Recent progress in fundamental constants and the International System of Units Precision Physics and Fundamental Physical Constants
advertisement

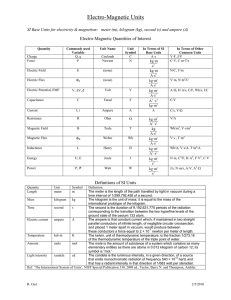
Third Workshop on Precision Physics and Fundamental Physical Constants 6 December 2010 Recent progress in fundamental constants and the International System of Units Peter Mohr National Institute of Standards and Technology Gaithersburg, MD, USA Related Publications Redefinition of the kilogram: a decision whose time has come Metrologia 42, 71 (2005). IM Mills, PJ Mohr, T Quinn, BN Taylor, M Williams Redefinition of the kilogram, ampere, kelvin and mole: a proposed approach to implementing CIPM recommendation 1 (CI­2005), Metrologia 43, 227 (2006). IM Mills, PJ Mohr, T Quinn, BN Taylor, M Williams Defining units in the quantum based SI Metrologia 45, 129 (2008). PJ Mohr Resource Letter FC-1: The physics of fundamental constants American Journal of Physics 78, 338 (2010). PJ Mohr and DB Newell URL: physics.nist.gov/constants International System of Units (SI) SI base units and symbols − − − − − − − Some SI derived units and symbols − − − − − meter m (length) kilogram kg (mass) second s (time) ampere A (electric current) kelvin K (thermodynamic temperature) mole mol (amount of substance) candela cd (luminous intensity) hertz Hz (frequency) newton N (force) joule J (energy) coulomb C (electric charge) volt V (electric potential difference) Non­SI units and symbols − − electron volt eV (energy) unified atomic mass unit u (mass) Kilogram International Kilogram prototype manufactured in 1880s: Cylinder of 90 % platinum and 10 % iridium 39 mm high and 39 mm in diameter 1st General Conference on Weights and Measures (CGPM) in 1889: This prototype shall henceforth be considered to be the unit of mass. 3rd CGPM in 1901: The kilogram is the unit of mass; it is equal to the mass of the international prototype of the kilogram Limitations of the current kilogram prototype definition The prototype definition is not linked to an unchanging property of nature. The mass of the international prototype appears to be changing relative to the mass of its copies. The drift of the kilogram prototype together with its copies (relative to an unchanging standard) could be as large as 20 ҳ 10­9 kg per year (Williams et al. 1998; Davis 2003). The prototype and its copies appear to gain mass over time and lose mass when washed for use in comparisons. The kilogram mass definition cannot be realized independently of the international prototype. Possible redefinitions of the kilogram The limitations on stability of the definition of the kilogram in terms of the international prototype could be eliminated if the kilogram were defined in terms of a fundamental constant in analogy with the definition of the meter. 17th CGPM in 1983: The meter is the length of the path traveled by light in vacuum during a time interval of 1/299 792 458 of a second. As a consequence for the velocity of light c: An alternative statement of the definition could be: The meter is the length scaled such that the velocity of light is 299 792 458 m/s. Experimental realization of the kilogram (watt­balance experiment) A horizontal circular coil is suspended in a radial magnetic field The current in the coil is adjusted so that the net magnetic forces equal the force of gravity on a 1 kg mass The coil is slowly moved with a measured velocity through the magnetic field to calibrate the field strength and the geometry factors Electrical measurements are made in terms of the Josephson and von Klitzing constants: KJ2RK = 4/h NIST 1998 Ed Williams Dave Newell Rich Steiner Ruimin Liu Experimental realization of the kilogram (watt­balance experiment) Current interpretation: − Alternative interpretation: − precise kilogram mass + watt­balance experiment → h unknown mass ← watt­balance experiment + h (defined value) This suggests a possible new definition of the kilogram: The kilogram is the unit of mass scaled such that the Planck constant is exactly 6.626 068 96 ҳ 10­34 J s. Experimental realization of the kilogram (X­ray­crystal­density method) Current interpretation: − Alternative interpretation: − known mass silicon sphere + volume measurement + lattice spacing measurement + silicon isotopic composition measurement → NA known mass silicon sphere ← volume measurement + lattice spacing measurement + silicon isotopic composition measurement + NA This suggests a possible new definition of the kilogram: The kilogram is the unit of mass scaled such that the Avogadro constant is exactly 6.022 141 79 ҳ 1023 mol­1. Relation between the Avogadro constant NA and the Planck constant h Rydberg constant definition: unified atomic mass unit u: Avagadro constant: Limitations of the present ampere definition 9th CGPM in 1948: The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross­section, and placed one metre apart in vacuum, would produce between these conductors a force equal to 2 x 10­7 newton per metre of length. Modern voltage measurements are based on the Josephson effect: KJ = 2e/h [2.5 x 10­8] Modern resistance measurements are based on the quantum Hall effect: RK = h/e2 [6.8 x 10­10] To express measurements with better accuracy, arbitrary exact units (not SI) are used: KJ → KJ­90 = 483 597.9 GHz/V RK → RK­90 = 25 812.807 Ω Possible ampere redefinition to make e exact The ampere is the electric current scaled such that the elementary charge is 1.602 176 487 ҳ 10­19 coulomb. Consequences: for ε0 Josephson constant (voltage measurements) von Klitzing constant (resistance measurements) (the latter two equalities are assumed to be exact) Possible kelvin redefinition to make k exact The kelvin, the unit of thermodynamic temperature, is scaled such that the Boltzmann constant is exactly 1.380 650 4 ҳ 10­23 joule per kelvin. Possible mole redefinition to make NA exact The mole, the unit of amount of substance, is scaled such that the Avogadro constant is exactly 6.022 141 79 ҳ 1023 per mole. Relative standard uncertainties for a selection of fundamental constants multiplied by 108 (i.e. in parts per hundred million) constant current SI new SI m(K ) 0 5.0 h 5.0 e 2.5 kB NA R F σ 170 5.0 170 2.5 700 constant current SI new SI α 0.068 0.068 0 KJ 2.5 0 0 RK 0.068 0 0 µ0 0 0.068 0 ε0 0 0.068 0 Z0 0 0.068 0 qP 2.5 0.034 0 J↔kg 0 0 me 5.0 0.14 J↔m–1 5.0 0 mu 5.0 0.14 J↔Hz 5.0 0 m(12C) 5.0 0.14 J↔K M(12C) 0 0.14 J↔eV 170 2.5 0 0 Quantum SI and Unit democracy In the Quantum SI, the fundamental constants, c, h, e, k, NA, νCs, ... are given fixed numerical values. These constants determine the scales of the base units. The defined set of base units determines all the units in the SI. The conclusion is: assigning fixed values to the fundamental constants, c, h, e, k, NA, νCs, ... determines all the units in the SI. The distinction between base units and other units is unnecessary. Time scale for possible redefinitions At its meeting in September 2010, the Consultative Committee on Units (CCU) prepared a draft of the form the redefined units might take. The draft was presented to the International Committee on Weights and Measures (CIPM) at its meeting in October 2010 for consideration. The General Conference on Weights and Measures (CGPM) next meets in 2011, and it could approve the new definitions for implementation when the results of the relevant experiments are satisfactory Conclusion The SI can be improved by modernizing the way units are defined. In particular, the current definitions of the kilogram, ampere, kelvin and mole are based on 19th century science and technology and can be replaced by ones that take into account subsequent progress in physics. If an update of the SI is done by specifying values of the fundamental constants as discussed, many constants would become exact and others would have smaller uncertainties. With such an update of the SI, the concepts of base units and derived units would not be not necessary.