LBNL MC252 Oil Leak Research Team
advertisement

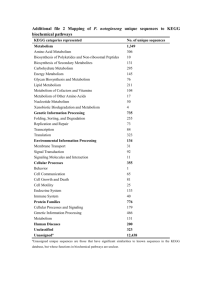
LBNL MC252 Oil Leak Research Team Terry C. Hazen, Olivia U. Mason, Eric A. Dubinsky, Todd Z. DeSantis, Gary L. Andersen, Yvette M. Piceno, Navjeet Singh, Janet K. Jansson, Alexander Probst, Sharon E. Borglin, Julian L. Fortney, William T. Stringfellow, Markus Bill, Mark S. Conrad, Lauren M. Tom, Krystle L. Chavarria, Thana R. Alusi, Regina Lamendella, Dominique C. Joyner, Chelsea Spier, Jacob Baelum, Manfred Auer, Marcin L. Zemla, Romy Chakraborty, Eric L. Sonnenthal, Patrik D'haeseleer, Hoi-Ying N. Holman, Shariff Osman, Zhenmei Lu, Joy D. Van Nostrand, Ye Deng, Jizhong Zhou, Kelly Wetmore, Jennifer Kuehl, Rachael Mackelprang, Cindy Wu, Jen Lim, Fran Reid, Joern Larson, Jenni Hultman, Susannah G. Tringe James Han, Tringe, Han Patrick Chain, Chain Christian Rinke, Rinke Tanja Woyke, Woyke Theresa Pollard, Pollard Edward M. M Rubin. Rubin http://vimss.lbl.gov/horizonwiki/ tchazen@lbl.gov http://www.nola.com/news/gulf-oil-spill/deepwater-disaster/index.ssf Hydrocarbon Composition Light Macondo Crude • • • • • • Macondo Oil is light crude specific gravity of 850 kg/m3 (API gravity 37.2°, Exxon V ld 29°) Valdes typical for the Gulf of Mexico oil reservoirs at 6,000 m carbon isotope signature is 27.42 ‰ ± 0.10 δ13CPDB 35% evaporates within 2 days in a wind tunnel 45% in 2 weeks North Slope Heavy Crude Where the oil went? The Federal Interagency Solutions Group, Oil Budget Calculator Science and Engineering Team (November, 2010) Systems Biology Approach ALS ALSsynchrotron imaging Hydrocarbon/Stable Isotope/Nutrient, Physical/Chemical analyses Lipids PLFA RNA Single cell isolation Metatranscriptomics (JGI) Isolation/phenotypic characterization, cell counts Cloning & sequencing Single cell sequencing ((JGI)) DNA PCR 16S rRNA genes Metagenomics (JGI) GeoChip (UO) PhyloChip 454 pyrotag sequencing (JGI) Research Vessels R/V Gyre R/V Brooks McCall R/V Ocean Veritas R/V Ferrel Missions and Sampling • • • • • • R/V Ocean Veritas – May 25 to June 11, Missions 1-3 R/V Brooks McCall – May 29 to June 27, Missions 5-9 R/V Ferrel – July 3 – August 29 29, Missions 1 1-9 9 R/V Gyre – September 11 – October 20, Missions 1-5 Filtered samples for ecogenomics (1-20 L) -80°C Fixed samples for direct cell counts, hydrocarbons, isotopes, metals, and nutrient analysis (10-125 ml) 4°C • Live samples for isolation and lab simulations (1-20 L) 4°C (1.2 km to 384 km in all directions from well head, collaborations with: University U es yo of Alabama, aba a, Florida o da S State aeU University, e s y, Gu Gulf Coas Coast Research esea c Lab – USM, Rutgers University, University of Oklahoma, Penn State University, University of Georgia, Scripps, University of Tulsa) http://vimss.lbl.gov/horizonwiki/index.php/Main p g p p _Page g Sample collection & processing Station BM58 Deep Plume Bathymetry Camilli et al. August 19, 2010, Sciencexpress Sampling sites around the MC252 well head from May 25 to June 7, 2010 Oil biodegradation Oxygen Oxygen Carbon dioxide pH Daughter Products Microbe Daughter aug te Products oducts Temperature Daughter Products Petroleum Fertilizer (N, P, Fe) Water Characteristic depth profiles for distances from the well head and one non-plume site (diamonds = cell density) Dispersed MC252 plume and non-plume parameters at 1099-1219 m Plume Non-Plume P Berkeley Lab PhyloChip3 detects 50,000 different bacteria and archaea in one test Location and intensity of fluorescence determines occurrence and relative abundance of 16S rRNA genes. 951 subfamilies were detected in 62 bacterial phyla. Only 16 subfamilies in -proteobacteria significantly enriched in plume -proteobacteria enriched in oil plume Plume samples are distinct from Pre-spill and Non-plume Pre-Spill samples from Dr. Hollibaugh, UGA ANOSIM Results Deepwater sites using nonmetric multidimensional scaling ordination of Bray-Curtis distances (stress = 3.98 and 4.55, respectively). Significantly different by permutational analysis of variance (p = 0.005 for both) 16S rRNA phospholipids Microbial community composition (Pyrotag) 93% 80% 3.0% Single Cell Sequence Hydrocarbon Biodegradation Pathways to Degrade the Compounds Found in Crude Oil by Marine Microbes Oil by Marine Microbes CH3 COOH n-Alkane OH Alcohol + H2O COOH OH OH OH OH Aldehyde COOH NH2 Fatty acid Catechol CH3 TCA Ringg fission FAD Fatty acyl CoA COOH COOH Acetyl CoA O CH3 -C-SCoA OH OH HOOC Protocatechuate CH3 O R-CH R CH2 -CH CH2 -C-SCoA C SCoA CO2 OH COOH COOH COOH HO OH OH O R-C-SCoA FADH2 O R-CH CH-C-SCoA H2O CoASH O O R-C-CH2 -C-SCoA OH O R-CH -CH2 -C-SCoA OH OH OCH3 OH H + + NADH + NAD + Slide courtesy of Dr. Ron Atlas Summary of GeoChip 4.0 probe and sequence information by functional gene category No. of gene categories 11 No. sequences retrieved No. of probes designed No. CDS covered 15754 3349 5547 Bacterial phage 40 3644 1100 2083 Carbon degradation 33 21529 9033 13667 Carbon fixation 5 5252 1762 3398 Methane metabolism 3 9718 507 1677 Nitrogen cycling 17 47988 7552 17550 Phosphorus utilization 3 3783 1378 2261 Stress response 45 75305 21574 41033 Sulfur cycling 6 8078 3254 4461 Metal resistance 44 25277 9478 17575 Contaminant degradation 184 44220 17919 30361 Energy process 4 1762 862 1131 Virulence 13 16762 3732 7444 Others ( gyrB, bchY) 2 7830 2492 4226 410 286,902 83,992 152,414 Functional process Antibiotic resistance Total • > 400 functional gene categories • Universal standards to allow data comparison across different experiments & times Hydrocarbon degradation genes are highly enriched Functional Gene correlations with oil hydrocarbons Dominant bacteria at 1099-1219 m, SEM and acridine orange stain inset with distance from wellhead BM58 10km, 1179m BM54 1.3km, 1194m Floc SR-FTIR spectral analysis SR-FTIR analysis of Surface Water Sample Protein (N-H) heat map Alkane (C-H) heat map Thin oil film Reflectance ( ) µ µ 100 80 60 40 C-H C-H 20 3800 3600 3400 3200 3000 2800 2600 2400 2200 Wavenumber 2000 1800 1600 1400 1200 1000 8 (cm-1) 0.0 100 Normalized intensity 1.0 80 60 Oxidation signatures N-H amide A off protein t i 40 20 3800 3600 3400 3200 3000 2800 2600 2400 Wavenumber 2200 2000 1800 1600 1400 1200 1000 Oxidation products (C=O) heat map 80 (cm-1) A typical spectrum of oil from MC252 Distance (micrometers)=137 µm Refleectance Reeflectance 3 micron 3 micron Microbial cells and oil degradation g 100 80 60 40 3800 3600 3400 3200 3000 2800 2600 2400 2200 2000 1800 Wavenumber (cm-1) 1600 1400 1200 1000 80 Oxidation products (O-H) (O H) heat map Bacteria and oil drop Bright-field, 100X MC-252 alkane half-life (days) from field and laboratory with currents of 2 - 5 days to move 10 km from source. Alkane Ratios by site C26/C15 C26/C16 C26/C17 3 50 3.50 3.00 2 50 2.50 2.00 3.40 1.50 2 84 2.84 1.00 1.80 1.61 0.50 0 85 0.85 1.02 0.56 0.43 0.37 OV01005 OV01104 1.00 0.66 1.48 00.91 91 0 89 0.89 0.00 MC252 BM053104 BM054104 BM057104 BM058104 1km 1km 5km 10km Systems biology “the omics” Metagenomics and Metatranscriptomics Emulsion PCR of samples Extract microbial b l DNA and RNA Sheer DNA/RNA Emulsion PCR Non‐oil samples Sample Sample Sample 1 2 3 Metagenomic sequences Metatranscriptomics reveals in situ reveals in situ activity Sequencing with Illumina sequencing platform Genes involved in hydrocarbon degradation 1 0.01 Proximal station metatranscriptome Distal plume Uncontaminated Proximal plume 0.001 Tolu uene Arom matic Ethylbenz nzene Par articulate metha ane monooxygen enase Ring hydroxyla R ating Cyclohexa anol dehydrogen enase n-alk kane Benz zoate Cyclohexan none Aldehy hyde dehydrogen nase 0.00001 Alco ohol dehydrogen nase 0.0001 FattyA Acid Gen ne relative abundan ance 0.1 Dominant genes in the metagenome and metatranscriptome code for cycloalkane and n-alkane (C6 to C20) degradation. Oceanospiralles model Alkanes CRISPR regions to protect from phage predation Metabolome (metabolic pathways) O Glycan biosynthesis O Mannosyl glycan biosynthesis N Glycan biosynthesis Glycan Biosynthesis and Metabolism High mannose type N glycan biosynthesis Glycosphingolipid biosynthesis globoseries Glycosphingolipid biosynthesis lact and neolacto series Butirosin and neomycin biosynthesis Lipopolysaccharide biosynthesis Glycosaminoglycan biosynthesis chondroitin sulfate Other glycan degradation Puromycin biosynthesis Biosynthesis of ansamycins Glycosaminoglycan biosynthesis heparan sulfate [B] Glycosyltransferases Nucleotide Metabolism Streptomycin biosynthesis Thiamine metabolism Purine metabolism Starch and sucrose metabolism • Oceanospirillales possess genes that code for chemotaxis and motility as well as those for degradation of C6-C20 alkanes and cycloalkanes. l lk • Monooxygenases decreased probably due to the abundance of easy to degrade alkanes initially. • Oceanospirillales exhibited a rapid chemotactic response to the presence of oil, oil aliphatic hydrocarbons, but not methane, which was very abundant. Glycosaminoglycan degradation Galactose metabolism [B] Proteoglycans Caffeine metabolism Ascorbate and aldarate metabolism Glycosylphosphatidylinositol (GPI) anchor biosynthesis Glycosaminoglycan Glycosphingolipid biosynthesis biosynthesis Arachidonic acid keratan sulfate ganglioseries metabolism Metabolism of Cofactors and Vitamins Folate biosynthesis beta Alanine metabolism alpha Linolenic acid metabolism Polyketide sugar unit biosynthesis Pyrimidine metabolism Pentose and glucuronate interconversions Riboflavin metabolism Vitamin B6 metabolism Tyrosine metabolism Biosynthesis of vancomycin group antibiotics Amino sugar and nucleotide sugar metabolism Pentose phosphate metabolism Fructose and mannose metabolism Biosynthesis of 12 , 14 16 membered macrolides and Phenylalanine , tyrosine and tryptophan biosynthesis Glycerophospholipid metabolism Linoleic acid metabolism One carbon pool by folate Phenylalanine metabolism Isoquinoline alkaloid biosynthesis Photosynthesis antenna proteins Phenylpropanoid biosynthesis [B] Phytochemical Compounds Lipid Metabolism Ether lipid metabolism Fatty acid biosynthesis Taurine and hypotaurine metabolism Sulfur metabolism Glycolysis / Gluconeogenesis Biosynthesis of siderophore group nonribosomal peptides [B] Photosynthesis Proteins Fatty acid metabolism Biosynthesis of type II polyketide products Retinol metabolism Acridone alkaloid biosynthesis Photosynthesis Inositol phosphate metabolism 3 Chloroacrylic acid degradation D Alanine metabolism Benzoxazinone biosynthesis Glycine, serine and Glycine threonine metabolism Anthocyanin biosynthesis Limonene and pinene degradation Monoterpenoid biosynthesis Selenoamino acid metabolism Propanoate metabolism Sesquiterpenoid biosynthesis Lipoic acid metabolism Terpenoid backbone biosynthesis Caprolactam degradation Methane metabolism Primary bile acid biosynthesis Clavulanic acid biosynthesis Nitrogen metabolism Cystein and methionine metabolism Citrate cycle (TCA cycle) Atrazine degradation Porphyrin and chlorophyll metabolism Valine, leucine and isoleucine degradation C5 Branched dibasic acid metabolism Carbon fixation Steroid biosynthesis Insect hormone biosynthesis Lysine degradation Reductive carboxylate cycle (CO2 fixation) Tetrachloroethene degradation Pantothenate and CoA biosynthesis Cyanoamino acid metabolism Fatty acid elongation in mitochondria Butanoate metabolism Valine, leucine and isoleucine biosynthesis Synthesis and degradation of Ketone bodies Biosynthesis of type II polyketide backbone Zeatin biosynthesis Ubiquinone and other terpenoid quinone biosynthesis Phosphonate and phosphinate metabolism Pyruvate metabolism Flavone and flavonol biosynthesis Indole alkaloid biosynthesis Tryptophan metabolism Energy Metabolism Peptidoglycan biosynthesis Carotenoid biosynthesis Flavonoid biosynthesis Amino Acid Metabolism Carbohydrate Metabolism Sphingolipid metabolism Biosynthesis of unsaturated fatty acids Isoflavonoid biosynthesis Biosynthesis y of Other Secondary Metabolites Novobiocin N bi i biosynthesis Glycerolipid metabolism [B] Lipids D Arginine and D ornithine metabolism Alanine , aspartate and glutamate metabolism Glyoxylate and dicarboxylate metabolism Steroid hormone biosynthesis Glucosinolate biosynthesis Tetracycline biosynthesis 1,4 Dichlorobenzene degradation Naphthalene and anthracene degradation Diterpenoid biosynthesis Biphenyl degradation Metabolism of Terpenoids and Polyketides Secondary bile acid Secondar biosynthesis Carbazole degradation 1,2 Dichloroethane degradation Fluorobenzoate degradation Oxidative O idati e phosphorylation Brassinosteroid biosynthesis 2,4 Dichlorobenzoate degradation Cytochrome Xenobiotics Biodegradation[B] P450 Substrates and Metabolism Bisphenol A degradation Glutathione metabolism Styrene degradation Fluorene degradation Benzoate degradation via CoA ligation Arginine and proline metabolism gamma Hexachlorocyclohexane degradation Toluene and xylene degradation Benzoate degradation via hydroxylation [B] Cytochrome P450 Ethylbenzene degradation 1,1,1 Trichloro 2,2 bis(4 chlorophenyl) ethane (DDT) degradation Tropane, piperidine and pyridine alkaloid biosynthesis Penicillin and cephalosporin biosynthesis D Glutamine and D glutamate metabolism beta Lactam resistance Metabolism of Other Amino Acid Biotin metabolism Nicotinate and nicotinamide metabolism Deep-Sea Plume 7/27-8/26/10 5-310 km All samples BDL CTDfluorescence (<1 ppm) and chemical analysis for all petroleum hydrocarbons (<2 ppb) b) N=170 N 170 Community analysis of deep-sea GOM samples • • • • 82 samples analyzed with PhyloChip so far from 5/27/10 – 8/24/10 Nearly all plume samples different from background Microbial community in several DO dip samples with no detected hydrocarbons similar to plume communities Some DO dip samples collected 6-weeks after well was capped still similar to plume samples 17 samples from Science paper have outlined symbols DO dips measured after well was capped are circled Cluster Analysis of PhyloChip OTU abundance from BioTraps incubated 28 Days Corexit Degradation -- mixed community of microbes cultured from plume depth, non-plume water 6 bis(2‐ethylhexyl)sulfosuccinate 90 4°C 5 Glycol compounds 25 4°C 80 Branched alkanes 25°C 25°C 4°C 20 70 25°C 4 60 50 mg/L mg/L mg//L 15 3 40 10 2 30 5 20 1 10 0 0 3 5 day 7 10 0 0 12 ( total glycol propylene ethylhexyl)sulf cmpds glycol osuccinate 0 0 3 5 all compounds day 7 10 branched alkanes 12 3 5 day 7 10 12 Corexit, 4°C k T 1/2 day days ‐1 0.021 32.2 0.071 9.7 ‐0.002 na ‐0.017 na 0.068 10.1 Corexit, 25°C k T 1/2 day days ‐1 0.011 62.3 0.083 8.4 ‐0.015 na ‐0.044 na 0.027 25.9 • Glycol compounds do not show any degradation in the laboratory • Bis Bi (2-ethylhexyl) (2 h lh l) sulfosuccinate lf i shows h more degradation d d i at 4°C • Branched alkanes degrade about the same at 4°C and 25°C Hydrocarbon mineralization Control (no addition) Oil Oil + Corexit Corexit Control (no addition) Killed control Killed control with oil + Fe Oil Oil + Corexit Oil + Fe F Oil + Corexit + Fe Degradation of Oil and COREXIT Oil degradation Oil Oil + Corexit Oil + Fe Oil + Corexit + Fe COREXIT degradation Sediment Analyses 120 multi-core sediment samples (64 R/V Gyre and 56 R/V Ocean Veritas). 29% showed potential indication of contamination by oil, qualitatively, only y 7 ((6%)) exceeded aquatic q life benchmarks all located within 0.33‐2.7 km of the wellhead Operational Science Advisory Team – Unified Area Command (OSAT, 12/17/10) Federal On-scene Coordinator U.S. Coast Guard “Other than (sediments near the wellhead and near shore tar mats) the OSAT found: • No deposits of liquid‐phase MC252 oil in sediments. • No exceedances of EPA’s Human H lth benchmark Health b h k in i water. t • No exceedances of EPA’s dispersant benchmarks. g , no exceedances off the • Since 3 August, aquatic life benchmark for PAHs in water that were consistent with MC252 oil. • Since 3 August, August no exceedances of the aquatic life benchmark for PAHs in sediment beyond 3 km of the wellhead” Slicing frozen sediment cores Flocs - remains of plume? Grains with alkane (C H) Grains with clay (Al Al OH) 1.0 SR‐FTIR analysis of sediment sample SE‐20101001‐GY‐LBNL1‐BC‐124‐core 3 100 CH Sulfate minerals (S O) Normalize ed intensity Tran nsmittance (%) Grains with cement and clinker phase components Carbonate minerals ( COO ) Al‐Al‐OH COO A typical sediment spectrum sho in alkane cla showing alkane, clay, cement‐ and clinker phase‐ like absorption features Al O S O Si O 20 4,000 3,000 2,000 1,500 1,000 Wavenumber (cm‐1) Tricalcium aluminates (Al‐O) 0.0 Orthosilicate minerals (Si (Si‐O) O) Scale bar = 50 µm Elmer’s Beach •Oil degrading bacteria were found in high abundance •Community C i structure changed h d spatially i ll and d temporally ll •Members of Rhodobacteraceae family seem to dominate in the high TPH beach samples. Day 0 Alcanivorax str. str Abu-1 Day 2 Day 7 GoM Dead Zones From DiMarco et al., 2010 Input of Oil in North America (Oil in the Sea III - National Academy of Science, 2003) Houston Chronicle – Monday, October 4, 2010 http://academy.asm.org/index.php/colloquia-reports/faq-series/291-faq-microbes-and-oil-spills http://vimss.lbl.gov/horizonwiki horizonwiki// “…I hope you still feel small. When you stand by the Ocean…”