, there are data in mammals suggesting that atrazine can act within the CNS by interacting with receptors for the inhibitory neurotransmitter gamma aminobutyric acid (Shafer et al., 1999) or by altering monoamine turnover (Das et al., 2000). There are only limited data on the potential effects of atrazine on behavior in non-mammalian vertebrates. The behaviors studied were not accompanied by neuroanatomical or pharmacological studies to identify mode of action and what neurotransmitter pathways may be affected; thus the responses reported are difficult to characterize in relation to those reported in mammals. A. Effects on Olfactory Neurons and Behavior in Fish Goldfish exhibited altered burst swimming, grouping and surfacing behavior in response to small concentrations of atrazine (Saglio and Trijasse, 1998). It is unclear whether the data were reported as a percentage or animals responding to the treatments. Moreover, the standard deviation measurements were large for the measured endpoints reported to be significantly different from controls, bringing into question whether appropriate statistical tests were used to analyze the data. For example, the authors reported that “control” fish exhibited no burst swimming (0.00 ± 0.00, SD) whereas fish exposed to 0.5 or 50 μg atrazine/L, but not 1 μg atrazine/L, exhibited greater burst swimming (3.38 ± 3.38 and 2.25 ± 3.15 for the 0.5- and 50-μg atrazine/L groups, respectively). Although test concentrations of atrazine in the exposure water were not confirmed analytically, the authors reported that baseline levels of atrazine in the tap water reached levels as great as 0.235 μg atrazine/L. Thus, there were no negative control groups employed in the study. Another issue is the fact that some behaviors in “control” animals differed nearly 15-fold between experiments: a difference that was greater than differences between atrazine treated and control animals in individual experiments. For example, sheltering behavior in “control” animals ranged from 4.63 ± 3.46 to 1.50 ± 1.91 to 15.50 ± 10.28 in three different experiments. Collectively, these issues make the Saglio and Trijasse (1998) paper impossible to interpret. A study by Moore and Waring (1998) on S. salar evaluated the effects of atrazine on pheromonally induced olfactory responses including the measurement of plasma steroid levels. Fish were exposed to graded concentrations of atrazine (0–20 μg/L) for 5 d and then were challenged with urine collected from ovulated female salmon. Blood samples were collected after 5 h, which is when the urine stimulated a pheromonallyinduced increase in plasma T, 11-ketotestosterone, or 17,20βdihydroxy-4-pregnen-3-one concentrations. Consistent with the suppression of olfactory responses to pheromones by atrazine, an exposure-dependent decrease in concentrations of plasma steroids (17,20β-dihydroxy-4-pregnen-3-one, testosterone, and 11-ketotestosterone) was observed (testosterone shown only, Fig. 12). The statistical analysis of the data was incorrect but a concentration response was evident. In the same study, Moore and Waring evaluated the in vitro release of free and conjugated steroids from fish primed with ovulated female urine alone or in combination with atrazine. These results failed to show a consistent effect of atrazine, although again the statistical analyses were inappropriate. The mechanism for this was most likely through a direct action of atrazine on olfactory neurons and not a direct action on the endocrine system. As discussed later, the explanation in the conclusion of the paper is not clear as it states that “atrazine is a known inhibitor of acetylcholinesterase,” a fact that is completely incorrect. A second experiment (Moore and Lower, 2001) showed that neither atrazine nor simazine, alone or in combination, appeared to alter concentrations of testosterone, K11-ketotestosterone, or 17,20β-dihydroxy-4-pregnen-3-one, compared to control fish that were also exposed to female priming pheromone (Fig. 13, testosterone shown only). Although this was a key question to ask, the authors did not actually test these differences for statistical significance. The authors reported statistical comparisons of treatment groups relative to the ethanol-treated control when the more appropriate comparisons are relative to fish treated with PGF2a (which was common to all treatment groups). The previously reported effects of atrazine on the priming pheromone response of plasma testosterone, 11-ketotestosterone, and 17,20βdihydroxy-4-pregnen-3-one concentrations (Moore and Waring, 1998) were not observed with either atrazine or simazine alone or in combination; however, smaller concentrations were tested Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES FIG. 12. Effect of atrazine on testosterone in mature male salmon parr. Data represent the mean + SEM of five fish per group (redrawn from data of Moore and Waring, 1998). than in the previous study (Moore and Waring, 1998). It is interesting that the apparent effect reported is an increase in concentration of plasma testosterone induced by atrazine and simazine: not a response consistent with the induction of aromatase. Inconsistency of responses and incorrect comparisons of treatments make these results difficult to interpret. Although effects on olfactory responses were reported, these were not reflected in changes in concentrations of the hormones measured in the studies. Further, the inconsistency of these results makes their significance at the population-level difficult to interpret. The study by Moore and Waring (1998) on S. salar reported concentrationdependent effects of atrazine on electrophysiology of the olfactory epithelium at nominal concentrations from 2 to 20 μg/L, but not at 0.5 μg/L. However, no analyses of exposure concentrations were conducted, no detail of how these solutions were prepared, and no information was given on whether solvents were used or not. Responses of the endocrine system showed inconsistent concentration responses but the authors did suggest that atrazine was affecting androgen metabolism. The authors reported that the priming effect on milt and plasma 17,20β-dihydroxy-4-pregnen-3-one concentrations were reduced at water atrazine concentrations at and above 0.04 μg/L. The mechanism for this was not clear and some of the statements in discussion are inconsistent with the known prop- 755 FIG. 13. The effects of the priming pheromone PGF2 alone and in combination graded concentrations of simazine and atrazine on plasma testosterone concentrations in mature male salmon parr. Control parr were treated ethanol alone. Data represent the mean + SEM of 7 fish/group (redrawn from Moore and Lower, 2001). erties of atrazine. The authors suggest that atrazine is a highly lipophilic substance that would bioaccumulate in the lipid-rich testes. But atrazine is not highly lipophilic, and the data for bioconcentration and bioaccumulation in fish (Giddings et al., 2005) and discussed earlier do not support this suggestion. It was reported that atrazine affected odor (amino acid Lhistidine)-evoked behavioral and neurophysiological responses in rainbow trout (Oncorhynchus mykiss) (Tierney et al., 2007). However, the actual exposure concentrations of atrazine in the tank water were not measured in this study. In these experiments, O. mykiss were allowed to acclimate to test troughs for 20 min prior to a 30-min exposure to the pesticide. After the 30-min pesticide exposure, fish were exposed to L-histidine dissolved in the water for 10 min on one side of the test trough (test side) and the percent time spent on the test side of the trough measured. Locomotor activity also was monitored for 10 min after the pesticide exposure. Exposure for 30 min to 100 but not 1 or 10 μg atrazine/L reduced the preference/avoidance response ratio to Lhistidine, whereas 1 and 10 μg atrazine/L increased locomotor activity (measured for 10 min after 30 min of atrazine exposure). Electro-olfactograms (EOG) also were measured in response to L-histidine as an indicator of olfactory neuron activity. Exposure 756 K. R. SOLOMON ET AL. to 10 and 100 μg atrazine, but not 1 μg atrazine/L, reduced the L-histidine-induced EOG. The population and reproductive significance of the reported behavioral effects are not clear. Three reports indicate that atrazine may affect locomotor behavior. Exposure for 24 h to 0.5 μg atrazine/L reportedly caused a significant increase in burst swimming reactions in goldfish (Saglio and Trijasse, 1998). Downloaded At: 15:43 28 October 2008 B. Effects on Behavior in Amphibians Although exposures of A. barbouri to atrazine in the range of 4–400 μg/L for 37 d had no effect on embryo survival or growth, Rohr et al. (2003) reported that exposure to the greatest concentration (400 μg/L) adversely affected antipredator behavior, which consisted of seeking refuge in response to a potential threat. Exposure of A. barbouri to >40 μg atrazine/L during the larval period resulted in postmetamorphic salamanders that displayed greater locomotor activity, fewer water-conserving behaviors, and greater desiccation (water loss) 4 and 8 months later (Rohr and Palmer, 2005). Although the control of motor patterns in urodeles is entirely dependent upon subcortical neuronal pathways and is, arguably, relatively primitive compared to mammals, the number of factors that can modulate motor command neurons and premotor areas of the brainstem and spinal cord are numerous and complex. The possible mechanisms underlying these reported effects and whether a direct effect of atrazine on nervous system activity is even plausible in these species are unknown. Because of our relative lack of understanding of role of behavior in amphibian field biology it is difficult to interpret the potential effects of atrazine, if any, on amphibian behavior. Overall, although several studies have reported effects of atrazine on the olfactory system in salmon, in many cases the responses are inconsistent between studies and do not show clear concentration-related responses. In at least one study, the behavioral responses are difficult to interpret based upon inadequacies in the experimental design (lack of a negative control). Effects on behavior of salamanders have been reported at concentrations ranging from 40 to 400 μg atrazine/L. The population and reproductive significance of the reported behavioral effects are not clear. XII. EFFECTS OF ATRAZINE AT THE POPULATION LEVEL Any contaminant that adversely alters biochemistry, physiology, development, reproduction, or behavior may alter reproductive success and subsequently affect population health. However, many of these responses do not result in ecologically relevant changes that affect the fitness of a population or its sustainability. Specifically, if the response does not affect the survival, growth, or reproduction of the individuals in a population, there would be no adverse effects on populations. In many cases, the changes at these levels of organization are adaptive responses that allow the individuals to adapt to exposures to stressors in a manner that does not translate into population-level effects. In addition, there are a number of density-dependent interactions between individuals in populations, between populations, and with the environment that they occupy. For this reason, subtle effects of a substance or effects on a small number of individuals would not necessarily be expected to result in a negative effect on the population. Finally, populations of animals are dynamic and fluctuate in response to the most important determinants of population sustainability—food supply, weather conditions, and habitat quality and quantity. This makes identification of clear effects difficult and, without clear and consistent effects, causality is very difficult to assign. There are no published population studies to suggest that atrazine is associated with declines in populations of amphibians, reptiles, and fish. Indeed, frogs from every continent where amphibians occur have suffered high mortality rates in recent times. It is generally agreed that no single cause can be invoked to explain the loss of diverse amphibian populations from several continents. The effect of habitat change on amphibian populations has been known for years but our understanding of the effects of chemicals on amphibians is comparatively recent. Chemical pollution does not totally explain the global amphibian declines in often montane areas or where agricultural chemicals are not used, and in recent years attention has shifted to five other possible stressors: increased exposure to UV radiation, direct exploitation, the spread of alien species, climate change, and emerging diseases. Chytridiomycosis, an emerging infectious disease, was identified as the most likely cause of massive population declines of frogs on multiple continents and is spreading globally, and rapidly. A. Atrazine and Reptiles There are no published population studies or other data to suggest that atrazine is associated with declines in the populations of reptiles. Even though atrazine has been reported to affect a steroidogenic enzyme in alligators in Lake Apopka, Florida, the researchers who reported these observations concluded that atrazine did not have any endocrine-disrupting effects on populations of alligators in that lake (Crain et al., 1997, 1999, and discussed earlier). The declines in populations of alligators in some states of the United States have been linked to excessive hunting and/or changes in habitat (Rhodes, 1998) and not to exposures to atrazine or other substances in the environment. Alligator populations in North Carolina have, in fact, recovered where hunting has been restricted (Rhodes, 1998). Based on this observed recovery, hunting in North Carolina was most likely responsible for the declines. B. Atrazine and Fish While some populations of fishes are threatened or in decline, most of these are the direct result of over-harvesting but some, such as salmon, are because of habitat alteration. As was the case for reptiles, there are no published studies of populations of fish that suggest that atrazine is associated with declines of fish. No adverse effects were observed in a number of microcosm studies of the effects of atrazine, except in situations where indirect ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES effects occurred through reduction in food supply for grazing fish or alterations in habitat through loss of macrophytes (reviewed in Solomon et al., 1996; Giddings et al., 2005). Downloaded At: 15:43 28 October 2008 C. Atrazine and Amphibians Frogs from every continent where amphibians occur have suffered high mortality rates in recent times, resulting in various species undergoing severe population reductions and some even becoming extinct (U.S. Fish and Wildlife Service, 1984; Fellers and Drost, 1993, Ingram and McDonald, 1993; Hines et al., 1999; Houlahan et al., 2000; Stuart et al., 2004; Mendelson et al., 2006). Before considering the limited data on atrazine and amphibian populations, it is valuable to briefly summarize what is known about the causes underlying amphibian declines and the evidence supporting a role of pesticides in these declines. Historical data indicate that amphibian declines began in the 1970s in northern Australia (Czechura and Ingram, 1990), the western United States (Sherman and Morton, 1993; Drost and Fellers, 1996), and Puerto Rico (Burrowes et al., 2004). Many declines took place in seemingly pristine and often montane areas (Pounds et al., 1997; Pounds and Crump, 1994; Young et al., 2001). The first reports of amphibian declines were received with skepticism since amphibian populations often fluctuate widely (Pechmann and Wilbur, 1994). A recent report from the IUCN’s Global Amphibian Assessment suggests that as many as a third of amphibian species (>5700) have undergone severe declines or extinction with many species on the brink of extinction (Stuart et al., 2004). Today it is generally agreed that no single cause can be invoked to explain the loss of diverse amphibian populations from several continents. Various amphibian species have specific habitat requirements and habitat loss has driven various species to the brink of extinction. For example, loss of migration routes has been implicated as a major cause for the reductions in the populations of two North American Bufonids to single populations (Bufo baxteri and Bufo houstonensis; U.S. Fish and Wildlife Service, 1984, 2001). Although the effects of, for example, habitat change have been known for many years, our understanding of the effects of chemicals on amphibians is comparatively recent (Collins and Storfer, 2003). Various studies have been conducted at the laboratory, microcosm, mesocosm, and field enclosure level, but proving deleterious effects of pesticides at the population level is a difficult problem (Beebee and Griffiths, 2005). Although the effects of atrazine treatment on aquatic communities have been well studied in a number of microcosm experiments (summarized in Giddings et al., 2005), few experimental studies of the effects of atrazine on amphibians at the population-level have been reported. One microcosm study reported on the effects of atrazine on community and food-web structure in small microcosms (11.3 L) containing Rana sylvatica tadpoles (Rohr and Crumrine, 2005). The treatment concentrations for atrazine in this study were two applications of 25 μg/L each spaced 2 weeks apart. Although exposure concentrations were not measured, this likely produced a nominal final concentration of 50 μg/L. The 757 authors reported direct effects of the atrazine treatment on the abundance of periphyton with indirect effects on chironomids, snails, and tadpoles. The effects on algae are not surprising at the stated nominal concentration—they are sensitive and the overall NOECcommunity reported in the review of over 20 microcosm studies was of the order of 20 μg/L. That grazers of phytoplankton may have been affected is also not unexpected but the relevance of this larger systems and the field should be tempered by the lack of realism in the size of the microcosms and the lack of power inherent in small systems with few replicates (Sanderson, 2002). Sophisticated analyses taking historical pesticide application data into account have strongly linked organophosphate and carbamate pesticides in agricultural use with the declines of four Californian anurans (Davidson, 2004). Another potential hazard that has been debated is pH shifts due to acid rain; however, there is no evidence to link acidification to amphibian declines (Vertucci and Corn, 1997). Chytridiomycosis, an emerging infectious disease, was identified as the most likely cause of massive population declines of frogs in Australia, New Zealand, Spain, Tanzania, and Meso America. Furthermore, recent evidence suggests that chytridiomycosis is spreading rapidly, sometimes resulting in the decline or disappearance of rare and endemic amphibians. This fungal disease was first described from moribund and dead amphibians that were collected at sites of mass deaths of frogs in Australia and Panama from 1993 to 1998 (Berger et al., 1998). The chytrid that infects Australian and Central American amphibians is similar in morphology, while analysis of zoospore ultrastucture and 18s rDNA sequence data placed the fungus in the order Chytridiales (Berger et al., 1998). The chytrid was subsequently described as a new genus and species, Batrachochytrium dendrobatidis (Longcore et al., 1999). Batrachochytrium dendrobatidis has low host specificity and is likely to infect any species of amphibian as infections have been detected globally in 15 amphibian families that include 94 species (Speare, 2001). Amphibian chytridiomycosis is an emerging infectious disease of amphibians and has been recognized as such on a global scale (Daszak et al., 1999, 2003; Mendelson et al., 2006) and was nominated for listing as a key threatening process under the Environment Protection and Biodiversity Conservation Act 1999 of New Zealand (Speare, 2001). Given the diverse habitats in which amphibian declines have been observed, a great deal of attention has been directed at prioritizing and classifying potential causes for declines. According to Collins and Storfer (2003), Class I hypotheses include factors such as habitat loss, introduction of nonindigenous species, and overexploitation and collection of amphibians. Normal variation in breeding success, which can be linked to the stochastic nature of rainfall and moisture availability, coupled with isolation of certain populations by reduction in ecological corridors, may be a primary cause of extinctions at the population level (Richter et al., 2003). Given the rapid urbanization of southern California and the limited natural water sources in 758 K. R. SOLOMON ET AL. TABLE 5 Relative sensitivity of reproductive endpoints to atrazine in fish and amphibians–laboratory studies Downloaded At: 15:43 28 October 2008 Endpoint Species Gonadal effects Testicular ovarian R. clamitans follicles R. pipiens R. pipiens X. laevis X. laevis X. laevis X. laevis X. laevis X. laevis X. laevis Intersex C. auratus P. promelas R. clamitans R. pipiens R. pipiens X. laevis X. laevis X. laevis X. laevis X. laevis X. laevis X. laevis Gonadal R. pipiens dysgenesis4 X. laevis Spermatogenesis C. auratus P. promelas P. promelas R. pipiens Oogenesis Seminiferous tubule diameter Sex ratio GSI Atrazine-specific effect Concentration response LOEC (μg/L)1 Reference No No >10 (Coady et al.; 2004) Yes No No No2 No No No No No No No No Yes No Yes Yes No3 No No No No Yes No No No No No No No No No No No No No Not tested No No No3 No No No No No <0.1 >15 >25 >25 >25 >100 >31 >25 >100 >859 50 >28 <0.1 >15 <0.1 <0.1 25 >25 >100 >31 >100 <0.1 (Hayes et al.; 2003) (Orton et al.; 2006) (Hayes et al.; 2002) (Carr et al.; 2003) (Coady et al.; 2005) (Kloas et al.; 2008) (Jooste et al.; 2005) (Du Preez et al.; 2008b) (Oka et al.; 2008) (Spanó et al.; 2004) (U.S. EPA 2005) (Coady et al.; 2004) (Hayes et al.; 2003) (Orton et al.; 2006) (Hayes et al.; 2002) (Hayes et al.; 2006b) (Carr et al.; 2003) (Coady et al.; 2005) (Kloas et al.; 2008) (Jooste et al.; 2005) (Oka et al.; 2008) (Hayes et al.; 2003) Yes No No No No 25 >859 >44 >224 15 (Carr et al.; 2003) (Spanó et al.; 2004) (Bringolf et al.; 2004) (U.S. EPA 2005) (Orton et al.; 2006) Yes 103 (Spanó et al.; 2004) P. promelas P. promelas Yes No No No Yes, slightly accelerated spermatogenesis Yes, follicular atresia No Yes, decrease No Yes >224 224 (U.S. EPA 2005) (U.S. EPA 2005) R. clamitans R. pipiens R. pipiens X. laevis X. laevis X. laevis X. laevis X. laevis X. laevis X. laevis C. auratus P. promelas No No No No No No No No No Yes No No No No No No No No No No No Yes No No >28 >25 >15 >200 >19 >25 >25 >100 >31 10 >859 >44 (Coady et al.; 2004) (Hayes et al.; 2003) (Orton et al.; 2006) (Hayes et al.; 2002) (Carr et al.; 2003) (Coady et al.; 2005) (Du Preez et al.; 2008b) (Kloas et al.; 2008) (Jooste et al.; 2005) (Oka et al.; 2008) (Spanó et al.; 2004) (Bringolf et al.; 2004) C. auratus 759 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES TABLE 5 Relative sensitivity of reproductive endpoints to atrazine in fish and amphibians–laboratory studies (Continued) Endpoint Species P. promelas X. laevis Downloaded At: 15:43 28 October 2008 Plasma sex steroids Plasma estradiol Atrazine-specific effect Concentration response LOEC (μg/L)1 No Yes, increase C. auratus Yes, increase M. salmoides Yes (formulated product only) P. promelas No X. laevis No X. laevis No Plasma testosterone C. auratus Yes, decrease M. salmoides No P. promelas No X. laevis Yes, decrease X. laevis No X. laevis Yes, decrease Plasma C. auratus Yes, decreased 11-ketotestosterone M. salmoides Yes, decrease P. promelas No Induction of X. laevis No aromatase CYP19 mRNA expression X. laevis No X. laevis No X. laevis No Secondary sex effects Laryngeal dilator X. laevis Yes, reduced muscle diameter X. laevis No X. laevis No Fecundity P. promelas No Fertilization P. promelas No Hatching success P. promelas No X. laevis No Plasma vitellogenin C. auratus No P. promelas No, when compared to vehicle controls P. promelas No M. salmoides No X. laevis No Transgenerational X. laevis No effects 1 Reference No No >224 12 (U.S. EPA 2005) (Hecker et al.; 2005a) Yes No 1000 100 (Spanó et al.; 2004) (Gross et al.; 1997) No No No Yes No No Not tested No Yes Yes >224 >259 >100 1000 >100 >224 25 >100 259 1000 (U.S. EPA 2005) (Hecker et al.; 2005b) (Hecker et al.; 2005a) (Spanó et al.; 2004) (Gross et al.; 1997) (U.S. EPA 2005) (Hayes et al.; 2002) (Hecker et al.; 2005a) (Hecker et al.; 2005b) (Spanó et al.; 2004) No No No 50 >224 >259 (Gross et al.; 1997) (U.S. EPA 2005) (Hecker et al.; 2005b) No No No >100 >259 >100 (Hecker et al.; 2005a) (Hecker et al.; 2005b) (Oka et al.; 2008) No 1 No No No No No No No No >20 >25 >44 >44 >44 >25 >859 >44 (Carr et al.; 2003) (Coady et al.; 2005) (Bringolf et al.; 2004) (Bringolf et al.; 2004) (Bringolf et al.; 2004) (Du Preez et al.; 2008b) (Spanó et al.; 2004) (Bringolf et al.; 2004) No No No No >224 >100 >1000 >25 (U.S. EPA 2005) (Gross et al.; 1997) (Oka et al.; 2008) (Du Preez et al.; 2008b) (Hayes et al.; 2002) As reported by the author, nominal or measured. No TOFs were observed in the atrazine-exposed frogs; however, they were observed in estradiol-exposed positive control frogs. In Table 2 of Hecker et al. (2006), the presence of TOFs in atrazine-exposed frogs is incorrect. 3 Based upon inability to determine phenotypic sex from the physical appearance of gonads in 4.7% of the animals. Histological evaluation revealed no mixture of testicular and ovarian tissue in these animals. 4 For the purposes of this review, gonadal dysgenesis also includes the term “discontinuous testes.” 2 760 K. R. SOLOMON ET AL. TABLE 6 Relative sensitivity of reproductive endpoints to atrazine in amphibians—Field studies Endpoint Larynx weight Downloaded At: 15:43 28 October 2008 Gonadal anomalies Testicular cell types GSI Sex ratio Species Response X. laevis Correlation with Concentration atrazine -response Reference No relationship between atrazine use and relative larynx mass in males and females. R. pipiens Hermaphroditism in frogs from sites with atrazine based on a single measurement in surface water at time of frog collection. X. laevis Gonadal anomalies in males and females. R. clamitans Gonadal anomalies in males and females. R. catesbeiana Gonadal anomalies in males and females. A. crepitans Intersex. No temporal relationship between incidence and historical use of atrazine. X. laevis No difference between exposed and reference sites. R. catesbeiana No No (Smith et al.; 2005) No No (Hayes et al.; 2003) No No No No No No No No No No No No X. laevis No No R. catesbeiana GSI or testicular cell types in males. No No X. laevis Sex ratio. No No R. catesbeiana Sex ratio. No No No differences between exposed and reference. Naturally present in one haplotype and absent in another. R. clamitans No differences between exposed and reference. Reported to occur well before the introduction of atrazine. R. catesbeiana No differences between exposed and reference. No No No NA No No No No (Smith et al.; 2005) (Murphy et al.; 2006a) (Smith, 2007 pers. com.) (Reeder et al.; 1998, Reeder et al.; 2005) (Smith et al.; 2005) (Smith, 2007 pers. com.) (Hecker et al.; 2004) (Smith, 2007 pers. com.) (Du Preez et al.; 2005b) (Smith, 2007 pers. com.) (Smith et al.; 2005) (Du Preez et al.; 2008a) (Murphy et al.; 2006a) (Witschi 1929) No No R. pipiens No No No No No No GSI in males and females. Testicular ovarian X. laevis follicles No differences between exposed and reference. Different between agricultural and non agricultural sites Found in males in sites where atrazine exposure may have occurred during development1 . (Murphy et al.; 2006a) (Smith, 2007 pers. com.) (Murphy et al.; 2006a) (McDaniel et al.; 2008) (Hayes et al.; 2003) (Continued on next page) 761 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES TABLE 6 Relative sensitivity of reproductive endpoints to atrazine in amphibians—Field studies Endpoint Aromatase Plasma testosterone Downloaded At: 15:43 28 October 2008 Plasma estradiol Species Correlation with Concentration atrazine -response Response Acris crepitans No temporal response, found before and after introduction of atrazine. X. laevis No differences between exposed and reference sites R. clamitans X. laevis R. clamitans No NA No No No No Testosterone in plasma of females. Yes Negative Testosterone in plasma of males and concentrations of the atrazine metabolite (DACT) in water. Plasma testosterone. Yes Negative No No R. catesbeiana Plasma testosterone. No No X. laevis Plasma estradiol and atrazine. No No Yes Negative R. clamitans Plasma estradiol and DEA concentrations. Plasma estradiol and atrazine. No No Yes Negative No No Plasma estradiol and DEA concentrations. R. catesbeiana Plasma estradiol and atrazine use. Reference (Reeder et al.; 2005) (Hecker et al.; 2004) (Murphy et al.; 2006a) (Hecker et al.; 2004) (Hecker et al.; 2004) (Murphy et al.; 2006b) (Smith, 2007 pers. com.) (Hecker et al.; 2004) (Murphy et al.; 2006b) (Smith, 2007 pers. com.) Note. Pers. com., personal communication. 1 Atrazine concentrations were measured when frogs were collected and exposures during development (if any) were not measured. the western United States, it is not surprising that 3 of the 10 anuran species currently listed as endangered or threatened in North America inhabit California (U.S. Fish and Wildlife Service, 2003). The introduction of aggressive anuran species such as bullfrogs also has been linked empirically to adverse effects on survival in the California Red-legged frog (Rana aurora draytonii) (Lawler et al., 1999; Department of the Interior Fish and Wildlife Service, 2000). In a long-term study, Vredenburg et al. (2004) demonstrated that the introduction of trout, which prey on larval anurans, into mountain ponds in the Sierras was responsible for the decline of mountain yellow-legged frogs (Rana mucosa). Class II hypotheses for amphibian declines include global changes in climate (UV radiation, global warming), emerging diseases (such as the chytrid fungus), and contaminants such as pesticides and industrial waste products (Berger et al., 1998). Amphibians have a thin and permeable integument and undergo embryonic development in eggs with relatively little protection from chemicals in the aquatic environment. Some have speculated that amphibians may be sensitive indicators of contaminant exposure (see Blaustein and Johnson, 2003) because of their unique morphology and life history patterns. However, the role of contaminants in amphibian declines has been hotly debated, especially since many reports of declining species have occurred in areas that should be protected, at least in theory, from widespread agricultural or industrial contamination (USGS, 2004). There are some data linking aerial drift of organophosphorus and other pesticides with amphibian declines in California and Costa Rica. Sparling et al. (2001) reported that surface waters in Sequoia National Park at an elevation (2000+ m) that had been associated with declining frog populations contained greater than 100 ng/L chlorpyrifos and greater than 65 ng/L diazinon. Furthermore, tree frog (Hyla regilla) tadpoles collected from populations in Sequoia and Yosemite National Parks, and located downwind from agricultural areas in the Sacramento and San Joaquin valleys, had body burdens of chlorinated pesticide residues 2–3 times greater than tadpoles from coastal areas of California (Sparling et al., 2001). Although chlorpyrifos, malathion, and diazinon, with 24-h LC50s of 2140 to 7490 μg/L, are not highly toxic to frogs at the measured Downloaded At: 15:43 28 October 2008 762 K. R. SOLOMON ET AL. environmental exposures to Rana boylii, their oxon metabolites were reported to be 10 to 100-fold more toxic (Sparling and Feller, 2007). Atmospheric transportation of current-use pesticides into montane regions of Costa Rica has been suggested as potential risk to wildlife (Daly et al., 2007), but no specific effects of these pesticides have been identified. There are only limited data on the potential relationship between atrazine and amphibian population dynamics. Amphibians inhabiting ponds on agricultural land in Minnesota and exposed to atrazine (0.1–0.5 μg/L) and de-ethyl atrazine (0.1– 0.3 μg/L) concentrations 5-fold greater than those reported to produce gonadal effects (Hayes et al., 2003) exhibited no differences in species richness or reproductive success (Knutson et al., 2004). This suggests that, if any gonadal anomalies exist, they do not appear to have effects at the population level. Du Preez et al. (2005b) examined populations of X. laevis inhabiting maize-growing areas with atrazine application versus non-maize-growing areas in South Africa and found no differences in several aspects of population structure including age and size classes. A study in R. catesbeiana in Iowa revealed no relationship between a number of population and reproductive parameters and concentrations of atrazine in the ponds (Smith, 2007 personal communication). Although the data are limited, the studies that are available to date do not make a compelling case for population-level effects of atrazine in amphibians. Additional well-controlled studies are needed before any conclusions regarding potential population-level effects of atrazine on amphibians can be reached. XIII. OVERALL CONCLUSIONS, AND RESEARCH DIRECTIONS A. Strengths and Uncertainties We have identified significant strengths as well as uncertainties in the studies we have reviewed. Strengths relate to experimental designs where a range of concentrations were tested and where exposure concentrations were verified. Strengths are also apparent in large numbers of animals used in some studies and in the consistency of observations of response across several laboratories. Several studies were carried out under full good laboratory practice (GLP) guidelines with quality assurance and quality control (QA/QC). Several others were conducted in the spirit of GLP with QA/QC. Two studies were conducted to specifically address issues raised by a U.S. EPA Science Advisory Panel (U.S. EPA, 2003b) and were conducted under full GLP QA/QC in separate laboratories, one in the United States, the other in the European Union (EU) (Kloas et al., 2008). Additional strengths are in the comprehensiveness of the totality of the studies. These include a good understanding of the exposure scenarios resulting from the use of atrazine, concentrations present in surface waters, and knowledge of the pharmacokinetics of uptake and depuration of atrazine in fish and amphibians. In terms of effects on the individual organism, there were data on acute lethality, physiological effects, and induc- tion of developmental abnormalities. In terms of reproductive endpoints, there were data for effects on reproduction and sexdependent processes, such as sex differentiation, sexual and gonadal development, and secondary sexual characteristics, as well as hormone titers, in exposed animals. There were also data related to putative mechanisms mediated through aromatase. In addition to effects mediated through the reproductive system and associated hormones, there have been some studies on stress physiology, immune function, and behavior. There were also some observations on the effects of atrazine at the population level that serve to integrate effects at all other levels. The strengths of the available data were tempered by uncertainty at several levels. Uncertainties in exposures were evident in several laboratory studies where nominal concentrations were used or exposures were not characterized analytically. In other studies, there was contamination of controls with low concentrations of atrazine. The same uncertainty was evident in some field studies where there was no temporal characterization of exposure concentrations. There were uncertainties resulting from poor experimental designs, such as the testing of too few concentrations, use of inappropriate methods for mixture studies, lack of clarity and/or complete description of methods, and, in a few cases, inconsistencies in the data between published studies on the same experiments. In some cases, incorrect or inappropriate statistical methods and comparisons were used. An important potential uncertainty was identified relating to variation between haplotypes within a species in terms of background incidence of responses. In addition to these, there were uncertainties in several overarching issues that include a lack of understanding of the relevance of some physiological responses at higher levels of organization, such as on populations. These relate in particular to responses occurring at low frequency, which do not show a monotonic concentration response, and also occur naturally. B. Conclusions The primary focus of these conclusions is on the putative effects of atrazine on reproduction in aquatic vertebrates. Effects on stress, behavior, and effects at the population level were discussed earlier. In formulating our conclusions, we used a subset of the Bradford–Hill guidelines (Hill, 1965) as modified to assess causality of endocrine-modulated effects (IPCS, 2002) and reproductive responses to atrazine. These guidelines are based on temporality; strength of association; consistency; biological plausibility; and recovery. The effects of atrazine on aquatic species tested under laboratory conditions summarized in Table 5 and those for field studies in Table 6. Some studies (such as those of Tavera-Mendoza, discussed earlier) have been omitted from these tables because of significant concerns about the quality of the data. Temporality In terms of temporality, the study on A. crepitans in Illinois showed the presence of indicators of effects in sexual development (TOFs) prior to the introduction of atrazine to the market ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES Downloaded At: 15:43 28 October 2008 in 1957 (Reeder et al., 2005). In addition, TOFs were observed decades prior to the introduction of atrazine in other species of frogs from other locations (Witschi, 1929). The number and frequency of occurrence of TOFs decrease in X. laevis as frogs mature, whether in the presence of atrazine or not (Jooste et al., 2005; Du Preez et al., 2008a). This same phenomenon had been observed earlier (Gallien, 1974). Thus, there is no temporal evidence of any association between atrazine and reproductive effects as indicated by the presence of TOFs in frogs. Strength of Association Strength of association is best assessed by concentration response. This is particularly relevant and one of the key theories as to the mechanism of action of atrazine in that it causes changes in concentration of estradiol and testosterone by causing induction of aromatase (Hayes et al., 2003). In work on tissue cultures, this induction has been observed to show a consistent, monotonic concentration response (Sanderson et al., 2000). Thus, if the aromatase theory is correct, responses mediated via this mechanism should increase with increasing atrazine concentration. As summarized earlier, very few laboratory studies have reported a monotonic concentration response to atrazine. In terms of frequency and number of TOFs, zero of nine studies reported monotonic concentration responses. Using gross morphology of testes as an endpoint, 1 of 10 studies reported a concentration response and this was only evident at the greatest concentration tested (25 μg/L). For sex ratio and gonado-somatic index, 1 of 10 and 0 of 4 studies reported a concentration response, respectively. Of four studies on spermatogenesis, only one reported effects related to atrazine exposure—an acceleration of the process. This effect did not show a concentration response. One study reported a concentration-related decrease in seminiferous tubule diameter in the fish, P. promelas. Oogenesis, which is apparently unaffected by atrazine in frogs, responded to atrazine in C. auratus but not in P. promelas. No effects were observed on fecundity, fertilization, and hatching success in P. promelas exposed to 44 μg/L and no effects on these processes were observed in the F2 generation of X. laevis exposed throughout their lifespan to atrazine at concentrations as great as 25 μg/L. Similarly, larynx size in male frogs, a developmental process mediated by androgens, was reported to show an atrazine-related effect in only one of three laboratory studies but with no concentration-response and was not observed in the field (Table 6). Overall, these studies show poor strength of association between atrazine exposure and concentration for a number of reproductive and developmental endpoints. This is further evidenced by the presence of robust populations of amphibians in areas where atrazine is widely used and is present in surface waters. Given the number of studies done, there is no evidence of any atrazine-related effect. Biochemical endpoints related directly to aromatase responded equivocally. In the fish, C. auratus, plasma testosterone, and 11-ketotestosterone decreased while plasma estradiol in- 763 creased. However, this was only observed at large concentrations (>859 μg/L), and no effects were observed at the highest concentration tested (224 μg/L) in another fish, P. promelas. Atrazine was reported to cause a decrease in plasma testosterone in male X. laevis in one study (based on 4 animals) at 25 μg/L and also at 259 μg/L in a more robust study (15 males) but not at 100 μg/L (42 males). The change in plasma testosterone was not correlated with changes in aromatase activity, which remained the same as the control at 100 and 259 μg/L and plasma estradiol was not increased (as would be expected if the aromatase theory was correct) at 100 or 259 μg atrazine/L. Although testosterone concentrations in plasma appeared to be affected only at large exposure concentrations (259 μg/L), these were not accompanied by changes in aromatase or increases in plasma estradiol. This suggests that another mechanism may have been responsible, but only at large exposures. Atrazine is rapidly metabolized in frogs (Edginton and Rouleau, 2005) and also induces mixed-function oxidases in cell lines (Oh et al., 2003) and in frogs (Murphy et al., 2006c). Thus, the decrease in testosterone and some other steroids at large exposure concentrations may be the result of increased rates of degradation but is certainly not consistent with the aromatase-induction theory. Consistency There is consistency in the studies that have reported on the effects of atrazine on reproductive development in amphibians. The common and consistent theme in most of studies (Table 5 and Table 6) is that atrazine has no effects on reproduction or reproductive development. With rare exceptions, the only studies that report adverse effects on amphibian development and reproduction are those from the Hayes laboratory. Even these are not internally consistent; for example, the initial observation of effects on larynx size has not been reported from any other study. Some effects have been reported in fish, but the consistency here is that the effects are only observed at large concentrations that are not relevant to those measured in the environment. Biological Plausibility There is no evidence that atrazine itself (or its metabolites) act directly at hormone receptors such as those for estrogen or thyroid hormones. The theory of aromatase induction would result in increases in titers of endogenous estradiol and is biologically plausible. It has been shown to apply at large concentrations in cancer cell lines tested in vitro. However, in amphibians and fish, it is not supported by experimental observations, either of increased transcription of mRNA (some using very sensitive methods), induction of aromatase activity, or changes in the ratios of testosterone and estradiol in exposed animals. Thus, this is not a mechanism by which atrazine could affect reproductive development or reproduction in amphibians and fish. There are other theories where biological plausibility is absent. If atrazine changes the relationship between estradiol and testosterone, this would result in clear downstream effects on larynx size. Likewise, the nonmonotonic concentration response, 764 K. R. SOLOMON ET AL. Downloaded At: 15:43 28 October 2008 which is inconsistent with the observed concentration-related induction of aromatase in cancer cell lines, has been invoked to explain anomalous results (Hayes et al., 2003) when parsimony would suggest that there are other explanations for the observed effects. The implausible theory that atrazine is bioconcentrated to a large extent in frogs is similarly invoked to explain differences between studies in different laboratories (Hayes, 2004) when this is not supported by the physicochemical properties of atrazine or prior observations of bioconcentration in fish and amphibians, including X. laevis. Recovery The guideline of recovery is applicable where a stressor has been removed from the affected environment and recovery of the affected organisms occurs. Recovery has been observed in experimental systems, such as microcosms, and is used as a criterion for classification of responses in communities and ecosystems (Brock et al., 2006). For recovery to be used as a guideline for causality in assessing the effects of atrazine on aquatic vertebrates, there must be an effect from which recovery can be observed. This effect also must be consistent, reproducible, and robust so that it can be studied in this context. No such direct (or indirect) effects of atrazine on reproductive development or reproduction in aquatic vertebrates have been observed; thus, the guideline of recovery cannot be tested. Overall, the central theory that environmentally relevant concentrations of atrazine affect reproduction and/or reproductive development in amphibians is not supported by the vast majority of observations. The same conclusion also holds for the supporting theories such as induction of aromatase. XIV. SUMMARY The herbicide atrazine is widely used in agriculture for the production of corn and other crops. Atrazine is found in surface waters and several reports on the effects of atrazine on aquatic organisms have been published in the literature. However, there is inconsistency in the effects reported and inconsistency between studies in different laboratories. Some studies reported adverse effects on sexual development of atrazine in frogs and other amphibians. To assess whether atrazine causes adverse effects in frogs through mechanisms mediated by endocrine and other pathways, several hypotheses were tested in laboratory and field studies, using guidelines for the identification of causative agents of disease and ecoepidemiology derived from Koch’s postulates and the Bradford–Hill guidelines. The hypotheses were that atrazine used in crop protection causes adverse effects in amphibians through: (1) estrogen-mediated mechanisms, (2) androgen-mediated mechanisms, (3) thyroid-mediated mechanisms, (4) adverse effects on gonadal development in amphibians, or (5) adverse effects at the population level in exposed amphibians. The biological plausibility of the proposed mechanisms of endocrine disruption was critically assessed in relation to results of controlled laboratory and microcosm studies as well as field observations. These data include DNA genotyping in relation to the haplotype specificity of a developmental response based on the presence of testicular ovarian follicles in male frogs and the potential for transgenerational effects resulting from exposure to atrazine in frogs. Based on a weight-of-evidence analysis of all of the data, the central theory that environmentally relevant concentrations of atrazine affect reproduction and/or reproductive development in amphibians is not supported by the vast majority of observations. The same conclusion also holds for the supporting theories such as induction of aromatase, the enzyme that converts testosterone to estradiol. We conclude that environmentally relevant concentrations of atrazine do not affect amphibian growth, sexual development, reproduction, and survival. Although fewer data are available, the same conclusions apply to fish and reptiles. ACKNOWLEDGMENTS This review was developed with a grant from Syngenta Crop Protection, Inc. The authors specifically thank the following individuals for their help in preparing this review: Dr. Norbert Kaminski of the Department of Pharmacology and Toxicology, Michigan State University, for his contributions to reviewing the immunotoxicity papers; the late Sir Richard Doll, Green College, Oxford, for his helpful discussions of the BradfordHill guidelines; Cathy Bens for advice on quality assurance and report data; Susanne Williamson for logistics, coordination, and meeting arrangements; and Robert Bruce of Ecorisk for his management support. REFERENCES Allran, J.W., and Karasov, W.H. (2000). Effects of atrazine and nitrate on northern leopard frog (Rana pipiens) larvae exposed in the laboratory from posthatch through metamorphosis. Environ. Toxicol. Chem. 19:2850–2855. Allran, J.W., and Karasov, W.H. (2001). Effects of atrazine on embryos, larvae, and adults of anuran amphibians. Environ. Toxicol. Chem. 20:769–775. Ankley, G.T., Diamond, S.A., Tietge, J., Holcombe, G.W., Jensen, K.M., Defoe, D.L., and Peterson, R. (2002). Assessment of the risk of solar ultraviolet radiation to amphibians. I. Dose-dependent induction of hindlimb malformations in the northern leopard frog (Rana pipiens). Environ. Sci. Technol. 36:2853–2858. ASTM. (1992). Standard guide for conducting the Frog Embryo Teratogenesis Assay—Xenopus (FETAX). E1439-91. In Annual Book of ASTM Standards, Vol 11.04. Philadelphia, PA, pp. 1199–1209. Baker, J.L., and Laflen, J.M. (1979). Runoff losses of surface-applied herbicides as affected by wheel tracks and incorporation. J. Environ. Qual. 8:602–607. Barron, M.G. (1986). Endocrine control of smoltification in anadromous salmonids. J. Endocrinol. 108:313–319. Battaglin, W.A., Furlong, E.T., Burkhardt, M.R., and Peter, C.J. (2000). Occurrence of sulfonylurea, sulfonamide, imidazolinone, and other herbicides in rivers, reservoirs and ground water in the Midwestern United States, 1998. Sci. Tot. Environ. 248:123–133. Beebee, T.J.C., and Griffiths, R. (2005). The amphibian decline crisis: A watershed for conservation biology? Biol. Conserv. 132:136–142. Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES Belden, L.K. (2006). Impact of eutrophication on wood frog, Rana sylvatica, tadpoles indected with Echinostoma tricolvis cercaria. Can. J. Zool. 84:1315–1321. Beldomenico, P.M., Rey, F., Prado, W.S., Villarreal, J.C., Munõz-deToro, M., and Luque, E.H. (2007). In ovum exposure to pesticides increases the egg weight loss and decreases hatchlings weight of Caiman latirostris (Crocodylia: Alligatoridae). Ecotoxicol. Environ. Safety. 68:246–251. Berger, L., Speare, R., Daszak, P., Green, D.E., Cunningham, A.A., Goggin, C.L., Slocombe, R., Ragan, M.A., Hyatt, A.D., McDonald, K.R., Hines, H.B., Lips, K.R., Marantelli, G., and Parkes, H. (1998). Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. USA. 95:9031–9036. Biagianti-Risbourg S. (1990). Contribution a l’etude du foie de juveniles de muges Teleosteens, (Mugilides) contamines experimentalment par l’atrazine (s-triazine, herbicide): interet en ecotoxicologie [PhD thesis]. Perpignan, France: Academie de Montpellier, University of Perpignan, France. Birge, W.J., Black, J.A., Westerman, A.G., and Ramey, B.A. (1983). Fish and amphibian embryos—A model system for teratogenicity. Fundam. Appl. Toxicol. 3:237–242. Bisson, M., and Hontela, A. (2002). Cytotoxic and endocrine-disrupting potential of atrazine, diazinon, endosulfan, and mancozeb in adrenocortical steroidogenic cells of rainbow trout exposed in vitro. Toxicol. Appl. Pharmacol. 180:110–117. Blackler, A.W., Fischberg, M., and Newth, D.R. (1965). Hybridization of two subspecies of Xenopus laevis (Daudin). Rev Suisse Zool. 72:841–857. Blackler, A.W., and Fischberg, M. (1968). Hybridization of Xenopus laevis petersi/poweri and X. l. laevis. Rev. Suisse Zool. 75:1023– 1103. Blaustein, A.R., and Johnson, P.T.J. (2003). The complexity of deformed amphibians. Front. Ecol. Environ. 1:87–94. Bögi, C., Schwaiger, J., Ferling, J.H., Mallow, U., Steineck, C., Sinowatz, F., Kalbfus, W., Negele, R.D., Lutz, I., and Kloas, W. (2003). Endocrine effects of environmental pollution on Xenopus laevis and Rana temporaria. Environ. Res. 93:195–201. Bridges, C., Little, E., Gardiner, D., Petty, J., and Huckins, J. (2004). Assessing the toxicity and teratogenicity of pond water in northcentral Minnesota to amphibians. Environ. Sci. Res. Int. 11:233–239. Bringolf, R.B., Belden, J.B., and Summerfelt, R.C. (2004). Effects of atrazine on fathead minnow in a short-term reproduction assay. Environ. Toxicol. Chem. 23:1019–1025. Brock, T.C.M., Arts, G.H.P., Maltby, L., and van den Brink, P.J. (2006). Aquatic risks of pesticides, ecological protection goals and common aims in EU legislation. Integr. Environ. Assess. 2:e20–e46. Brockway D.L., Smith P.D., and Stancil F.E. (1984). Fate and effects of atrazine in small aquatic microcosms. Bull Environ Contam Toxicol. 32:345–353. Brodkin, M.A., Madhoun, H., Rameswaran, M., and Itzicj, V. (2007). Atrazine is an immune disruptor in adult nothern leopard frogs (Rana pipiens). Environ. Toxicol. Chem. 26:80–84. Burrowes, P.A., Joglar, R.L., and Green, D.E. (2004). Potential causes for amphibian declines in Puerto Rico. Herpetologica 60:141–154. Carr, J.A., Gentles, A., Smith, E.E., Goleman, W.L., Urquidi, L.J., Thuett, K., Kendall, R.J., Giesy, J.P., Gross, T.S., Solomon, K.R., and Van Der Kraak, G.J. (2003). Response of larval Xenopus lae- 765 vis to atrazine: Assessment of gonadal and laryngeal morphology. Environ. Toxicol. Chem. 22:396–405. Carr, J.A., and Norris, D.O. (2006). The hypothalamus–pituitary axis. In: Norris, D.O., and Carr, J.A., ed. Endocrine Disruption: The Biological Basis for Health Effects in Wildlife and Humans, pp. 87–110. Oxford University Press, New York. Chang, C.Y., and Witschi, E. (1956). Genetic control and hormonal reversal of sex differentiation in Xenopus. Proc. Soc. Exp. Biol. Med. 93:140–144. Christin, M.-S., Gendron, A.D., Brousseau, P., Ménard, L., Marcogliese, D.J., Cyr, D., Ruby, S., and Fournier, M. (2003). Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance to parasitic infection. Environ. Toxicol. Chem. 22:1127–1133. Christin, M.-S., Ménard, L., Gendron, A.D., Ruby, S., Cyr, D., Marcogliese, D.J., Rollins-Smith, L., and Fournier, M. (2004). Effects of agricultural pesticides on the immune system of Xenopus laevis and Rana pipiens. Aquat. Toxicol. 67:33–43. Clements, C., Ralph, S., and Petras, M. (1997). Genotoxicity of select herbicides in Rana catesbeiana tadpoles using the alkaline singlecell gel DNA electrophoresis (Comet) assay. Environ. Mol. Mutagen. 29:277–288. Coady, K.K., Murphy, M.B., Villeneuve, D.L., Hecker, M., Jones, P.D., Carr, J.A., Solomon, K.R., Smith, E.E., Van Der Kraak, G.J., Kendall, R.J., and Giesy, J.P. (2004). Effects of atrazine on metamorphosis, growth, and gonadal development in the green frog (Rana clamitans). J. Toxicol. Environ. Health A 67:941– 957. Coady, K.K., Murphy, M.B., Villeneuve, D.L., Hecker, M., Carr, J.A., Solomon, K.R., Van Der Kraak, G.J., Smith, E.E., Kendall, R.J., and Giesy, J.P. (2005). Effects of atrazine on metamorphosis, growth, laryngeal and gonadal development, aromatase activity, and plasma sex steroid concentrations in Xenopus laevis. Ecotoxicol. Environ. Safety. 62:160–173. Collins, J.P., and Storfer, A. (2003). Global amphibian declines: Sorting the hypotheses. Div. Distrib. 9:89–98. Cooper, R.L., Stoker, T.E., Tyrey, L., Goldman, J.M., and McElroy, W.K. (2000). Atrazine disrupts the hypothalamic control of pituitary– ovarian function. Toxicol. Sci. 53:297–307. Cooper, R.L., Laws, S.C., Das, P.C., Narotsky, M.G., Goldman, J.M., Tyrey, E.L., and Stoker, T.E. (2007). Atrazine and reproductive function: Mode and mechanism of action studies. Brth Defects Res. (B) 80:98–112. Cossarinidunier, M. (1987). Effects of the pesticides atrazine and lindane and of manganese ions on cellular-immunity of carp, Cyprinus carpio. J. Fish. Biol. 31:67–73. Cossarinidunier, M., Demael, A., Riviere, J.L., and Lepot, D. (1988). Effects of oral doses of the herbicide atrazine on carp (Cyprinus carpio). Ambio 17:401–405. Cossarinidunier, M., and Hattenberger, A.M. (1988). Effect of pesticides (atrazine and lindane) on the replication of spring viremia of carp virus in vitro. Ann. Res. Vet. 19:209–211. Crain, D.A., Guillette, L.J., Rooney, A.A., and Pickford, D.B. (1997). Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ. Health Perspect. 105:528–553. Crain, D.A., Spiteri, I.D., and Guillette, L.J., Jr. (1999). The functional and structural observations of the neonatal reproductive system of Downloaded At: 15:43 28 October 2008 766 K. R. SOLOMON ET AL. alligators exposed in ovo to atrazine, 2,4-D, or estradiol. Toxicol. Ind. Health 15:180–185. Czechura, G.V., and Ingram, G. (1990). Taudactylus diurnus and the case of the disappearing frogs. Mem. Queenl. Mus. 29:361–365. Daly, G.L., Lei, Y.D., Teixeira, C., Muir, D.C.G., Castillo, L.E., and Wania, F. (2007). Accumulation of current-use pesticides in neotropical montane forests. Environ. Sci. Technol. 41:1118–1123. Das, P.C., McElroy, W.K., and Cooper, R.L. (2000). Differential modulation of catecholamines by chlorotriazine herbicides in pheochromocytoma (PC12) cells in vitro. Toxicol. Sci. 56:324–331. Daszak, P., Berger, L., Cunningham, A.A., Hyatt, A.D., Green, D.E., and Speare, R. (1999). Emerging infectious diseases and amphibian population declines. Emerg. Infect. Dis. 5:745–748. Daszak, P., Cunningham, A.A., and Hyatt, A.D. (2003). Infectious disease and amphibian population declines. Div. Distrib. 9:141–150. Davidson, C. (2004). Declining downwind: Amphibian population declines in California and historical pesticide use. Ecol. Appl. 14:1892– 1902. Davis, D.L., Bradlow, H.L., Wolff, M., Woodruff, T., Hoel, D.G., and Anton-Culver, H. (1993). Medical hypothesis: Xenoestrogens as preventable causes of breast cancer. Environ. Health Perspect. 101:372– 377. De Solla, S.R., Martin, P.A., Fernie, K.J., Park, B.J., and Mayne, G. (2006). Effects of environmentally relevant concentrations of atrazine on gonadal development of snapping turtles (Chelydra serpentina). Environ. Toxicol. Chem. 25:520–526. Department of the Interior Fish and Wildlife Service. (2000). 50 CFR Part 17. Endangered and threatened wildlife and plants; Proposed designation of critical habitat for the California Red-legged Frog (Rana aurora draytonii). Fed. Reg. 65:54892. Detenbeck, N.E., Hermanutz, R., Allen, K., and Swift, M.C. (1996). Fate and effects of the herbicide atrazine in flow-through wetland mesocosms. Environ. Toxicol. Chem. 15:937–946. Devine, M.D., Duke, S.O., and Fedtke, C. (1993). Physiology of Herbicide Action. Englewood Cliffs, NJ: Prentice Hall. Dhabhar, F.S., McEwen, B.S., and Spencer, R.L. (1993). Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels—A comparison between Sprague-Dawley, Fischer 344 and Lewis rats. Brain Res. 616:89–98. Dhabhar, F.S., Miller, A.H., Stein, M., McEwen, B.S., and Spencer, R.L. (1994). Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav. Immunol. 8:66–79. Diamond, S.A., Peterson, G.S., Tietge, J., and Ankley, G.T. (2002). Assessment of the risk of solar ultraviolet radiation to amphibians. III. Prediction of impacts in selected Northern Midwestern wetlands. Environ. Sci. Technol. 36:2866–2874. Diana, S.G., Resetarits, W.J., Jr., Schaeffer, D.J., Beckman, K.B., and Beasley, V.R. (2000). Effects of atrazine on amphibian growth and survival in artificial aquatic communities. Environ. Toxicol. Chem. 19:2961–2967. Drost, C.A., and Fellers, G.M. (1996). Collapse of a regional frog fauna in the Yosemite area of the California Sierra Nevada. Conserv. Biol. 10:410–425. Du Preez, H.H., and van Vuren, H.J. (1992). Bioconcentration of atrazine in the banded tilapia, Tilapia sparrmanii. Comp. Biochem. Physiol. C 101:651–655. Du Preez, H.H., Kunene, N., Hanner, R., Evans, B.J., Giesy, J.P., Hosmer, A.J., Solomon, K.R., and Van Der Kraak, G.J. (2008a). Clade- specific occurrence of testicular oocytes in Xenopus laevis from South Africa. Aquat. Toxicol., in press. Du Preez, L.H., Jansen van Rensburg, P.J., Jooste, A.M., Carr, J.A., Giesy, J.P., Gross, T.S., Kendall, R.J., Smith, E.E., Van Der Kraak, G., and Solomon, K.R. (2005a). Seasonal exposures to triazine and other pesticides in surface waters in the western Highveld corn-production region in South Africa. Environ. Pollut. 135:131–141. Du Preez, L.H., Solomon, K.R., Carr, J.A., Giesy, J.P., Gross, T.S., Kendall, R.J., Smith, E.E., Van Der Kraak, G.J., and Weldon, C. (2005b). Population structure of the African clawed frog (Xenopus laevis) in maize-growing areas with atrazine application versus nonmaize-growing areas in South Africa. Afr. J. Herpatol. 54:61–68. Du Preez, L.H., Kunene, N., Everson, G.J., Carr, J.A., Giesy, J.P., Gross, T.S., Kendall, R.J., Smith, E.E., Solomon, K.R., and Van Der Kraak, G.J. (2008b). Reproduction, larval growth, and reproductive development in African clawed frogs (Xenopus laevis) exposed to atrazine. Chemosphere 71:546–552. Dunier, M., and Siwicki, A.K. (1993). Effects of pesticides and other organic pollutants in the aquatic environment on immunity of fish— A review. Fish Shellfish Immunol. 3:423–438. Edginton, A.N., and Rouleau, C. (2005). Toxicokinetics of 14 C-atrazine and its metabolites in stage-66 Xenopus laevis. Environ. Sci. Technol. 39:8083–8089. Edwards, W.M., Shipitalo, M.J., Lal, R., and Owens, L.B. (1997). Rapid changes in concentration of herbicides in corn field surface depressions. J. Soil Water Conserv. 52:227–281. Fan, W., Yanase, T., Morinaga, H., Gondo, S., Okabe, T., Nomura, M., Komatsu, T., Morohashi, K.-I., Hayes, T.B., Takayanagi, R., and Nawata, H. (2007). Atrazine-induced aromatase expression is SF1 dependent: Implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ. Health Perspect. 115:720– 727. Fasano, S., Minucci, S., Di Matteo, L., D’Antonio, M., and Pierantoni, R. (1989). Intratesticular feedback mechanisms in the regulation of steroid profiles in the frog, Rana esculenta. Gen. Comp. Endocrinol. 75:335–342. Fellers, G.M., and Drost, C.A. (1993). Disappearence of the Cascades frog Rana cascadae at the southern end of its range, California. Biol. Conserv. 65:177–181. Fernandez, M., L’Hardidon, J., Gautnier, L., and Zoll-Moreux, C. (1993). Amphibian micronucleus test(s): A simple and reliable method for evaluating in vivo genotoxic effects of freshwater pollutants and radiation. Initial assessment. Mutat. Res. 292:83–99. Filipov, N.M., Pinchuk, L.M., Boyd, B.L., and Crittenden, P.L. (2005). Immunotoxic effects of short-term atrazine exposure in young male C57BL/6 mice. Toxicol. Sci. 86:324–332. Fournier, M., Friborg, J., Girard, D., Mansour, S., and Krzystyniak, K. (1992). Limited immunotoxic potential of technical formulation of the herbicide atrazine (Aatrex) in mice. Toxicol. Lett. 60:263–274. Frank, R., Braun, H.E., Ripley, B.D., and Clegg, B.S. (1990). Contamination of rural ponds with pesticide, 1971–1985, Ontario, Canada. Bull. Environ. Contam. Toxicol. 44:401–409. Freeman, J.L., and Rayburn, A.L. (2004). In vivo genotoxicity of atrazine to anuran larvae. Mutat. Res. 560:69–78. Freeman, J.L., and Rayburn, A.L. (2005). Developmental impact of atrazine on metamorphing Xenopus laevis as revealed by nuclear analysis and morphology. Environ. Toxicol. Chem. 24:1648–1653. Fridgen, C.M., Pauli, B.D., Berrill, M., Doe, K., and Jackman, P. (2005). Chronic effects of atrazine herbicide on the development of Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES Northern leopard frog (Rana pipiens) tadpoles. SETAC National Meeting, Baltimore, MD. SETAC, Pensacola, FL. Gallien, L. (1974). Intersexuality. In: Lofts, B., ed. Physiology of the Amphibia. pp. 523–549. Academic Press, New York. Gammon, D.W., Aldous, C.N., Carr, W.C., Jr., Sanborn, J.R., and Pfeifer, K.F. (2005). A risk assessment of atrazine use in California: Human health and ecological aspects. Pestic. Manage. Sci. 61:331– 355. Gendron, A.D., Marcogliese, D.J., Barbeau, S., Christin, M.S., Brousseau, P., Ruby, S., Cyr, D., and Fournier, M. (2003). Exposure of leopard frogs to a pesticide mixture affects life history characteristics of the lungworm Rhabdias ranae. Oecologia 135:469–476. Giddings, J.M., Anderson, T.A., Hall, L.W., Jr, Kendall, R.J., Richards, R.P., Solomon, K.R., and Williams, W.M. (2005). A Probabilistic Aquatic Ecological Risk Assessment of Atrazine in North American Surface Waters. Pensacola, FL: SETAC Press. Gluth, G., and Hanke, W.A. (1985). Comparison of physiological changes in carp, Cyprinus carpio, induced by several pollutants at sublethal concentrations. I. The dependency on exposure time. Ecotoxicol. Environ. Safety. 9:179–188. Görge, G., and Nagel, R. (1990). Kinetics and metabolism of 14 Clindane and 14 C-atrazine in early life stages of zebrafish (Brachydanio rerio). Chemosphere 21:1125–1137. Goulet, B.N., and Hontela, A. (2003). Toxicity of cadmium, endosulfan, and atrazine in adrenal steroidogenic cells of two amphibian species, Xenopus laevis and Rana catesbeiana. Environ. Toxicol. Chem. 22:2106–2113. Grady, J., Wieser, C., Wiebe, J., and Gross, T.S. (1998). An evaluation of atrazine as a potential endocrine disruptor in largemouth bass. SETAC Annual Meeting, November 15–19, 1998; Charlotte, NC. Grim, K.C., Wolfe, M., Hawkins, W., Johnson, R.D., and Wolf, J. (2007). Intersex in Japanese medaka (Oryzias latipes) used as negative controls in toxicological bioassays: A review of 54 vases from 41 studies. Environ. Toxicol. Chem. 26:1636–1643. Groombridge, B. (1982). The IUCN Amphibia–Reptilia Red Data Book. Gland, Switzerland: International Union for Conservation of Nature and Natural Resources. Gross, T.S., Shrestha, S., Wieser, C.M., Wiebe, J.J., Denslow, N.D., Chow, C., Johnson, W.E., and Stout, R. (1997). Evaluation of potential endocrine-disrupting effects of water-soluble herbicides in largemouth bass. SETAC Annual Meeting, November 16–20, 1997; San Francisco, CA. Gross, T.S. (1999a). Determination of Potential Effects of 10 Day Neonatal Exposure of Atrazine on Histological and Hormonal Sex Determination in Incubated American Alligator (Alligator mississippiensis) Eggs. Gainesville, FL: University of Florida, Wildlife Reproductive Toxicology Laboratory. No. NOVA98.02a. 22 p. Gross, T.S. (1999b). Determination of potential effects of 10 day neonatal exposure of atrazine on histological and hormonal sex determination in incubated red-eared slider (Psuedemys elegans) eggs. Gainesville: University of Florida, Wildlife Reproductive Toxicology Laboratory. No. NOVA98.02b. Guillette, L.J., Jr, Gross, T.S., Masson, G.R., Matter, J.M., and Woodward, A.R. (1994). Developmental abnormalities of the gonad of juvenile alligators from contaminated and control lakes in Florida. Environ. Health Perspect. 102:680–688. Guillette, L.J., Jr, Pickford, D.B., Crain, D.A., Rooney, A.A., and Percival, H.F. (1996). Reduction in penis size and plasma testos- 767 terone concentrations in juvenile alligators living in a contaminated environment. Gen. Comp. Endocrinol. 101:32–42. Gunkel, G., and Streit, B. (1980). Mechanisms of bioaccumulation of a herbicide (atrazine, s-triazine) in a freshwater mollusc (Ancylus fluviatilis Muell.) and a fish (Coregonus fera Jurine.). Water Res. 14:1573–1584. Guyton, A.C., and Hall, J.E. (1996). Human Physiology and Mechanisms of Disease. Philadelphia, PA: W.B. Saunders. Hamala, J.A., and Kollig, H.P. (1985). The effects of atrazine on periphyton communities in controlled laboratory ecosystems. Chemosphere 14:1391–1408. Hayes, T.B. (1998). Sex determination and primary sex differentiation in amphibians: Genetic and developmental mechanisms. J. Exp. Zool. 281:373–399. Hayes, T.B., Stuart, A.A., Vonk, A., and Liu, R. (2001). Atrazine disrupts sex differentiation in the African clawed frog (Xenopus laevis) at ecologically relevant doses. SETAC 22nd Annual Meeting, November 12–15 2001; Baltimore, MD. SETAC. Abstract 421 p. Hayes, T.B., Collins, A., Mendoza, M., Noriega, N., Stuart, A.A., and Vonk, A. (2002). Hermaphroditic, demasculinized frogs exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl. Acad. Sci. USA 99:5476–5480. Hayes, T.B., Haston K., Tsui, M., Hoang, A., Haeffele, C., and Vonk, A. (2003). Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): Laboratory and field evidence. Environ. Health Perspect. 111:568–575. Hayes, T.B. (2004). This is no denying this: Defusing the confusion about atrazine. Bioscience 54:1138–1149. Hayes, T.B., Case, P., Chui, S., Chung, D., Haefele, C., Haston, K., Lee, M., Mai, V.P., Marjuoa, Y., Parker, J., and Tsui, M. (2006a). Pesticide mixtures, endocrine disruption, and amphibian declines: Are we underestimating the impact? Environ. Health Perspect. 114 Sup. 1:40–50. Hayes, T.B., Stuart, A.A., Mendoza, M., Collins, A., Noriega, N., Vonk, A., Johnston, G., Liu, R., and Kpodzo, D. (2006b). Characterization of atrazine-induced gonadal malformations in African clawed frogs (Xenopus laevis) and comparisons with effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (17ß-estradiol): Support for the demasculinization/feminization hypothesis. Environ. Health Perspect. 114 Sup. 1:134–141. Hecker, M., Coady, K.K., Villeneuve, D.L., Murphy, M.B., Jones, P.D., and Giesy, J.P. (2003). Response of Xenopus laevis to atrazine exposure: assessment of the mechanism of action of atrazine. East Lansing: Michigan State University–Aquatic Toxicology Laboratory. Syngenta. No. 1165–02. 143 p. Hecker, M., Giesy, J.P., Jones, P.D., Jooste, A.M., Carr, J.A., Solomon, K.R., Smith, E.E., Van Der Kraak, G.J., Kendall, R.J., and Du Preez, L.H. (2004). Plasma sex steroid concentrations and gonadal aromatase activities in African clawed frogs (Xenopus laevis) from the corn-growing region of South Africa. Environ. Toxicol. Chem. 23:1996–2007. Hecker, M., Kim, W.J., Park, J.-W., Murphy, M.B., Villeneuve, D., Coady, K.K., Jones, P.D., Solomon, K.R., Van Der Kraak, G.J., Carr, J.A., Smith, E.E., du Preez, L.H., Kendall, R.J., and Giesy, J.P. (2005a). Plasma concentrations of estradiol and testosterone, gonadal aromatase activity, and ultrastructure of the testis in Xenopus laevis exposed to estradiol and atrazine. Aquat. Toxicol. 72:383– 396. Downloaded At: 15:43 28 October 2008 768 K. R. SOLOMON ET AL. Hecker, M., Park, J.-W., Murphy, M.B., Jones, P.D., Solomon, K.R., Van Der Kraak, G.J., Carr, J.A., Smith, E.E., Du Preez, L.H., Kendall, R.J., and Giesy, J.P. (2005b). Effects of atrazine on CYP19 gene expression and aromatase activity in testes and on sex steroid concentrations in plasma of male African clawed frogs (Xenopus laevis). Toxicol. Sci. 86:273–280. Hecker, M., Murphy, M.B., Coady, K.K., Villeneuve, D.L., Jones, P.D., Carr, J.A., Solomon, K.R., Smith, E.E., Van Der Kraak, G.L., Gross, T.S., du Preez, L.H., Kendall, R.J., and Giesy, J.P. (2006). Terminology of gonadal anomalies in fish and amphibians resulting from chemical exposures. Rev. Environ. Contam. Toxicol. 187:103– 132. Heneweer, M., van den Berg, M., and Sanderson, J.T. (2004). A comparison of human H295R and rat R2C cell lines as in vitro screeing tools for effects on aromatase. Toxicol. Lett. 146:183–194. Hill, A.B. (1965). The environment and disease: association or causation? Proc. R. Soc. Med. 58:295–300. Hines, H., Mahony, M., and McDonald, K. (1999). An assessment of frog declines in wet subtropical Australia. In: Campbell A., ed. Declines and Disappearances of Australian Frogs, pp. 44–63. Environment Australia, Canberra. Hoagland, K.D., Drenner, R.W., Smith, J.D., and Cross, D.R. (1993). Freshwater community responses to mixtures of agricultural pesticides: Effects of atrazine and bifenthrin. Environ. Toxicol. Chem. 12:627–637. Hoberg, J.R. (2007). The Toxicity of Atrazine to the Freshwater Macrophyte Elodea canadensis at Three Light Intensities for 14 Days. Greensboro, NC: Syngenta Crop Protection, Inc. No. 1781.6691. 96 p. Hooghe, R.J., Devos, S., and Hooghe-Peters, E.L. (2000). Effects of selected herbicides on cytokine production in vitro. Life Sci. 66:2519– 2525. Houck, A., and Sessions, S.K. (2006). Could atrazine affect the immune system of the frog, Rana pipiens? Bios 77:107–112. Houlahan, J.E., Findlay, C.S., Schmidt, B.R., Meyer, A.H., and Kuzmin, S.L. (2000). Quantitative evidence for global amphibian population declines. Nature 404:752–755. Howard, P.H. (1991). Handbook of Environmental Fate and Exposure Data for Organic Chemicals. Chelsea, MI: Lewis. Howe, G.E., Gillis, R., and Mowbray, R.C. (1998). Effects of chemical synergy and larval stage on the toxicity of atrazine and alachlor to amphibian larvae. Environ. Toxicol. Chem. 17:519–525. Hu, F., Smith, E.E., and Carr, J.A. (2008). Effects of larval exposure to estradiol on spermatogenesis and in vitro gonadal steroid secretion in African clawed frogs, Xenopus laevis. Gen. Comp. Endocrinol. 155:190–200. Ingram, G.J., and McDonald, K.R. (1993). An update on the decline of Queenslands frogs. In: Lunney D., and Ayers D., eds. Herpetology in Australia: A Diverse Discipline, pp. 297–303. Royal Zoological Society of NSW, Mosman. IPCS. (2002). Global Assessment of the State-of-the-Science of Endocrine Disruptors. Geneva, Switzerland: International Programme on Chemical Safety of the World Health Organization. No. WHO/PCS/EDC/02.2. http://www.who.int/pcs Jensen, K.I.N., Stephenson, G.R., and Hunt, L.A. (1977). Detoxification of atrazine in three gramineae subfamilies. Weed Sci. 25:212–220. Johnson, P.T.J., Lunde, K.B., Richie, E.G., and Launer, A.E. (1999). The effect of tematode infection on amphibian limb development and survivorship. Science 284:802–804. Johnson, P.T.J., Lunde, K.B., Thurman, E.M., Ritchie, E.G., Wray, S.N., Sutherland, D.R., Kapper, J.M., Frest, T.J., Bowerman, J., and Blaustein, A.R. (2002). Parasite (Ribeiroia ondatrae) infection linked to amphibian malformation in the western United States. Ecol. Monogr. 72:151–168. Johnson, P.T.J., Chase, J.M., Dosch, K.L., Hartson, R.B., Gross, J.A., Larson, D.J., Sutherland, D.R., and Carpenter, S.R. (2007). Aquatic eutrophication promotes pathogenic infection in amphibians. Proc. Natl. Acad. Sci. USA 104:15781–15786. Jooste, A.M., Du Preez, L.H., Carr, J.A., Giesy, J.P., Gross, T.S., Kendall, R.J., Smith, E.E., Van Der Kraak, G.J., and Solomon, K.R. (2005). Gonadal development of Xenopus laevis larvae exposed through larval development to atrazine in outdoor microcosms. Environ. Sci. Technol. 39:5255–5261. Kang, L., Marin, M., and Kelley, D. (1995). Androgen biosynthesis and secretion in developing Xenopus laevis. Gen. Comp. Endocrinol. 100:293–307. Karrow, N.A., Mccay, J.A., Brown, R.D., Musgrove, D.L., Guo, T.L., Germolec, D.R., and White, K.L. (2005). Oral exposure to atrazine modulates cell-mediated immune function and decreases host resistance to the B16F10 tumor model in female B6C3F1 mice. Toxicology 209:15–28. Kazeto, Y., Place, A.R., and Trant, J.M. (2004). Effects of endocrine disrupting chemicals on the expression of CYP19 genes in zebrafish (Danio rerio) juveniles. Aquat. Toxicol. 69:25–34. Kendall, R.J., and Lacher, T.E., Jr, eds. (1994). Wildlife Toxicology and Population Modeling: Integrated Studies of Agroecosystems. Lewis, Chelsea, MI. Kiesecker, J.M. (2002). Synergism between trematode infection and pesticide exposure: A link to amphibian limb deformities in nature? Proc. Natl. Acad. Sci. USA 99:9900–9904. Klaasen, H.E., and Kadoum, A.H. (1979). Distribution and retention of atrazine and carbofuran in farm pond ecosystems. Arch. Environ. Contam. Toxicol. 8:345–353. Kloas, W., Lutz, I., Springer, T., Krueger, H., Wolf, J., Holden, L., and Hosmer, A. (2008). Atrazine does not induce gonadal feminization in Xenopus laevis. Toxicol. Sci., accepted. Knutson, M.G., Richardson, W.B., Reineke, D.M., Gray, B.R., Parmelee, J.R., and Weick, S.E. (2004). Agricultural ponds support amphibian populations. Ecol. Appl. 14:669–684. Koch, R. (1942). The aetiology of tuberculosis (translation of Die Aetiologie der Tuberculose [1882]). In: Clark, D.H., ed. Source Book of Medical History, pp. 392–406. Dover, New York. Koprivnikar, J., Forbes, M.R., and Baker, R.L. (2006). Effects of atrazine on cercarial longevity, activity, and infectivity. J. Parasitol. 92:306–311. Koprivnikar, J., Forbes, M.R., and Baker, R.L. (2007). Contaminant effects on host–parasite interactions: Atrazine, frogs, and trematodes. Environ. Toxicol. Chem. 26:2166–2170. Larson, D.L., McDonald, S., Fivizzani, A.J., Newton, W.E., and Hamilton, S.J. (1998). Effect of the herbicide atrazine on Ambystoma tigrinum metamorphosis: Duration, larval growth and hormonal response. Physiol. Zool. 71:671–679. Lawler, S.P., Dritz, D., Strange, T., and Holyoak, M. (1999). Effects of introduced mosquitofish and bullfrogs on the threatened California red-legged frog. Conserv. Biol. 13:613–622. LeBlanc, G.A., and Wang, G. (2006). Chemical mixtures: Greaterthan-additive effects? Environ. Health Perspect. 114:A517– A518. Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES Lenkowski, J.R., Reed, J.M., Deininger, L., and McLaughlin, K.A. (2008). Perturbation of organogenesis by the herbicide atrazine in the amphibian Xenopus laevis. Environ. Health Perspect. 116:223– 230. Levy, G., Lutz, I., Kruger, A., and Kloas, W. (2004). Bisphenol A induces feminization in Xenopus laevis tadpoles. Environ. Res. 94:102– 111. L’Haridon, J., Fernandez, M., Ferrier, V., and Bellan, J. (1993). Evaluation of the genotoxicity of N -nitrosoatrazine, N nitrosodiethanolamine and their precursors in vivo using the newt micronucleus test. Water Res. 28:855–862. Licht, P., McCreery, B.R., Barnes, R., and Pang, R. (1983). Seasonal and stress related changes in plasma gonadotropins, sex steroids, and corticosterone in the bullfrog, Rana catesbeiana. Gen. Comp. Endocrinol. 50:124–145. Lipnick, R.L. (1993). Baseline toxicity QSAR models: A means to assess mechanism of toxicity for aquatic organisms and mammals. In: Gorsuch, J., Dwyer, F., Ingersoll, C., and La Point, T.W., eds. Environmental Toxicology and Risk Assessment STP 1216, Volume 2, pp. 610–619. American Society for Testing and Materials, Philadelphia, PA. Longcore, J.E., Pessier, A.P., and Nichols, D.K. (1999). Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227. Lutz, I., Kloas, W., Springer, T., Holden, L., Wolf, J., Kruger, H., and Hosmer, A. (2008). Development, standardization and refinement of procedures for evaluating effects of endocrine active compounds on development and sexual differentiation of Xenopus laevis. Anal. Bioanal. Chem. 390:2031–2048. Mazanti, L., Sparling, D.W., Rice, C., Bialeck, K., Stevenson, C., and Teels, B. (2003). Synergistic effects of a combined exposure to herbicides and an insecticide in Hyla versicolor. In: Linder E., Krest S.K., Sparling D.W., and Little E.E., eds. Multiple Stressor Effects in Relation to Declining Amphibian Populations, ASTM STP 1443, pp. 111–129. ASTM, West Conshohocken, PA. McClelland B.E., Wilczynski W., and Ryan M.J. (1996). Correlations between call characteristics and morphology in male cricket frogs (Acris crepitans). J. Exp. Biol. 199:1907–1919. McClelland, B.E., Wilczynski, W., and Ryan, M.J. (1998). Intraspecific variation in laryngeal and ear morphology in male cricket frogs (Acris crepitans). Biol. J. Linn. Soc. Lond. 63:51–67. McConkey, B.J., Dixon, G.G., and Greenburg, B.M. (2000). Fractional simplex designs for mixture interaction screening in complex mixtures. Biometrics 56:824–833. McDaniel, T., Martin, P.A., Struger, J., Sherry, J., Marvin, C.H., McMaster, M.E., Clarence, S., and Tetreault, G. (2008). Potential endocrine disruption of sexual development in free ranging male northern leopard frogs (Rana pipiens) and green frogs (Rana clamitans) from areas of intensive row crop agriculture. Aquat. Toxicol. 88:230–242. Mendelson, J.R. III, Lips, K.R., Gagliardo, R.W., Rabb, G.B., Collins, J.P., Diffendorfer, J.E., Daszak, P., Ibáñez, D R., Zippel, K.C., Lawson, D.P., Wright, K.M., Stuart, S.N., Gascon, C., da Silva, H.R., Burrowes, P.A., Joglar, R.L., La Marca, E., Lötters, S., du Preez, L.H., Weldon, C., Hyatt, A., Rodriguez-Mahecha, J.V., Hunt, S., Robertson, H., Lock, B., Raxworthy, C.J., Frost, D.R., Lacy, R.C., Alford, R.A., Campbell, J.A., Parra-Olea, G., Bolaños, F., Domingo, J.J.C., Halliday, T., Murphy, J.B., Wake, M.H., Coloma, L.A., Kuzmin, S.L., Price, M.S., Howell, K.M., Lau, M., Pethiyagoda, 769 R., Boone, M., Lannoo, M.J., Blaustein, A.R., Dobson, A., Griffiths, R.A., Crump, M.L., Wake, D.B., and Brodie, E.D., Jr. (2006). Confronting amphibian declines and extinctions. Science 313:48. Moore, A., and Waring, C.P. (1998). Mechanistic effects of a triazine pesticide on reproductive endocrine function in mature male Atlantic salmon (Salmo salar L.) parr. Pestic. Biochem. Physiol. 62:41–50. Moore, A., and Lower, N. (2001). The impact of two pesticides on olfactory-mediated endocrine function in mature male Atlantic salmon (Salmo salar L.) parr. Comp. Biochem. Physiol. B 129:269– 276. Morgan, M.K., Scheuerman, P.R., Bishop, C.S., and Pyles, R.A. (1996). Teratogenic potential of atrazine and 2,4-D using FETAX. J. Toxicol. Environ. Health 48:151–168. Mosconi, G., Yamamoto, K., Carnevali, O., Nabissi, M., PolzonettiMagni, A., and Kikuyama, S. (1994). Seasonal changes in plasma growth hormone and prolactin concentrations of the frog Rana esculenta. Gen. Comp. Endocrinol. 93:380–387. Mosconi, G., Crnevali, O., Franzoni, M.F., Cottone, E., Lutz, I., Kloas, W., Yamamoto, K., Kikuyama, S., and Polzonetti-Magni, A.M. (2002). Environmental estrogens and reproductive biology in amphibians. Gen. Comp. Endocrinol. 126:125–129. Muller, J.K., Gross, T.S., and Borgert, C.J. (2007a). Topical dose delivery in the reptilian egg treatment model. Environ. Toxicol. Chem. 26:914–919. Muller, J.K., Scarborough, J.E., Sepúveda, M.S., Casella, G., Gross, T.S., and Borgert, C.J. (2007b). Dose verification after topical treatment of alligator (Alligator mississippiensis) eggs. Environ. Toxicol. Chem. 26:908–913. Murphy, M.B., Hecker, M., Coady, K.K., Tompsett, A.R., Du Preez, L.H., Everson, G.J., Solomon, K.R., Carr, J.A., Smith, E.E., Kendall, R.J., Van Der Kraak, G.J., and Giesy, J.P. (2006a). Atrazine concentrations, gonadal gross morphology and histology in ranid frogs collected in Michigan agricultural areas. Aquat. Toxicol. 76:230–245. Murphy, M.B., Hecker, M., Coady, K.K., Tompsett, A.R., Higley, E.B., Jones, P.D., Du Preez, L.H., Solomon, K.R., Carr, J.A., Smith, E.E., Kendall, R.J., van der Kraak, G.J., and Giesy J.P. (2006b). Plasma steroid hormone concentrations, aromatase activities and GSI in ranid frogs collected from agricultural and non-agricultural sites in Michigan (USA). Aquat. Toxicol. 77:153–166. Murphy, M.B., Hecker, M., Coady, K.K., Tompsett, A.R., Jones, P.D., Solomon, K.R., Van Der Kraak, G., Carr, J.A., Smith, E.E., Kendall, R.J., and Giesy, J.P. (2006c). Sediment TCDD-EQs and EROD and MROD activities in Ranid frogs from agricultural and nonagricultural sites in Michigan (USA). Arch. Environ. Contam. Toxicol. 51:467– 477. Napier, J.D., Scheuerman, P.R., and Pyles, R.A. (1998). The effect of water hardness and humic acid on the teratogenicity and toxicity of atrazine using FETAX. SETAC Annual Meeting, November 18, 1988; Charlotte, NC. Nieuwkoop, P.O., and Faber, J. (1967). Normal table of Xenopus laevis(Daudin). North Holland, Amsterdam, the Netherlands: North Holland. Nieves-Puigdoller, K., Björnsson, B.T., and McCormick, S.D. (2007). Effects of hexazinone and atrazine on the physiology and endocrinology of smolt development in Atlantic salmon. Aquat. Toxicol. 84:27– 37. Nikkilä, A., Paulsson, M., Almgren, K., Blanck, H., and Kukkonen, J.V.K. (2001). Atrazine uptake, elimination, and bioconcentration by periphyton communities and Daphnia magna: Effects Downloaded At: 15:43 28 October 2008 770 K. R. SOLOMON ET AL. of dissolved organic carbon. Environ. Toxicol. Chem. 20:1003– 1011. Novartis. (2000). Summary of Environmental Fate of Atrazine. Greensboro, NC: Novartis Crop Protection, Inc. No. Study No. 1213-99. OECD. (2004). Detailed Review Paper on Amphibian Metamorphosis Assay for the Detection of Thyroid Active Substances. Paris, France: OECD. OECD Environment Health and Safety Publications Series on Testing and Assessment No. 46. No. ENV/JM/MONO(2004)17. http://www.epa.gov/scipoly/oscpendo/pubs/edmvac/oecd amphibian drp.pdf Oh, S.M., Shim, S.H., and Chung, K.H. (2003). Antiestrogenic action of atrazine and its major metabolites in vitro. J. Health Sci. 49:65–71. Oka, T., Tooi, O., Mitsui, N., Miyahara, M., Ohnishi, Y., Takase, M., Kashiwagi, A., Santo, N., and Iguchi, T. (2008). Effect of atrazine on metamorphosis and sexual differentiation in Xenopus laevis. Aquat. Toxicol. 87:215–226. Orton, F., Carr, J.A., and Handy, R.D. (2006). Effects of nitrate and atrazine on larval development and sexual differentiation in the Northern leopard frog Rana pipiens. Environ. Toxicol. Chem. 25:65– 71. Park, J.-W., Hecker, M., Murphy, M.B., Jones, P.D., Solomon, K.R., van der Kraak, G.J., Carr, J.A., Smith, E.E., Du Preez, L.H., Kendall, R.J., and Giesy, J.P. (2006). Development and optimization of a Q-RT PCR method to quantify CYP19 mRNA expression in testis of male adult Xenopus laevis: Comparisons with aromatase enzyme activity. Comp. Biochem. Physiol. B 144:18–28. Pastoor, T., Stevens, J.T., and Breckenridge, C.B. (2008). Evaluation of the potential effects of atrazine and its metabolites on the immune system: Routine toxicity studies conducted in rat, mouse and dog. Society of Toxicology; Seattle, WA, USA. Society of Toxicology, Poster 2140. Pechmann, J.E., and Wilbur, H.M. (1994). Putting declining amphibian populations in perspective: Natural fluctuations and human impacts. Herpetologica 50:65–84. Peterson, G., Johnson, L., Axler, R.P., and Diamond, S.A. (2002). Assessment of the risk of solar ultraviolet radiation to amphibians. II. In situ characterization of exposure in amphibian habitats. Environ. Sci. Technol. 36:2859–2865. Pieau, C., Dorizzi, M., and Richard-Mercier, N. (1999). Temperaturedependent sex determination and gonadal differentiation in reptiles. Cell Mol. Life Sci. 55:887–900. Pistl, J., Kovalkovicova, N., Legath, J., Novotny, J., Holovska, V., and Mikula, I. (2002). Metabolic activity of sheep peripheral blood phagocytes after exposure to selected pesticides in vitro. Bull. Vet. Inst. Pulawy 46:247–253. Pounds, J.A., and Crump, M.L. (1994). Amphibian declines and climate disturbance: The case of the golden toad and the harlequin frog. Conserv. Biol. 8:72–85. Pounds, J.A., Fogden, M.P.L., Savage, J.M., and Gorman, G.C. (1997). Tests of null models for amphibian declines on a tropical mountain. Conserv. Biol. 11:1307–1322. Pruett, S.B., Fan, R., Zheng, Q., Myers, L.P., and Hebert, P. (2003). Modeling and predicting immunological effects of chemical stressors: Characterization of a quantitative biomarker for immunological changes caused by atrazine and ethanol. Toxicol. Sci. 75:343–354. Qin, Z.F., Zhou, J.M., Chu, S.G., and Xu, X.B. (2003). Effects of Chinese domestic polychlorinated biphenyls (PCBs) on gonadal differentiation in Xenopus laevis. Environ. Health Perspect. 111:553–556. Reeder, A.L., Foley, G.L., Nichols, D.K., Hansen, L.G., Wikoff, B., Faeh, S., Eisold, J., Wheeler, M.B., Warner, R., Murphy, J.E., and Beasley, V.R. (1998). Forms and prevalence of intersexuality and effects of environmental contaminants on sexuality in cricket frogs (Acris crepitans). Environ. Health Perspect. 106:261–266. Reeder, A.L., Ruiz, M.O., Pessier, A., Brown, L.E., Levengood, J.M., Phillips, C.A., Wheeler, M.B., Warner, R.E., and Beasley, V.R. (2005). Intersexuality and the cricket frog decline: Historic and geographic trends. Environ. Health Perspect. 113:261–265. Rhodes, W.E. (1998). Health of alligator populations in South Carolina. In: Kendall R.J., Dickerson R., Giesy J.P., and Suk W., eds. Principles and Processes for Evaluating Endocrine Disruption in Wildlife, pp. 301–310. SETAC Press, Pensacola, FL. Richter, S.C., Young, J.E., Johnson, G.N., and Seigel, R.A. (2003). Stochastic variation in reproductive success of a rare frog, Rana sevosa: Implications for conservation and for monitoring amphibian populations. Biol. Conserv. 111:171–177. Robertson, J.C., and Kelley, D.B. (1996). Thyroid hormone controls the onset of androgen sensitivity in the developing larynx of Xenopus laevis. Dev. Biol. 176:108–123. Rohr, J.R., Elskus, A., A, Shepherd, B.S., Crowley, P.H., McCarthy, T.M., Neidzwiecki, J.H., Sager, T., Shi, A., and Palmer, B.D. (2003). Lethal and sublethal effects of atrazine, carbaryl, endosulfan, and octylphenol on the streamside salamander (Ambystoma barbouri). Environ. Toxicol. Chem. 22:2385–2392. Rohr, J.R., Elskus, A.A., Shepherd, B.S., Crowley, P.H., McCarthy, T.M., Niedzwiecki, J.H., Sager, T., Sih, A., and Palmer, B.D. (2004). Multiple stressors and salamanders: Effects of an herbicide, food limitation, and hydroperiod. Ecol. Appl. 14:1028–1040. Rohr, J.R., and Crumrine, P.W. (2005). Effects of an herbicide and an insecticide on pond community structure and processes. Ecol. Appl. 15:1135–1147. Rohr, J.R., and Palmer, B.D. (2005). Aquatic herbicide exposure increases salamander desiccation risk eight months later in a terrestrial environment. Environ. Toxicol. Chem. 24:1253–1258. Rohr, J.R., Sager, T., Sesterhenn, T.M., and Palmer, B.D. (2006). Exposure, postexposure, and density-mediated effects of atrazine on amphibians: Breaking down net effects into their parts. Environ. Health Perspect. 114:46–50. Saglio, P., and Trijasse, S. (1998). Behavioral responses to atrazine and diuron in goldfish. Arch. Environ. Contam. Toxicol. 35:484–491. Sanderson, H. (2002). Pesticide studies: Replicability of micro/mesocosms. Environ. Sci. Pollut. Res. 9:429–435. Sanderson, J.T., Seinen, W., Giesy, J.P., and van den Berg, M. (2000). 2-Chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: A novel mechanism for estrogenicity? Toxicol. Sci. 54:121–127. Sanderson, J.T., Letcher, R.J., Heneweer, M., Giesy, J.P., and van den Berg, M. (2001). Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitelllogenin production in male carp hepatocytes. Environ. Health Perspect. 109:1027–1031. Sassoon, D., and Kelley, D.B. (1986). Androgen-induced myogenesis and chondorgenesis in the larynx of Xenopus laevis. Am. J. Anat. 177:457–472. Sessions, S.K., Franssen, R.A., and Horner, V.L. (1999). Morphological clues from multilegged frogs: Are retinoids to blame? Science 284:800–801. Downloaded At: 15:43 28 October 2008 ATRAZINE EFFECTS ON FISH, AMPHIBIANS, REPTILES Shafer, T.J., Ward, T.R., Meacham, C.A., and Cooper, R.L. (1999). Effects of the chlorotriazine herbicide, cyanazine, on GABA(A) receptors in cortical tissue from rat brain. Toxicology 142:57–68. Sherman, C.K., and Morton, M.L. (1993). Population declines of Yosemite toads in the eastern Sierra Nevada of California. J. Herpetol. 27:186–198. Smith, E.E., du Preez, L.H., Gentles, B.A., Solomon, K.R., Tandler, B., Carr, J.A., Van Der Kraak, G.J., Kendall, R.J., Giesy, J.P., and Gross, T.S. (2005). Assessment of laryngeal muscle and testicular cell types in Xenopus laevis (Anura Pipidae) inhabiting maize and non-maize growing areas of South Africa. Afr. J. Herpatol. 54:69– 76. Solomon, K.R., Baker, D.B., Richards, P., Dixon, K.R., Klaine, S.J., La Point, T.W., Kendall, R.J., Giddings, J.M., Giesy, J.P., Hall, L.W.J., Weisskopf, C., and Williams, M. (1996). Ecological risk assessment of atrazine in North American surface waters. Environ. Toxicol. Chem. 15:31–76. Solomon, K.R., and Takacs, P. (2002). Probabilistic risk assessment using species sensitivity distributions. In: Posthuma L., Suter G.W., and Traas T., eds. Species Sensitivity Distributions in Ecotoxicology, pp. 285–313. CRC Press, Boca Raton, FL. Spanó, L., Tyler, C.R., Van Aerle, R., Devos, P., Mandiki, S.N.M., Thomé, J.-P., and Kestemont, P. (2004). Effects of atrazine on sex steroid dynamics, plasma vitellogenin concentration and gonad development in adult goldfish (Carasius auratus). Aquat. Toxicol. 66:369–379. Sparling, D.W., Feller, G.M., and McConnell, L.L. (2001). Pesticides and amphibian declines in California. Environ. Toxicol. Chem. 20:1591–1595. Sparling, D.W., and Feller, G.M. (2007). Comparative toxicity of chlorpyrifos, diazinon, malathion and their oxon derivatives to larval Rana boylii. Environ. Pollut. 147:535–539. Speare, R. (2001). Recommendations from Workshop in Getting the Jump on Amphibian Disease. In: Speare R., ed. Getting the Jump on Amphibian Disease, Developing Management Strategies to Control Amphibian Diseases: Decreasing the Risks Due to Communicable Diseases, pp. 131–147. School of Public Health and Tropical Medicine, James Cook University, Townsville, NZ. Stoker, T.E., Guidici, D.L., Laws, S.C., and Cooper, R.L. (2002). The effects of atrazine metabolites on puberty and thyroid function in the male Wistar rat. Toxicol. Sci. 67:198–206. Storrs, S.I., and Kiesecker, J.M. (2004). Survivorship patterns of larval amphibians exposed to low concentrations of atrazine. Environ. Health Perspect. 112:1054–1057. Stuart, S.N., Chanson, J.S., Cox, N.A., Young, B.E., Rodrigues, A.S.L., Fischman, D.L., and Waller, R.W. (2004). Status and trends of amphibian declines and extinctions worldwide. Science 306:1783– 1786. Sullivan, K.B., and Spence, K.M. (2003). Effects of sublethal concentrations of atrazine and nitrate on metamorphosis of the African clawed frog. Environ. Toxicol. Chem. 22:627–635. Suzaw,a M., and Ingraham, H.A. (2008). The herbicide atrazine activates endocrine gene networks via non-steroidal NR5A nuclear receptors in fish and mammalian cells. PLoS ONE. 3:e2117. doi:2110.1371/journal.pone.0002117. Tavera-Mendoza, L., Ruby, S., Brousseau, P., Fourier, M., Cyr, D., and Marcogliese, D. (2002a). Response of the amphibian tadpole (Xenopus laevis) to atrazine during sexual differentiation of the testis. Environ. Toxicol. Chem. 21:527–531. 771 Tavera-Mendoza, L., Ruby, S., Brousseau, P., Fourier, M., Cyr, D., and Marcogliese, D. (2002b). Response of the amphibian tadpole Xenopus laevis to atrazine during sexual differentiation of the ovary. Environ. Toxicol. Chem. 21:1264–1267. Tavera-Mendoza, L.E. (2001). Influences of atrazine on gonadal differentiation in Xenopus laevis tadpoles during metamorphosis [MSc thesis]. Montreal, PQ, Canada: Concordia University. 73 p. Tierney, K.B., Singh, C.R., Ross, P.S., and Kennedy, C.J. (2007). Relating olfactory neurotoxicity to altered olfactory-mediated behaviors in rainbow trout exposed to three currently-used pesticides. Aquat. Toxicol. 81:55–64. Tobias, M.L., Marin, M.L., and Kelley, D.B. (1993). The roles of sex, innervation, and androgen in laryngeal muscle of Xenopus laevis. J. Neurosci. 13:324–333. Trentacoste S.V., Friedman A.S., Youker R.T., Breckenridge C.B., and Zirkin B.R. (2001). Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. J Androl. 22:142–148. U.S. Fish and Wildlife Service. (1984). Houston Toad Recovery Plan. Albuquerque, NM: United States Fish & Wildlife. U.S. Fish and Wildlife Service. (2001). Wyoming Toad (Bufo baxteri)Population and Habitat Viability Assessment (PHVA). Laramie, WY: U.S. Fish and Wildlife Service. U.S. Fish and Wildlife Service. (2003). Threatened and Endangered Species System (TESS). United States Fish and Wildlife Service web site http://ecos.fws.gov/tess public/servlet/ gov.doi.tess public.servlets.VipListed?code=V&listings=0#D, accessed July 31 2005. U.S. Environmental Protection Agency. (1989). Methods for the Determination of Organic Compounds in Drinking Water. Cincinnati, OH: U.S. Environmental Protection Agency. No. EPA-600/4-88/039. U.S. Environmental Protection Agency. (1998). Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC) Final Report. Washington, DC: Endocrine Disruptor Screening and Testing Advisory Committee. http://www.epa.gov/scipoly/ oscpendo/edspoverview/finalrpt.htm U.S. Environmental Protection Agency. (2000). Atrazine: Evaluation of Carcinogenic Potential. Washington, DC: U.S. Environmental Protection Agency. Memorandum, Office of Prevention, Pesticides, and Toxic Substances. No. HED DOC. NO 014431. http://www.epa.gov/pesticides/reregistration/atrazine/carc-rpt.pdf U.S. Environmental Protection Agency. (2003a). White Paper on Potential Developmental Effects of Atrazine in Amphibians. Washington, DC: U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, Environmental Fate and Effects Division. www.epa.gov/scipoly/sap/index.htm U.S. Environmental Protection Agency. (2003b). A Set of Scientific Issues Being Considered by the Environmental Protection Agency Regarding: Potential Developmental Effects of Atrazine on Amphibians. Washington, DC: United States Environmental Protection Agency. Report of the FIFRA Scientific Advisory Panel Meeting, June 17–20, 2003,. No. SAP Report No. 2003-01. www.epa.gov/scipoly/sap/index.htm U.S. Environmental Protection Agency. (2003c). Office of Prevention, Pesticides and Toxic Substances. Interim Reregistration Eligibility Decision for Atrazine. U.S. EPA web site http://www.epa.gov/REDs/atrazine ired.pdf, accessed July 28 2005. Downloaded At: 15:43 28 October 2008 772 K. R. SOLOMON ET AL. U.S. Environmental Protection Agency, Biological and Economic Analysis Division Office of Pesticide Programs Office of Prevention, Pesticides, and Toxic Substances. (2004). Pesticides Industry Sales and Usage 2000 and 2001: Market Estimates. U.S. EPA web site http://www.epa.gov/oppbead1/pestsales/index.htm, accessed July 28 2005. U.S. Environmental Protection Agency. (2005). Draft Final Report on Multi-Chemical Evaluation of the Short-Term Reproduction Assay with the Fathead Minnow. Washington, DC: U.S. Environmental Protection Agency. No. 68-W-01-023. http://www.epa.gov/ scipoly/oscpendo/docs/edmvac/draft fish repro multichemical.pdf U.S. Geological Survey. (2004). Where Have All the Frogs Gone? USGS website http://www.usgs.gov/amphibian faq.html, accessed October 5 2004. van Tienhoven, A. (1983). Reproductive Physiology of Vertebrates. Ithaca, NY: Cornell University Press. Vertucci, F.A., and Corn, P.S. (1997). Evaluation of episodic acidification and amphibian declines in the Rocky Mountains. Ecol. Appl. 6:449–457. Vredenburg, V.T. (2004). Reversing introduced species effects: Experimental removal of introduced fish leads to rapid recovery of a declining frog. Proc. Natl. Acad. Sci. USA 101:7646–7650. Wallace, H., Badawy, G.M.I., and Wallace, B. (1999). Amphibian sex determination and sex reversal. Cell Mol. Life Sci. 55:901–909. Walsh, A.H., and Ribelin, W.E. (1975). The pathology of pesticide poisoning. In: Ribelin W.E., and Migaki E., eds.The Pathology of Fish, pp. 515–557. Madison: University of Wisconsin Press. Waring, C.P., and Moore, A. (2004). The effect of atrazine on Atlantic salmon (Salmo salar) smolts in fresh water and after sea water transfer. Aquat. Toxicol. 66:93–104. Weed, D.L. (1997). Methodologic guidelines for review papers. JNCI 89:6–7. Whale, G.F., Sheahan, D.A., and Kirby, M.F. (1994). Assessment of the value of including recovery periods in chronic toxicity test guidelines for rainbow trout (Oncorhynchus mykiss). In: Sublethal and Chronic Effects of Pollutants on Freshwater Fish, pp. 175–187. Fishing News (Books) Ltd, London. Whale, M.M., Loganathan, G., Yamashita, N., and Saito, T. (2003). Immunomodulation of human natural killer cell cytotoxic function by triazine and carbamate pesticides. Chem. Biol. Interact. 145:311– 319. Wiegand, C., Pflugmacher, S., Giese, M., Frank, H., and Steinberg, C. (2000). Uptake, toxicity, and effects on detoxication enzymes of atrazine and trifluoroacetate in embryos of zebrafish. Ecotoxicol. Environ. Safety. 45:122–131. Wiegand, C., Krause, E., Steinberg, C., and Pflugmacher, S. (2001). Toxicokinetics of atrazine in embryos of the zebrafish (Danio rerio). Ecotoxicol. Environ. Safety. 49:199–205. Winkelmann, D.A., and Klaine, S.J. (1991). Degradation and bound residue formation of four atrazine metabolites, deethylatrazine, deisopropylatrazine, dealkylatrazine and hydroxyatrazine, in a western Tennessee soil. Environ. Toxicol. Chem. 10:347– 354. Witschi, E. (1929). Studies on sex differentiation and sex determination in amphibians. III. Rudimentary hermaphrodites and Y chromosome in Rana temporaria. J. Exp. Zool. 54:157–222. Wolf, J.C. (2007). Supplementary analysis of X. laevis gonadal histology from Carr et al., 2003 and Coady et al., 2005 investigations. Greensboro, NC: Syngenta Crop Protection, Inc. Draft. No. T000799-07. Young, B.E., Lips, K.R., Reaser, J.K., Ibáñez, R., Salas, A.W., Cedeño, J.R., Coloma, L.A., Ron, S., La Marca, E., Meyer, J.R., Muñoz, A., Bolaños, F., Chaves, G., and Romo, D. (2001). Population declines and priorities for amphibian conservation in Latin America. Conserv. Biol. 15:1213–1223. Zeeman, M.G., and Brindley, W.A. (1981). Effects of toxic agents upon fish immune systems: A review. In: Sharma R.P., ed. Immunologic Consideration in Toxicology, pp. 1–47. CRC Press, Boca Raton, FL.