OTHER HALOGENATED POPs OF CONCERN
advertisement

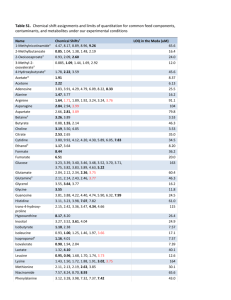
OTHER HALOGENATED POPs OF CONCERN LEVELS OF PERFLUOROOCTANE SULFONATE (PFOS) AND OTHER RELATED COMPOUNDS IN THE BLOOD OF JAPANESE PEOPLE Shigeki Masunaga1, Kurunthachalam Kannan2, Rikuo Doi3, Junko Nakanishi1, 4 and John P. Giesy2 1 Graduate School of Environment and Information Sciences, Yokohama National University, 79-7, Tokiwadai, Hodogaya, Yokohama 240-8501, Japan 2 National Food Safety and Toxicology Center, Michigan State University, East Lansing, MI 48824, U.S.A. 3 Department of Hygiene, School of Medicine, Yokohama City University, 3-9, Fuku-ura, Kanazawa, Yokohama 236-0004, Japan. 4 Research Center for Chemical Risk Management, National Institute of Advanced Industrial Science and Technology, 16-1 Onogawa, Tsukuba, Ibaraki 305-8569, Japan Introduction Despite of the situation that fluorinated organic compounds has been widely used for various purposes, contamination by these compounds in environmental or biological media has not been well documented compared with chlorinated or brominated compounds. Recently, however, with the development of analytical technique for these compounds, biological surveys became possible. Olsen et al. 1) found perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in the blood sera of employees in the fluorochemical manufacturing industry. Hansen et al. 2) reported the levels of PFOS, PFOA, perfluorooctanesulfonamide (PFOSA) and perfluorohexane sulfonate (PFHS) in human sera. The structures of these compounds are shown in Fig. 1. Studies of these compounds in wildlife from various places in the world have been performed 3, 4, 5,). They revealed that PFOS was the most prevalent in wildlife tissues among the fluorinated organics studied (PFOS, PFOSA, PFHS, and PFOA). PFOS was detectable in most samples (lever and plasma) including those from remote marine regions at concentrations greater than 1 ng/g, suggesting widespread distribution of these compounds on a global scale. Perfluorooctane sulfonate (PFOS) Perfluorohexane sulfonate (PFHS) Perfluorooctanesulfonylamide (PFOSA) Perfluorooctanoic acid (PFOA) CF3-CF2-CF2-CF2-CF2-CF2-CF2-CF2-SO3CF3-CF2-CF2-CF2-CF2-CF2-SO3CF3-CF2-CF2-CF2-CF2-CF2-CF2-CF2-SO2-NH2 CF3-CF2-CF2-CF2-CF2-CF2-CF2-COOH Figure 1. Structures of PFOS and related compounds In this study, we measured the PFOS, PFOSA and PFOA in Japanese blood samples. In Japan, major fluorochemical manufacturers produce derivatives of PFOA, not PFOS derivatives. This is different from that in the US, where PFOS derivatives are primarily used. Thus, the fluorochemical exposures in Japanese population may be different from that in the US. Materials and methods Ethical approval of the research plan As this study involves collection of human blood samples, study plan was submitted to the ethics ORGANOHALOGEN COMPOUNDS Vol. 59 (2002) 319 OTHER HALOGENATED POPs OF CONCERN committee belonging to the School of Medicine, Yokohama City University. The plan was approved by the committee on Feb. 19, 2001. Blood samples Volunteers of blood donor were from the Yokohama National University and Yokohama City University. Thus, the most of the donors are students or workers in these two universities. About 10 ml of blood was drawn into a Vacutainer (Becton Dickinson, Rutherford, NJ, USA) from each volunteer’s arm by a professional nurse at Kanagawa Prefectural Institute of Preventive Medicine on March 6, 2002. The samples were refrigerated without any preservatives and analyzed for fluorochemicals. Analysis Extraction and analytical procedure were basically the same as those of Hansen et al. 2). One ml of whole blood, 5 ml of internal standard, 1 ml of 0.5 M tetrabutylammonium solution (adjusted to pH 10), and 2 ml of 0.25 M sodium carbonate buffer were added to 15-ml polypropylene tube for extraction. After through mixing, 5 ml of methyl tert-butyl ether (MTBE) were added to the solution and mixed for 20 min. The organic and aqueous layers were separated by centrifugation and 4.0 ml of MTBE was removed from the solution. The aqueous mixture was rinsed with MTBE and separated two more times. Then all the rinses were combined in a second polypropylene tube. The solvent was allowed to evaporate under nitrogen gas before being reconstituted in 0.5 ml of methanol. The sample was vortex mixed for 30 seconds and passed through a 0.2-mm nylon mesh filter into a vial. Ten ml of sample was, then, injected into high performance liquid chromatograph (Hewlett-Packard HP1100) equipped with Keystone Betasil C18 column (50 x 2 mm, particle size 5 mm). The mobile phase was 2 mM ammonium acetate/methanol (300 ml/min) with gradient from 45% methanol to 90% in 9 min. A tandem mass spectrometer (Micromass Quattro II) was coupled with the liquid chromatograph and operated in the atmospheric pressure electrospray negative ion mode. Results and discussion Blood samples from 26 people, 24 Japanese and two foreigners from Asian countries, were analyzed. Two foreigners had lived in Japan more than a few years. The analytical results are shown in Table 1. Levels of PFOS and PFOSA were in the range of 2.0 - 20.2 and <1.3 - 4.8 ng/ml whole blood, respectively. PFOA concentrations were less than limit of quantitation (LOQ) of 3.35 ng/ml in whole blood. The distribution of PFOS concentration for all samples is shown in Figure 1. The arithmetic and geometric means of PFOS concentrations were 8.1 and 6.9 ng/ml, respectively. Average concentrations in female samples were slightly higher than that in male samples. The difference, however, was not significant. Figures 2 shows the age dependence of PFOS and PFOSA concentrations. PFOS concentration increased as the blood donor became older, especially for females. Its correlation, however, was week. Age dependent accumulation was more uncertain for PFOSA because more than half of the samples did not contain quantifiable concentrations. The relationship between PFOS and PFOSA was not significant. Hansen et al. 2) measured PFOS, PFOA, PFHS and PFOSA levels of 65 human serum samples. Their results showed that range of PFOS concentrations was 6.7 - 81.5 (average: 28.4) ng/ml serum. For comparison, our data were converted into serum basis. Human blood consists of about half volume of fluid (serum) and half volume of haemocytes. Thus, we can multiply whole blood based data by a factor of two, if the target compound is assumed not present in the haemocyte fraction of blood. As it is probable that haemocyte fraction also contains PFOS, multiplication by a factor of two may overestimate the serum based data. So, the arithmetic mean of 8.1 ng/ml on a whole blood basis for PFOS can be approximately 16.2 mg/ml on a serum basis. This value is about half of that reported for 320 ORGANOHALOGEN COMPOUNDS Vol. 59 (2002) OTHER HALOGENATED POPs OF CONCERN Table 1. PFOS, PFOSA and PFOA concentrations in whole blood from Japan* Sample No. Male/ Female PFOS (ng/ml) PFOSA*** (ng/ml) Sample No. Male/ Female PFOS (ng/ml) PFOSA*** (ng/ml) 1 2 3 4 5 6 7 8 9 10 11 12 13 Male Female Female Female Female Male Male Male Female Female Male** Male Male 10.2 20.2 12.8 12.8 3.1 8.1 15.2 6.8 6.1 8.9 6.3 2.3 9.0 4.3 < LOQ 3.0 2.4 < LOQ 3.2 2.7 2.3 1.4 2.9 < LOQ < LOQ 4.8 14 15 16 17 18 19 20 21 22 23 24 25 26 Female Male Male Male** Male Male Male Male Female Female Female Female Male 3.3 7.2 8.6 2.0 6.2 5.2 2.4 5.9 9.1 11.7 7.7 15.0 4.1 < LOQ < LOQ < LOQ < LOQ 1.5 < LOQ < LOQ < LOQ 3.6 NA NA < LOQ < LOQ Range (All samples) Arithmetic Mean (All samples) Geometric Mean (All samples) Arithmetic Mean (Japanese only) Geometric Mean (Japanese only) Arithmetic Mean (Japanese males) Arithmetic Mean (Japanese females) PFOS (ng/ml) PFOSA (ng/ml) 2.0 - 20.2 8.1 (n=26) 6.9 (n=26) 8.4 (n=24) 7.3 (n=24) 7.0 (n=13) 10.1 (n=11) <1.3 - 4.8 1.7 (n=24)**** 1.2 (n=24)**** 1.8 (n=22)**** 1.3 (n=22)**** 1.8 (n=13)**** 1.8 (n= 9)**** * PFOA was less than the LOQ (limit of quantitation) of 3.35 ng/ml for all samples. ** Foreigner from Asian country who had lived in Japan for a few years. *** NA = not analyzed. LOQ (limit of quantitation) = 1.28 ng/ml. **** Mean was calculated assuming that <LOQ samples had half of LOQ concentration (0.64 ng/ml). Figure 2. Distribution of PFOS concentration ORGANOHALOGEN COMPOUNDS Vol. 59 (2002) 321 OTHER HALOGENATED POPs OF CONCERN the US sera 2). This shows, on the one hand, that the Japanese are less exposed to PFOS compared with Americans, probably because major Japanese producers of perfluorinated surfactants do not use PFOS derivative as intermediates. On the other hand, it shows that Japanese are exposed to PFOS probably through imported products and/or global scale environmental transport. Further study of exposure route is necessary to understand the causes. The samples measured in this study are quite limited in number, both area-wise and age-wise. Thus, the present results may not necessarily represent general Japanese population. However, this is the first study of PFOS level in Japanese people and provides a rough estimate of present status. Figure 3. Age dependence and PFOS (left) and PFOSA (right) concentration in blood Acknowledgement The authors thank those students and workers in the Yokohama National University and Yokohama City University who volunteered to donate their blood. This study (sample collection part) was supported by Grant-in-Aid for the Development of Innovative Technology, Ministry of Education, Culture, Sports, Science and Technology. References 1. Olsen G.W., Burris, J.M., Burlew, M.M., Mandel, J.H. (2000) Drug Chem. Toxicol. 23, 603-620. 2. Hansen, K.J. Clemen, L.A., Ellefson, M.E., Johnson, H.O. (2001) Environ. Sci. Technol. 35, 766770. 3. Giesy, J.P, Kannan, K. (2001) Environ. Sci. Technol. 35, 1339-1342. 4. Kannan, K., Koistinen, J., Beckmen, K., Evans, T., Gorzelany, J.F., Hansen, K.J., Jones, P.D., Helle, E., Nyman, M., Giesy, J.P. (2001) Environ. Sci. Technol. 35, 1593-1598. 5. Kannan, K., Hansen, S.P., Franson, C.J., Bowerman, W.W., Hansen, K.J., Jones, P.D., Giesy, J.P. (2001) Environ. Sci. Technol. 35, 3065-3070. 322 ORGANOHALOGEN COMPOUNDS Vol. 59 (2002)